1. Introduction
Conch is a common name that is applied to a number of medium to large-sized shells of large snails (
Turbinella pyrum) from the family Turbinellidae [
1]. Structurally, conch is a porcelaneous shell of an oblong or conical form with bulging in the middle and tapering at each end [
2]. Conch (
Shankha) prepared as conch shell ash, known in Ayurvedic literature as
Shankha Bhasma, is traditionally used in Ayurveda to treat many ailments [
3]. According to Ayurveda, conch has cooling (
sheetal), alkaline (
kshariya) and adsorbent (
grahi) properties; has detoxifying (
vishahara), complexion enhancing (
varnya) and strengthening (
balya) actions and is useful for hyperacidity (
amlapitta), loss of appetite (
agnimandya), irritable bowel syndrome (
grahani), pain in the abdomen (
parinaam shool) and acne vulgaris (
tarunya pidika) when administered within the maximum therapeutic dose of 250 mg per dose [
4]. It is also used internally against dysentery, gonorrhoea, colic, dyspepsia and jaundice [
3] in various classical formulations such as
Kaphaketu rasa (Vol. 1, p. 286),
Chandrodaya Varti (Vol. 2, p. 193),
Shankha Vati (Vol. 5, p. 104),
Sutashekhar Rasa (Vol. 5, p. 371), as one of the major ingredients [
5]. Recent research on incinerated conch has focused on pharmaceutical standardization of compressed tablets along with estimation of calcium content and acid neutralization capacity as an antacid [
6]. Another comparative clinical study of
Shankha Bhasma, prepared after purifying with two different methods, was conducted, but no attempt was made to standardize and characterize the incinerated conch. In this clinical study, lemon purified
Shankha Bhasma revealed significant resolution (
p < 0.005) of Gastroesophageal Reflux Disease (GERD) symptoms as compared to sour gruel purified
Shankha Bhasma [
7]. Various classical references of
Shankha Bhasma and its efficacy reports have been reviewed.
Shankha Bhasma prepared traditionally, and in a muffle furnace, depicted an acid neutralization capacity of 9.5 mE and 7.05 mE, respectively [
8]. It also showed in vivo dose dependent protection against gastric ulcer and anti-peroxidative effect without altering serum calcium level, but additional mucin production as compared to standard Ranitidine [
9]. It has also shown antispasmodic effect on acetylcholine induced excised rat ileum compared to atropine, therapeutic response to acetic acid induced writhing test in rats and in vitro anti-inflammatory activity related to inhibition of protein denaturation [
10]. However, protocol standardization and characterization data which are the basis of further investigations are found to be insufficient in the literature.
Rasashastra is the science of mercury and the branch of Ayurvedic Pharmaceuticals that deals with the preparation of medicines from metals like gold, silver and so forth, and minerals like mica, chalcopyrite and so forth.
Shankha (Conch) is mentioned in Rasashastra as a mineral under
Sudha Varga (Calcium group) category [
11] and is one of the important Bhasmas used in daily practice by Ayurvedic physicians.
Shankha Bhasma is generally described to be prepared by soaking the shell in lime juice and calcination in covered crucibles ten to twelve times and finally reducing it to powder [
2]. However, the classical texts of Ayurveda describe several methods of incineration of conch using various media like borax [
5] (Vol. 5, p. 103),
Citrus limon juice [
12],
Citrus medica juice [
13] (p. 310–311) and Aloe Vera juice [
14]. Ayurvedic Formulary of India published by Health and Family Welfare Ministry, Government of India has stated to boil pieces of conch in sour gruel (
Kanjika) for 3 h and to incinerate 2 times by placing in earthen casserole. Additional use of lemon or aloe vera juice for trituration has also been suggested in these guidelines [
15]. Moreover, authoritative books in traditional Unani medicine have mentioned several methods to incinerate conch which includes media like sulphur or
Tribulus terristris paste or cow milk or
Calotropis procera latex or lemon juice [
16]. Thus, it is clear that various methods of preparation of
Shankha Bhasma, have been reported. However, the complete pharmaceutical standardization with analytical results except specifications like description, loss on drying, acid insoluble ash, loss on ignition and calcium assay, is not available [
17].
The present study was, therefore, undertaken to develop the protocol for preparation of Shankha Bhasma. In this method, purification was done using juice of Citrus limon (Nimbuk) and incineration by the pulp of aloe vera leaf (Ghrut-kumari) among all the media suggested. Physico-chemical characterization of replicated batches of the final product was performed using modern methods such as X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Thermogravimetric Analysis (TGA), Particle size analysis, Fourier Transform Infra-Red (FTIR) and ICPOES to understand identity, purity and strength of the product. This has established standard operating procedures (SOP) to commercially manufacture Shankha Bhasma and developed its complete specifications for Quality Control and Quality Assurance (QCQA).
3. Discussion
Incinerated conch (
Shankha Bhasma) is one of the important medicines used in various Ayurvedic formulations. The characteristics of Molluscs are described as outer horny layer, a median prismatic layer of lime salts and an inner pearly nacreous layer. It is greatly variable in shape, structure and colour, forming an outstanding external feature in the animal [
20]. Being a natural marine animal origin product, there are several forms of conches found in nature. In Indian market, conch is available in three forms viz., whole, central stalk and pieces (personal observation). As per the
Rasashastra literature, conch which is whole, white in colour, shining and heavy in weight, is recommended for Ayurvedic use [
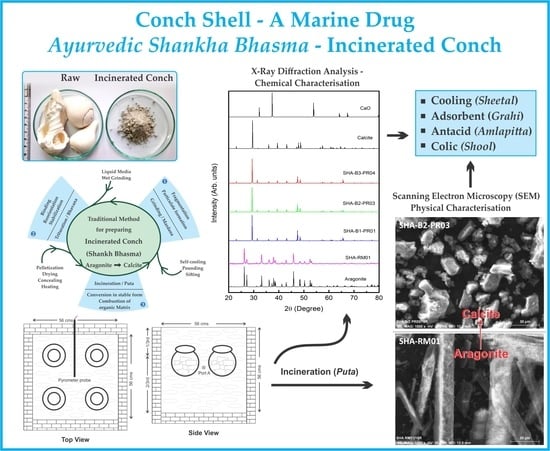
4] and hence whole form of conch was used in the present study. Raw conch is animal conch that contained 90% of Calcium as Calcium carbonate estimated by titration and XRD (
Figure 2) while rest being organic matrix, soil and moisture.
Shankha is sold without cleaning; hence it was washed after soaking in hot water. This helps to remove the physical impurities like sand deposits. Further any sour liquid is recommended for complete purification of conches [
21]. Also sour media is mentioned to be used for purification of metals and minerals [
13] (p. 158). Lemon (
Citrus limon) was selected as purification medium being readily available. The fresh juice of lemons is highly acidic (pH 2–2.5), so when poured on conch readily reacts with calcium carbonate, thus forming citrates and evolution of carbon dioxide. Hence, to avoid this, fresh juice was diluted to adjust pH, so that only impurities like organic matter on surface of conch would get eliminated. They were again thoroughly washed with hot water and dried for further use. This made the conch clean, bright white coloured, with no smell and lustre.
Fresh aloe vera pulp used for trituration had moisture not less than 98%
w/
w, pH 4.25–5, specific gravity 1.0040–1.0060 g/cc and viscosity from 2−4 mP as reported earlier [
22,
23]. It contains an inner clear gel like pulp with 98.5% water and the remaining with glucomannans, amino acids, lipids, sterols, vitamins [
24] and polysaccharides [
25]. Thus, aloe vera fresh juice helped to wet grind the calcium carbonate particles smoothly, bind them easily during pellets formation and got dried in a short duration without leaving substantial inorganic residue after incineration. Most importantly, modern pharmaceutical industry has been using biopolymers like chitosan, alginate or K
-carrageenan [
26] and xanthan, sodium alginate and pectin [
27] as substrate to contribute crystallization of calcium carbonate. Aloe vera pulp rich in polysaccharides may have similar function of biopolymer during incineration of conch. Role of aloe vera juice as a triturating medium has been studied in another
Sudha varga marine drug called
Shouktik (Mother pearl) by incinerating the material triturated with and without aloe vera juice. It was found that trituration prevents formation of CaO by interfering with heat transfer and maintaining the product in calcite form. Additionally, it helps to reconvert Ca(OH)
2 to CaCO
3 by maintaining CO
2 atmosphere due to burning of organic matter in aloe vera juice [
28]. Time dependent XRD and SEM analysis could have confirmed this observation in the present study also. However, arresting of the reaction at various time points was not possible in the current protocol.
The traditional texts have only mentioned the use of conch as raw material, aloe vera as liquid media and cow-dung cakes for incineration. However, no quantitative data for any of these steps are available in the literature. Hence, the amount of conch (in kg), liquid media (in L), quantity of cow-dung cakes (in kg) and number of earthen pots (count) were standardized. In this study, the size and quantity of crushed conches and liquid media quantity were taken into consideration while fixing the quantity of cow-dung cakes during the 1st incineration, after conducting pilot experiments. The main objective of the 1st incineration was to burn the organic matter and aloe vera juice as detailed in Material and Method section. During the 2nd incineration, the traditional sequence was followed as shown in
Figure 6. During second incineration, the quantity of cow-dung cakes was reduced to avoid overheating leading to conversion of calcium carbonate to calcium oxide. The observed value 39.05% of calcium as calcium carbonate (Molecular Weight 100.42 g/mole) determined by titration in present study is in accordance with the theoretical value of 40% rather than the reported values 44–46% [
29] and 48.6% [
30] of incinerated conch. Moreover, as reported previously [
31] similar method of incineration but without adding aloe vera juice was attempted, which showed incinerated conch as calcium oxide. However, incinerated conch (
Shankha Bhasma) does not give pungent taste character as shown by calcium oxide. Thus, it is necessary to have appropriate calcium carbonate form in
Shankha Bhasma rather than calcium oxide [
32].
The most critical step of synthesis of
Shankha Bhasma is the incineration step. Three Phases during both the incinerations observed in our studies are detailed in
Table 2. The temperature and its pattern were needed and sufficient to transform aragonite structure of calcium carbonate to calcite form. The temperature attained inside the pots initially evaporated water and evolved gases from the breakdown of organic matrix. This makes the aragonite free from extraneous matter and makes it easy to rearrange itself to calcite form. The first incineration was more intended to break down the layered structure, destruction of organic matrix and water evaporation followed by conversion of aragonite to calcite form. The second incineration was required for conversion of remaining aragonite form to calcite and its stability by avoiding overheating beyond 750 °C. The highest temperature attained outside earthen pot during the 1st incineration was around 900–1000 °C and during the 2nd incineration 800–900 °C. However, the inner temperature can be assumed to be between 700–800 °C for the 1st incineration and 650–750 °C for the 2nd incineration, the pots being of earthen nature. This is in conformity with other studies conducted on aragonite to calcite transformation [
33]. In this reported study, the structural and mechanical stability of conch shell was studied at 310, 500 and 900 °C. At 310 °C, the low content biopolymer burned out easily, phase transformation from aragonite to calcite look place at 500 °C while calcite to lime conversion was observed at 900 °C which induced structural modification and deteriorated mechanical stability, respectively.
To validate these observations, various analytical tools viz., XRD, FTIR, TGA and SEM were employed in the present study.
The XRD pattern of both raw and finished conch (
Figure 2) when simulated with standards, clearly indicated that raw conch is aragonite in nature, while after incineration this aragonite structure got rearranged to calcite form. Moreover, absence of any other major peaks indicated that incinerated conch was in pure calcite form. Previous X ray diffraction studies of aragonite crystal showed that aragonite was successfully transformed to calcite by heating above 488 °C [
34]. It has been reported that calcium in calcium carbonate calcite form is better absorbed as compared to its aragonite form [
35]. Furthermore, FTIR analysis of incinerated conch (
Figure 5) showed common characteristic peaks present at wavenumbers 1410, 874 and 712 cm
−1 which are more similar to CO
32− vibrations (symmetric and asymmetric) and are comparable with recent researches on calcium carbonate nanoparticles [
36].
This was further confirmed by TGA analysis of raw and incinerated conch (
Figure 4). Conch is a biogenic aragonite. For the biogenic sample the effect of thermal treatment is that, it reduces the intrinsic strain through the degradation of the macromolecules forming the organic matrix of the nacre [
37]. The TGA curve in our study showed negligible (1–2%
w/
w) weight loss till 600 °C. Previous DTA-TG study conducted on several aragonites clarified the transformation of aragonite to calcite. It has concluded that biogenic aragonites contain flowing water upto 0.1–1.3% which evaporates during heating along with CO
2 evolved from the combustion of other organic material. The study showed that water seems to play significant role of making some hydrothermal conditions, where calcite nucleation occurs thus triggering the transformation [
38]. In our method of
Shankha Bhasma preparation, conch is incinerated with aloe vera pulp as an added biopolymer for complete conversion of aragonite to calcite. A further significant weight loss with higher slope between 600–750 °C was recorded in our study. This weight loss ranged from 42% to 43%
w/
w which indicated decomposition of calcite to calcium oxide and CO
2. When heated above 800 °C, profile remained stable up to 1000 °C. This was in accordance with the previous findings of TGA analysis of Calcite [
39,
40].
SEM analysis of raw conch and finished product (
Figure 3) also confirmed the structural transformation of aragonite to calcite. The rhomboidal structure of calcite and a needle like shape of aragonite was found as expected morphologies [
41].
The particle size distribution study showed the range. The values are unique for this method of preparation and may help for quality control and quality assurance. Calcium carbonate nanoparticles have wide range of applications in drug delivery system due to its unique properties like accessibility, low cost, safety, biocompatibility, pH sensitive properties and slow biodegradability [
42].
As per quality parameters of Ayurvedic Pharmacopoeia of India, elemental Ca is reported to be in the range of 38–40%
w/
w while heavy metals need to be within the permissible limits (Hg: 1 ppm, As: 3 ppm, Cd: 0.3 ppm and Pb: 10 ppm) in the finished product of conch [
15,
17]. It has also been reported to have presence of Mg as trace element (<1.5%
w/
w) in the incinerated conch [
32,
43]. In the present study, conventional titration method detected elemental Ca as 39.02%
w/
w keeping in line with the pharmacopoeia guidelines. Moreover, an additional sensitive method of ICPOES used in the present study revealed simultaneous and precise estimation of Ca, Mg and heavy metals to be within the permissible limits in both, raw and incinerated conch (
Table 3).
All these experimental studies conducted through advanced instruments are helpful to understand the pharmaceutical transformation of aragonite conch into better absorbed calcite known as
Shankha Bhasma in Ayurveda. Rasashastra texts have mentioned
Shuddha Shankha/purified conch (aragonite form) for external applications as coryllium in eye diseases and Shankha Bhasma/incinerated conch (calcite form) for internal use in several diseases ([
4], pp. 285–289).
Shankha Bhasma being a traditional drug, the authors is clinically investigating the role of
Shankha Bhasma as adjunct treatment in GI malignancies, mainly stomach and colo-rectal cancers, by assessing the GI symptoms like nausea, vomiting, anorexia, flatulence, indigestion, mucositis, hyperacidity caused due to these malignancies as well as toxicities of chemotherapy. Further experimental studies to reveal the pharmacodynamics and pharmacokinetics of incinerated conch can be designed using this preliminary data in the form of SOP to manufacture quality drug.