Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of Triphlorethol A on Pentobarbital-Induced Sleep in Imprinting Control Region (ICR) Mice
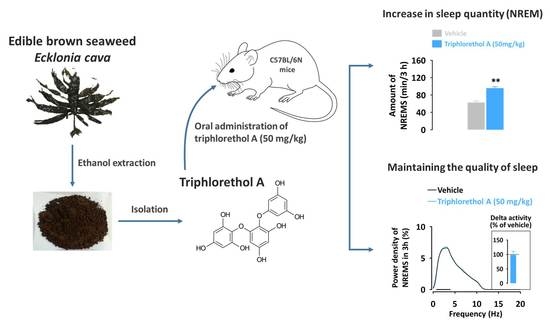
2.2. Effects of Triphlorethol A on Sleep Latency and the Amounts of Rapid Eye Movement Sleep (REMS) and Non-REMS (NREMS) in C57BL/6N Mice
2.3. Effects of Triphlorethol A on Time-Course Changes of NREMS, REMS, and Wake in C57BL/6N Mice
2.4. Effects of Triphlorethol A on Sleep-Wake Episode Profiles and Delta Activity
3. Discussion
4. Materials and Methods
4.1. Isolation of Triphlorethol A
4.2. Drugs
4.3. Animals
4.4. Pentobarbital-Induced Sleep Test
4.5. Polygraphic Recordings and Vigilance State Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Krueger, J.M.; Rector, D.M.; Roy, S.; Van Dongen, H.P.; Belenky, G.; Panksepp, J. Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci. 2008, 9, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Borja, N.L.; Daniel, K.L. Ramelteon for the treatment of insomnia. Clin. Ther. 2006, 28, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Doghramji, K. The epidemiology and diagnosis of insomnia. Am. J. Manag. Care 2006, 12, S214–S220. [Google Scholar] [PubMed]
- Meletis, C.D.; Zabriskie, N. Natural approaches for optimal sleep. Altern. Complement. Ther. 2008, 14, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Shimizu, M. Natural sleep aids and polyphenols as treatments for insomnia. In Bioactive Nutriceuticals and Dietary Supplements in Neurological and Brain Disease: Prevention and Therapy; Watson, R.R., Preedy, V., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2015; pp. 141–151. ISBN 978-0-12-411462-3. [Google Scholar]
- Fernández, S.P.; Wasowski, C.; Loscalzo, L.M.; Granger, R.E.; Johnston, G.A.R.; Paladini, A.C.; Marder, M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006, 539, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Back, N.I.; Kim, D.S.; Kang, S.M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoon, M.; Pae, A.N.; Jin, Y.H.; Cho, N.C.; Takata, Y.; Urade, Y.; Kim, S.; Kim, J.S.; Yang, H.; et al. Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor. Psychopharmacology 2014, 231, 2825–2837. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Kim, J.S.; Jo, J.H.; Han, D.; Cho, S. Sleep-promoting effect of Ecklonia cava: Ethanol extract promotes non-rapid eye movement sleep in C57BL/6N mice. Fish. Aquat. Sci. 2014, 17, 19–25. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakayama, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Um, M.; Kim, J.Y.; Han, J.K.; Kim, J.; Yang, H.; Yoon, M.; Kim, J.; Kang, S.W.; Cho, S. Phlorotannin supplement decreases wake after sleep onset in adults with self-reported sleep disturbance: A randomized, controlled, double-blind clinical and polysomnographic study. Phytother. Res. 2018, 32, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, K.; Kim, B.J.; Shin, T.; Park, J.W.; Lee, N.H.; et al. Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 inhibition. J. Toxicol. Environ. Health A 2008, 71, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Lee, K.H.; Park, J.W.; Lee, N.H.; Na, H.K.; Surh, Y.J.; You, H.J.; Chung, M.H.; Hyun, J.W. Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor. FEBS Lett. 2007, 581, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lee, I.K.; Kang, K.A.; Piao, M.J.; Ryu, M.J.; Kim, J.M.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava up-regulates the oxidant sensitive 8-oxoguanine DNA glycosylase 1. Mar. Drugs 2014, 12, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.; Wafford, K.A.; Deacon, S. Treating insomnia: Current and investigational pharmacological approaches. Pharmacol. Ther. 2006, 112, 612–629. [Google Scholar] [CrossRef] [PubMed]
- Bateson, A.N. Further potential of the GABA receptor in the treatment of insomnia. Sleep Med. 2006, 7, 3–9. [Google Scholar] [CrossRef]
- Trevor, A.J.; Way, W.L. Sedative-hypnotic Drugs. In Basic and Clinical Pharmacology, 12th ed.; Katzung, B.G., Ed.; McGraw-Hill Medical: New York, NY, USA, 2007; pp. 373–388. ISBN 978-0-07-176402-5. [Google Scholar]
- Stephenson, F.A. The GABAA receptors. Biochem. J. 1995, 310, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Erman, M.K. Therapeutic options in the treatment of insomnia. J. Clin. Psychiatry 2005, 66, 18–23. [Google Scholar] [PubMed]
- Liu, Z.; Xu, X.H.; Liu, T.Y.; Hong, Z.Y.; Urade, Y.; Huang, Z.L.; Qu, W.M. Safranal enhances non-rapid eye movement sleep in pentobarbital-treated mice. CNS Neurosci. Ther. 2012, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N.I.; Jo, J.; Cho, C.W.; Park, J.H.; Shimizu, M.; et al. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem. Biophys. Res. Commun. 2011, 413, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.R.; Zhou, X.Z.; Luo, Y.J.; Huang, Z.L.; Urade, Y.; Qu, W.M. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, induces sleep via the benzodiazepine site of GABA(A) receptor in mice. Neuropharmacology 2012, 63, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Aritake, K.; Tanaka, H.; Shoyama, Y.; Huang, Z.L.; Urade, Y. Crocin promotes non-rapid eye movement sleep in mice. Mol. Nutr. Food Res. 2012, 56, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.M.; Yue, X.F.; Sun, Y.; Fan, K.; Chen, C.R.; Hou, Y.P.; Urade, Y.; Huang, Z.L. Honokiol promotes non-rapid eye movement sleep via the benzodiazepine site of the GABA(A) receptor in mice. Br. J. Pharmacol. 2012, 167, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kagami, Y.; Yokoyama, C.; Moriyama, T.; Matsumoto, N.; Masaki, M.; Nakamura, H.; Kamasaka, H.; Shiraishi, K.; Kometani, T.; et al. Promotion of non-rapid eye movement sleep in mice after oral administration of ornithine. Sleep Biol. Rhythm. 2012, 10, 38–45. [Google Scholar] [CrossRef]
- Tobler, I.; Kopp, C.; Deboer, T.; Rudolph, U. Diazepam induced changes in sleep: Role of the α1 GABAA receptor subtype. Proc. Natl. Acad. Sci. USA 2001, 98, 6464–6469. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, M.; Cho, S. Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Mar. Drugs 2018, 16, 139. https://doi.org/10.3390/md16050139
Yoon M, Cho S. Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Marine Drugs. 2018; 16(5):139. https://doi.org/10.3390/md16050139
Chicago/Turabian StyleYoon, Minseok, and Suengmok Cho. 2018. "Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice" Marine Drugs 16, no. 5: 139. https://doi.org/10.3390/md16050139
APA StyleYoon, M., & Cho, S. (2018). Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Marine Drugs, 16(5), 139. https://doi.org/10.3390/md16050139