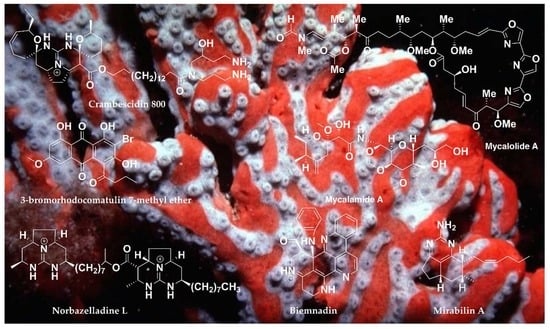
Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria
Abstract
:1. Introduction
2. Chemistry and Biological Activities of the Secondary Metabolites Isolated from the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria
2.1. Guanidine-Containing Alkaloids
2.2. Pyridoacridine, Pteridine, Tetrahydroquinolizine and N-methylpyrrolidone Alkaloids
2.3. Monoindole Alkaloids
2.4. Pyrrole-Containing Alkaloids
2.5. Bromine-Containing Amides
2.6. Cyclic Peptides/Thiopeptides
2.7. Nucleotides
2.8. Fatty Acids
2.9. Polyketides Derivatives
2.10. Anthraquinones
2.11. Macrolides
2.12. Terpenoids
2.13. Steroidal Compounds
2.14. Miscellaneous Compounds
3. Conclusions and Prospects
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| EC50 | Half maximal Effective Concentration |
| GI50 | Half maximal Growth Inhibition |
| HIV-1 | Human Immunodeficiency Virus 1 |
| IC50 | Half maximal Inhibitory Concentration |
| MIC | Minimum Inhibitory Concentration |
| MAD | Minimum active Dose |
| MID | Minimal Infective Dose |
| LD50 | Lethal Dose 50 (median concentration of a toxicant that will kill 50% of the test animals within a designated period |
References
- Miller, J.H.; Field, J.J.; Kanakkanthara, R.; Owen, J.G.; Singh, A.J.; Northcote, P.T. Marine invertebrate natural products that target microtubules. J. Nat. Prod. 2018, 81, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, A.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine fungi: A source of potential anticancer compounds. Front. Microbiol. 2018, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Hilàrio, A.; Munro, M.H.G.; Blunt, J.W.; Calado, R. Natural products discovery needs improved taxonomic and geographic information. Nat. Prod. Rep. 2016, 33, 747–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs. Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Romminger, S. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2016, 33, 456–490. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Bertonha, A.F.; Takaki, M.; Rodriguez, J.P.G. The chemistry and biology of guanidine natural products. Nat. Prod. Rep. 2017, 34, 1264–1301. [Google Scholar] [CrossRef] [PubMed]
- Gros, E.; Al-Mourabit, A.; Martin, M.T.; Sorres, J.; Vacelet, J.; Frederick, M.; Aknin, M.; Kashman, Y.; Bialecki, A.G. Netamines H–N, tricyclic alkaloids from the marine sponge Biemna laboutei and their antimalarial Activity. J. Nat. Prod. 2014, 77, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Gros, E.; Martin, M.T.; Sorres, J.; Moriou, C.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Bialecki, Y.G.; Al-Mourabit, A. Netamines O–S, five new tricyclic guanidine alkaloids from the Madagascar sponge Biemna laboutei, and their antimalarial activities. Chem. Biodivers. 2015, 12, 1725–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudi, A.; Yosief, T.; Loya, S.; Hizi, A.; Schleyer, M.; Kashman, Y. Clathsterol, a novel anti-HIV-1 RT sulfated sterol from the sponge Clathria species. J. Nat. Prod. 2001, 64, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Northcote, P.T.; Webb, V. Clathriol, a novel polyoxygenated 14 steroid isolated from the New Zealand marine sponge Clathria lissosclera. J. Nat. Prod. 2002, 65, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Northcote, P.T.; Berridge, M.V. Clathriol B, a new 14β marine sterol from the New Zealand sponge Clathria lissosclera. Aust. J. Chem. 2003, 56, 279–282. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Palumbo, F.; Costantini, M. Marine sponges and bactéria as challenging sources of enzyme inhibitors for pharmacological applications. Mar. Drugs 2017, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, U.; Schulz, T.C.; McLean, A.B.; Robins, A.J.; West, L.M. Bicyclic C21 terpenoids from the marine sponge Clathria compressa. J. Nat. Prod. 2012, 75, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, S.; Ference, C.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. A potent antimicrobial compound isolated from Clathria cervicornis. Bioorg. Med. Chem. Lett. 2015, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Atanasov, A.G.; Bishayee, A.; Abdel-Mogib, M.; Hooper, J.N.A.; Al-Mourabit, A. Batzella, Crambe and Monanchora: Highly prolific marine sponge genera yielding compounds with potential applications for cancer and other therapeutic areas. Nutrients 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera Database. 2018. Available online: http://www.marinespecies.org/porifera/ (accessed on 28 April 2018).
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Rodrigues-Stien, A.; Petek, S.; Demoy-Schnider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic guanidine alkaloids from a French Polynesian Monanchora n. sp. sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A. Isolation of Bioactive Marine Natural Products and Bio-Inspired Synthesis of Fused Guanidinic Tricyclic Analogues. Unpublished Ph.D. Thesis, University of Paris-Saclay, Paris, France, May 2016. [Google Scholar]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Petek, S.; Debitus, C.; Al-Mourabit, A. Unguiculins A–C: Cytotoxic bis-guanidine alkaloids from the French Polynesian sponge, Monanchora n. sp. Nat. Prod. Res. 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sfecci, E.; Lacour, T.; Amad, P.; Mehiri, M. Polycyclic guanidine alkaloids from Poecilosclerida marine sponges. Mar. Drugs 2016, 14, 77–101. [Google Scholar] [CrossRef] [PubMed]
- Kasmiati, K.; Yoshioka, Y.; Okamoto, T.; Ojika, M. New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Mar. Drugs 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Laville, R.; Thomas, O.; Berrué, F.; Marquez, D.; Vacelet, J.; Amade, D. Bioactive guanidine alkaloids from two Caribbean marine sponges. J. Nat. Prod. 2009, 72, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Barrow, R.A.; Murray, L.M.; Lim, T.K.; Capon, R.J. Mirabilins (A–F): New Alkaloids from a Southern Australian Marine Sponge, Arenochalina mirabilis. Aust. J. Chem. 1996, 49, 767–773. [Google Scholar] [CrossRef]
- Sorek, H.; Rudi, A.; Gueta, S.; Reyes, F.; Martin, M.J.; Aknin, M.; Gaydou, E.; Vacelet, J.; Kashman, Y. Netamines A–G: Seven new tricyclic guanidine alkaloids from the marine sponge Biemna laboutei. Tetrahedron 2006, 62, 8838–8843. [Google Scholar] [CrossRef]
- Capon, R.; Miller, M.; Rooney, F. Mirabilin G: A new alkaloid from a southern Australian marine sponge, Clathria species. J. Nat. Prod. 2001, 64, 643–644. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Conte, M.; Capon, R. Mirabilins revisited: Polyketide alkaloids from a southern Australian marine sponge, Clathria sp. Org. Biomol. Chem. 2010, 8, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Henriksen, N.; Skalicky, J.; Harper, M.; Cheatham, T.; Ireland, C.; Wagoner, R. Araiosamines A–D: Tris-bromoindole cyclic guanidine alkaloids from the marine sponge Clathria (Thalysias) araiosa. J. Org. Chem. 2011, 76, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Kijjoa, A. Pyridoacridine alkaloids from marine Origin: Sources and Anticancer Activity. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 771–802. ISBN 978-3-319-07144-2. [Google Scholar]
- Ibrahim, S.R.M.; Mohamed, G.A. Marine pyridoacridine alkaloids: Biosynthesis and biological activities. Chem. Biodivers. 2016, 13, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.M.; Ishibashi, M.; Matsumoto, K.; Nakaike, S.; Kobayashi, J. Two new polycyclic aromatic alkaloids from the Okinawan marine sponge Biemna sp. Tetrahedron 1993, 49, 8337–8342. [Google Scholar] [CrossRef]
- Aoki, S.; Wei, H.; Matsui, K.; Rachmat, R.; Kobayashi, M. Pyridoacridine alkaloids inducing neuronal differentiation in a neuroblastoma cellLine, from marine sponge Biemna fortis. Bioorg. Med. Chem. 2003, 11, 1969–1973. [Google Scholar] [CrossRef]
- Ueoka, R.; Ise, Y.; Okada, S.; Matsunaga, S. Cell differentiation inducers from a marine sponge Biemna sp. Tetrahedron 2011, 67, 6679–6681. [Google Scholar] [CrossRef]
- Morana, D.A.P.; Takadaa, K.; Ise, Y.; Bontemsc, N.; Davis, R.A.; Furihatae, K.; Okada, S.; Matsunaga, S. Two cell differentiation inducing pyridoacridines from a marine sponge Biemna sp and their chemical conversions. Tetrahedron 2015, 71, 5013–5018. [Google Scholar] [CrossRef]
- Zuleta, I.; Vitelli, M.; Baggio, R.; Garland, M.; Seldes, A.; Palermo, J. Novel pteridine alkaloids from the sponge Clathria sp. Tetrahedron 2002, 58, 4481–4486. [Google Scholar] [CrossRef]
- Sperry, S.; Crews, P. A novel alkaloid from the Indo-Pacific sponge Clathria basilana. Tetrahedron Lett. 1996, 37, 2389–2390. [Google Scholar] [CrossRef]
- Radhika, G.; Venkatesan, R.; Kathiroli, S. N-methylpyrrolidone: Isolation and characterization of the compound from the marine sponge Clathria frondifera (class:Demospongiae). Indian J. Mar. Sci. 2007, 36, 235–238. [Google Scholar]
- Wang, R.P.; Lin, H.W.; Li, L.Z.; Gao, P.Y.; Xu, Y.; Song, S.J. Monoindole alkaloids from a marine sponge Mycale fibrexilis. Biochem. Syst. Ecol. 2012, 43, 210–213. [Google Scholar] [CrossRef]
- Ortega, M.J.; Zubia, E.; Carballo, J.L.; Salva, J. New cytotoxic metabolites from the sponge Mycale micracanthoxea. Tetrahedron 1997, 53, 331–340. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Oliveri, M.C.; Pina, I.C.; Marques, S.; Rangel, H.R.; Dagger, F.; Suarez, A.I.; Gomez, M. 5-alkylpyrrole-2-carboxaldehydes from the Caribbean sponges Mycale Microsigmatosa and Desmapsamma Anchorata. Nat. Prod. Lett. 1999, 13, 203–211. [Google Scholar] [CrossRef]
- Venkatesham, U.; Rao, M.R.; Venkateswarlu, Y. New 5-alkylpyrrole-2-carboxaldehyde derivatives from the sponge Mycale tenuispiculata. J. Nat. Prod. 2000, 63, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.J.; Zubia, E.; Sanchez, M.C.; Salva, J.; Carballo, J.L. Structure and cytotoxicity of new metabolites from the sponge Mycale cecilia. Tetrahedron 2004, 60, 2517–2524. [Google Scholar] [CrossRef]
- Xue, D.-Q.; Liu, H.-L.; Chen, S.-H.; Mollo, E.; Gavagnin, M.; Li, J.; Li, X.-W.; Guo, Y.-W. 5-Alkylpyrrole-2-carboxaldehyde derivatives from the Chinese sponge Mycale lissochela and their PTP1B inhibitory activities. Chin. Chem. Lett. 2017, 28, 1190–1193. [Google Scholar] [CrossRef]
- Ohta, S.; Okada, H.; Kobayashi, H.; Oclarit, J.; Ikegami, S. Clathrynamides A, B, and C: Novel amides from a marine sponge Clathria sp. that inhibit cell division of fertilized starfish eggs. Tetrahedron Lett. 1993, 34, 5935–5938. [Google Scholar] [CrossRef]
- Davis, R.; Mangalindan, G.; Bojo, Z.; Antemano, R.; Rodriguez, N.; Concepcion, G.; Samson, S.; Guzman, D.; Cruz, L.; Tasdemir, D.; et al. Microcionamides A and B, bioactive peptides from the Philippine sponge Clathria (Thalysias) abietina. J. Org. Chem. 2004, 69, 4170–4176. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Jeon, J.; Kim, C.; Sim, C.; Oh, D.; Oh, K.; Shin, J. Gombamide A, a cyclic thiopeptide from the sponge Clathria gombawuiensis. J. Nat. Prod. 2013, 76, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshida, S.; Matsunaga, S.; Shindoh, N.; Terada Koji, N.; Yamashita, J.K.; Ganesan, A.; van Soest, R.W.M.; Fusetani, N. Azumamides A–E: Histone Deacetylase Inhibitory Cyclic Tetrapeptides from the Marine Sponge Mycale izuensis. Angew. Chem. Int. Ed. 2006, 45, 7553–7557. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Fusetani, N.; Matsunaga, S.; Hashimoto, K. Bioactive marine metabolites IX. Mycalisines A and B, novel nucleosides which inhibit cell division of fertilized starfish eggs, from the marine sponge Mycale sp. Tetrahedron Lett. 1985, 26, 3483–3486. [Google Scholar] [CrossRef]
- Firsova, D.; Calabro, K.; Lasserre, P.; Reyes, F.; Thomas, O.P. Isoguanosine derivatives from the Northeastern Atlantic sponge Clathria (Microciona) strepsitoxa. Tetrahedron Lett. 2017, 58, 4652–4654. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Negron, V.; Reyes, E.D. Novel monounsaturated fatty acids from the sponges Amphimedon compressa and Mycale laevis. J. Nat. Prod. 1992, 55, 333–339. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Negron, V.; Reyes, E.D. Novel naturally occurring α-methoxy acids from the phospholipids of Caribbean sponges. Tetrahedron 1992, 48, 1053–1058. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Zbd-Elrazek, A.E.E.; Hassanean, H.A.; Alhdal, A.M.; Almohammadi, A.; Youssef, D.T.A. New fatty acids from the Red Sea sponge Mycale euplectellioides. Nat. Prod. Res. 2014, 28, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G.; Pannell, L.K. Mycalamide A, an antiviral compound from a New Zealand sponge of the genus Mycale. J. Am. Chem. Soc. 1988, 110, 4850–4851. [Google Scholar] [CrossRef]
- Perry, N.B.; Blunt, J.W.; Munro, M.H.G.; Thompson, A.M. Antiviral and antitumor agents from a New Zealand sponge, Mycale sp. Structures and solution conformations of mycalamides A and B. J. Org. Chem. 1990, 55, 223–227. [Google Scholar] [CrossRef]
- West, L.M.; Northcote, P.T.; Hood, K.A.; Miller, J.H.; Page, M.J. Mycalamide D, a new cytotoxic amide from the New Zealand marine sponge Mycale species. J. Nat. Prod. 2000, 63, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Mayol, L.; Notaro, G.; Piccilli, V.; Sica, D. Structure and absolute configuration of two new Polybrominated CI5 acetogenins from the sponge Mycale rotalis. J. Chem. Soc. Chem. Commun. 1990, 1559–1561. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D.; Mayol, L.; Giordano, F. A further C15 nonterpenoid Polybromoether from the Encrusting sponge Mycale rotalis. J. Nat. Prod. 1992, 55, 626–632. [Google Scholar] [CrossRef]
- Khokhar, S.; Pierens, G.; Hooper, J.; Ekins, M.; Feng, Y.; Davis, R. Rhodocomatulin-type anthraquinones from the Australian marine invertebrates Clathria hirsuta and Comatula rotalaria. J. Nat. Prod. 2016, 79, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Yasumuro, K.; Matsunaga, S.; Hashimoto, K. Mycalolides A–C, hybrid macrolides of ulapualides and halichondramide, from a sponge of the genus Mycale. Tetrahedron Lett. 1989, 30, 2809–2812. [Google Scholar] [CrossRef]
- Fusetani, N.; Sugawara, T.; Matsunaga, S. Bioactive marine metabolites. Part 35. Cytotoxic metabolites of the marine sponge Mycale adhaerens Lambe. J. Org. Chem. 1991, 56, 4971–4974. [Google Scholar] [CrossRef]
- Northcote, P.T.; Blunt, J.W.; Munro, M.H.G. Pateamine: A potent cytotoxin from the New Zealand Marine sponge, Mycale sp. Tetrahedron Lett. 1991, 32, 6411–6414. [Google Scholar] [CrossRef]
- Matsunaga, S.; Nogata, Y.; Fusetani, N. Thiomycalolides: New cytotoxic trisoxazole-containing macrolides isolated from a marine sponge Mycale sp. J. Nat. Prod. 1998, 61, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Sugawara, T.; Fusetani, N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J. Nat. Prod. 1998, 61, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Liu, P.; Celatka, C.A.; Panek, J.S.; Fusetani, N. Relative and absolute stereochemistry of mycalolides, bioactive macrolides from the marine sponge Mycale magellanica. J. Am. Chem. Soc. 1999, 121, 5605–5606. [Google Scholar] [CrossRef]
- West, L.M.; Northcote, P.T.; Battershill, C.N. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Phuwapraisirisan, P.; Matsunaga, S.; van Soest, R.W.M.; Fusetani, N. isolation of a new mycalolide from the marine sponge Mycale izuensis. J. Nat. Prod. 2002, 65, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Koimaru, K.; Ohta, T. Secomycalolide A: A new proteasome inhibitor isolated from a marine sponge of the genus Mycale. Mar. Drugs 2005, 3, 29–35. [Google Scholar] [CrossRef]
- Singh, A.J.; Xu, C.X.; Xu, X.; West, L.M.; Wilmes, A.; Chan, A.; Hamel, E.; Miller, J.H.; Northcote, P.T.; Ghosh, A.K. Peloruside B, A potent antitumor Macrolide from the New Zealand marine sponge Mycale hentscheli: Isolation, structure, total synthesis, and bioactivity. J. Org. Chem. 2010, 75, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Suo, R.; Takada, K.; Kohtsuka, H.; Ise, Y.; Okada, S.; Matsunaga, S. Miuramides A and B, trisoxazole macrolides from a Mycale sp. marine sponge that induce a protrusion phenotype in cultured mammalian cells. J. Nat. Prod. 2018, 81, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Razzak, M.; Teesdale-Spittle, P.; Gaitanos, T.N.; Wilmes, A.; Paterson, I.; Goodman, J.; Miller, J.H.; Northcote, P.T. Structure–activity studies of the pelorusides: New congeners and semi-synthetic analogues. Org. Biomol. Chem. 2011, 9, 4456–4466. [Google Scholar] [CrossRef] [PubMed]
- Kanakkanthara, A.; Northcote, P.T.; Miller, J.H. Peloruside A: A lead non-taxoid-site microtubule-stabilizing agent with potential activity against cancer, neurodegeneration, and autoimmune disease. Nat. Prod. Rep. 2016, 33, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.A.; West, L.M.; Rouwe, B.; Northcote, P.T.; Berridge, M.V.; Wakefield, S.J.; Miller, J.H. Peloruside A, a novel antimitotic agent with paclitaxel-like microtubule- stabilizing activity. Cancer Res. 2002, 62, 3356–3360. [Google Scholar] [PubMed]
- Ganguly, A.; Cabral, F.; Yang, H.; Patel, K.D. Peloruside A is a microtubule-stabilizing agent with exceptional anti-migratory properties in human endothelial cells. Oncoscience 2015, 2, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Capon, R.J.; Nadeson, R.; Beveridge, A.A. Aromatic bisabolenes from an Australian marine sponge, Arenochalina sp. J. Nat. Prod. 1991, 54, 619–623. [Google Scholar] [CrossRef]
- Wright, A.E.; Pomponi, S.A.; McConnell, O.J.; Kohmoto, S.; McCarthy, P.J. (+)-Curcuphenol and (+)-curcudiol, sesquiterpene phenols from shallow and deep water collections of the marine sponge Didiscus flavus. J. Nat. Prod. 1987, 50, 976–978. [Google Scholar] [CrossRef]
- Peng, J.; Franzblau, S.G.; Zhang, F.; Hamann, M.T. Novel sesquiterpenes and a lactone from the Jamaican sponge Myrmekioderma styx. Tetrahedron Lett. 2002, 43, 9699–9702. [Google Scholar] [CrossRef]
- Gaspar, H.; Feio, S.S.; Rodrigues, A.I.; van Soest, R.W.M. Antifungal activity of (+)-curcuphenol, a metabolite from the marine sponge Didiscus oxeata. Mar. Drugs 2004, 2, 8–13. [Google Scholar] [CrossRef]
- Capon, R.; Miller, M.; Rooney, F. Clathrins A–C: Metabolites from a southern Australian marine sponge, Clathria species. J. Nat. Prod. 2000, 63, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Kim, C.; Kim, S.; Kim, H.; Oh, D.; Oh, K.; Shin, J. Gombaspiroketals A–C sesterterpenes from the sponge Clathria gombawuiensis. Org. Lett. 2014, 16, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Kim, C.; Ahn, C.; Oh, D.; Oh, K.; Shin, J. Additional sesterterpene and a nortriterpene saponin from the sponge Clathria gombawuiensis. J. Nat. Prod. 2015, 78, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Corriero, G.; Madaio, A.; Mayol, L.; Piccialli, V.; Sica, D. Rotalin A and B, two novel diterpene from the encrusting Mediterranean sponge Mycale Rotalis (Bowerbank). Tetrahedron 1989, 45, 277–288. [Google Scholar] [CrossRef]
- Rudi, A.; Benayahu, Y.; Kashman, Y. Mycgranol, a new diterpene from the marine sponge Mycale aff. Graveleyi. J. Nat. Prod. 2005, 68, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Macleod, J.K. Structural and stereochemical studies on marine norterpene cyclic peroxides, Part 2. J. Nat. Prod. 1987, 50, 225–229. [Google Scholar] [CrossRef]
- Capon, R.J. Two new norsesterterpene cyclic peroxides from a marine sponge, Mycale (Carmia) cf. spongiosa. J. Nat. Prod. 1991, 54, 190–195. [Google Scholar] [CrossRef]
- Tanaka, J.-C.; Higo, T.; Suwanborirus, K.; Kokpol, U.; Bernardinelli, G.; Jefford, C.W. Bioactive norsesterterpene 1,2-dioxanes from a Thai sponge, Mycale sp. J. Org. Chem. 1993, 58, 2999–3002. [Google Scholar] [CrossRef]
- Capon, R.J.; Rochfort, S.J.; Ovenden, S.P.B. Cyclic peroxides and related norterpenes from a Southern Australian marine sponge, Mycale sp. J. Nat. Prod. 1997, 60, 1261–1264. [Google Scholar] [CrossRef]
- Capon, R.J.; Rochfort, S.J.; Ovenden, S.P.B.; Metzger, R.P. Mycaperoxides F and G and a related norterpene ketone from Southern Australian marine sponges, Mycale Species. J. Nat. Prod. 1998, 61, 525–528. [Google Scholar] [CrossRef]
- Phuwapraisirisan, P.; Matsunaga, S.; Fusetani, N.; Chaitanawisuti, N.; Kritsanapuntu, S.; Menasveta, P. Mycaperoxide H, a new cytotoxic norsesterterpene peroxide from a Thai marine sponge Mycale sp. J. Nat. Prod. 2003, 66, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Katyama, T. Biochemical studies on the carotenoids in Porifera. The structure of clathriaxanathin in sea sponge Clathria frondifera (Bower bank). Bull. Jpn. Soc. Sci. Fish. 1976, 42, 801–805. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujita, Y.; Katayama, T. Biochemical studies on the carotenoids in Porifera. Identification of the aromatic ketocarotenoid in Clathria frondifera and Tedania digitata. Bull. Jpn. Soc. Sci. Fish. 1977, 43, 767–772. [Google Scholar] [CrossRef]
- Zeng, C.M.; Ishibashi, M.; Kobayashi, J. Biemnasterol, a new cytotoxic sterol with the rare 22,25-diene side chain isolated from the marine sponge Biemna sp. J. Nat. Prod. 1993, 56, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-C.; Guo, Y.-W.; Song, G.-Q. Fortisterol, a novel steroid with an unusual seven-membered lactone ring B from the Chinese marine sponge Biemna fortis Topsent. J. Asian Nat. Prod. Res. 2006, 8, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Bensemhoun, J.; Bombarda, I.; Aknin, M.; Vacelet, J.; Gaydou, E.M. 5α, 8α-epidioxy-24(S)-ethylcholest-6-en-3β-ol from the marine sponge Biemna triraphis Topsent. Nat. Prod. Commun. 2008, 3, 843. [Google Scholar]
- Huang, X.-C.; Guo, Y.-W. Chemical constituents of marine sponge Biemna fortis Topsent. Chin. J. Nat. Med. 2008, 6, 348–353. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Bader, J.M.; Shaala, L.A.; Mohamed, G.A.; Bamanie, F.H. Ehrenasterol and biemnic acid; new bioactive compounds from the Red Sea sponge Biemna ehrenbergi. Phytochem. Lett. 2015, 12, 296–301. [Google Scholar] [CrossRef]
- Kalinovsky, A.I.; Antonov, A.S.; Afiyatullov, S.S.; Dmitrenok, P.S.; Evtuschenko, E.V.; Stonic, V.A. Mycaloside A, a new steroid oligoglycoside with an unprecedented structure from the Caribbean sponge Mycale laxissima. Tetrahedron Lett. 2002, 43, 523–525. [Google Scholar] [CrossRef]
- Antonov, A.S.; Afiyatullov, S.S.; Kalinovsky, A.I.; Ponomarenko, L.P.; Dmitrenok, P.S.; Aminin, D.L.; Agafonova, I.G.; Stonic, V.A. Mycalosides B–I, eight new spermostatic steroid oligoglycosides from the sponge Mycale laxissima. J. Nat. Prod. 2003, 66, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Phuwapraisirisan, P.; Matsunaga, S.; Fusetani, N. Mycapolyols A–F, new cytotoxic metabolites of mixed biogenesis from the marine sponge Mycale izuensis. Org. Lett. 2005, 7, 2233–2236. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.; Macleod, J. 5-Thio-D-mannose from the marine sponge Clathria pyramida (Lendenfeld). The first example of a naturally occurring 5-thiosugar. J. Chem. Soc. Chem. Commun. 1987, 15, 1200–1201. [Google Scholar] [CrossRef]
- Venkateshwar, G.T.; Krishnaiah, P.; Malla, R.S. Chemical Investigation of the marine sponges Clathria reinwardtii and Haliclona cribicutus. Indian J. Chem. Sect. B 2005, 44B, 607–610. [Google Scholar]
- Coello, L.; Martín, M.J.; Reyes, F. 1,5-Diazacyclohenicosane, a New cytotoxic metabolite from the marine Sponge Mycale sp. Mar. Drugs 2009, 7, 445–450. [Google Scholar] [CrossRef] [PubMed]























| Name | Compound Class | Marine Sponges | Collection | Bioactivities | Ref. |
|---|---|---|---|---|---|
| Crambescidin 800 (1) | Pentacyclic guanidine | Clathria (Thalysias) cervicornis | - | Antimicrobial | 21 |
| Crambescidins 1–6 | Pentacyclic guanidine | C. bulbotoxa | Indonesia | Cytotoxic, antifungal | 28 |
| Norbatzelladine L (7) | Tricyclic guanidine | C. (Microciona) calla | Caribbean | Cytotoxic | 29 |
| Clathriadic acid (8) | Tricyclic guanidine | C. (Microciona) calla | Caribbean | Cytotoxic, antimalarial | 29 |
| Mirabilins A–F (9–14) | Tricyclic guanidine | Mycale (Arenochalina) mirabilis | Australia | Nr | 30 |
| Netamines A–G (15–21) | Tricyclic guanidine | Biemna laboutei | Madagascar | Cytotoxicity | 31 |
| Netamines H–N (22–28) | Tricyclic guanidine | B. laboutei | Madagascar | Cytotoxic, antimalarial | 14 |
| Netamines O–S (29–33) | Tricyclic guanidine | B.laboutei | Madagascar | Cytotoxic, antimalarial | 15 |
| Mirabilin G (34) | Tricyclic guanidine | Clathria sp. | Australia | Antibacterial, antifungal | 32 |
| Mirabilins H–J (35–37) | Tricyclic guanidine | Clathria sp. | Australia | Cytotoxic | 33 |
| Araiosamines A–D (38–41) | Indole cyclic guanidine | C. (Thalysias) araiosa | Vanuatu | Antibacterial, Anti-HIV-1 | 34 |
| 42–45 | Pyridoacridine | Biemna sp. | Okinawa | Cytotoxicity | 37 |
| 46 and 47 | Pyridoacridine | Biemna sp. | Indonesia | Enzyme inhibitor | 38 |
| 48 and 49 | Pyridoacridine | Biemna sp. | Japan | Cytotoxic | 39 |
| 50–53 | Pyridoacridine | Biemna sp. | Japan | Cytotoxic | 40 |
| Pseudoanchnazines A–C (54–56) | Pteridine alkaloid | Clathria sp. | Argentina | Antibacterial | 41 |
| Clathryimine A (57) | Quinolizine alkaloid | C. (Clathria) basilana | Indo-Pacific | Nr | 42 |
| N-methylpyrrolidone (58) | Pyrrolodine Alkaloid | C. frondifera | India | Nr | 43 |
| 59–69 | Indole alkaloids | M. fibrexilis | China | Nr | 44 |
| 70–83 | Pyrrole alkaloids | M. micracanthoxea | Spain | Cytotoxic | 45 |
| 84–94 | Pyrrole alkaloids | M. micracanthoxea | Venezuela | Cytotoxic | 46 |
| 95–97 | Pyrrole alkaloids | M. tenuispiculata | India | Nr | 47 |
| 98–111 | Pyrrole alkaloids | M. cecilia | California | Cytotoxic | 48 |
| 112 and 113 | Pyrrole alkaloids | M. lissochela | China | Enzyme inhibitor | 49 |
| Clathrynamides A–C (114–116) | Bromine-containing amide | Clathria sp. | Sad-Misaki, Japan | Cytotoxic, inhibitors of starfish eggs | 50 |
| Microcionamides A&B (117&118) | Cyclic thiopeptide | C. (Thalysias) abietina | Philippines | Cytotoxic, antibacterial | 51 |
| Gombamide A (119) | Cyclic thiopeptide | C. (Clathria) gombawuiensis | Korea | Cytotoxic, enzyme inhibitor | 52 |
| Azumamides (120–124) | Cyclic peptides | Mycale izuensis | Japan | Histone Deacetylase | 53 |
| Mycalisines (125–126) | Nucleotides | Mycale sp. | Japan | Inhibitors of starfish eggs | 54 |
| 127 and 128 | Nucleotides | C. (Microciona) strepsitoxa | Atlantic | Nr | 55 |
| 129 and 130 | Fatty acid | M. laevis | Caribbean | Nr | 56 |
| 131 | Fatty acid | M. laxissima | Caribbean | Nr | 57 |
| 132–134 | Fatty acid | M. euplectellioides | Red Sea | Cytotoxic | 58 |
| Mycalamides A&B (135&136) | Polyketide | Mycale sp. | New Zealand | Cytotoxic, antiviral | 59–60 |
| Mycalamide D (137) | Polyketide | Mycale sp. | New Zealand | Cytotoxic | 61 |
| 138–140 | Polyketide | M. rotalis | Mediterranean | Nr | 62–63 |
| 141–146 | Anthraquinone | C. (Thalysias) hirsuta | Australia | Nr | 64 |
| 147–149 | Macrolide | Mycale sp. | Japan | Antifungal, cytotoxic | 65 |
| 150 | Macrolide | M. adhaerens Lamb | Japan | Cytotoxic | 66 |
| Pateamine (151) | Macrolide | Mycale sp. | New Zealand | Cytotoxic | 67 |
| 152 and 153 | Macrolide | Mycale sp. | Japan | Cytotoxic | 68 |
| 154–156 | Macrolide | M. magellanica | Japan | Cytotoxic | 69–70 |
| Peloruside A (157) | Macrolide | Mycale sp. | New Zealand | Cytotoxic | 71 |
| 158 | Macrolide | M. izuensis | Japan | Cytotoxic | 72 |
| 159 | Macrolide | Mycale sp. | Japan | Cytotoxic | 73 |
| Peloruside B (160) | Macrolide | M. hentscheli | New Zealand | Cytotoxic | 74 |
| 161 and 162 | Macrolide | Mycale sp. | Japan | Cytotoxic | 75 |
| Peloruside C&D (163&164) | Macrolide | M. hentscheli | New Zealand | Cytotoxic | 76 |
| 165-169 | Sesquiterpene | M. (Arenochalina) sp | Australia | Antitumor, antifungal | 80–83 |
| Clathrin A–C (170–172) | Sesterterpene | Clathria sp | Australia | - | 84 |
| Clathric acid (173) | C21 terpenoid | C. compressa | Florida | Antimicrobial | 20 |
| Clathrimide A&B (174&175) | C21 -terpenoid | C. compressa | Florida | Antimicrobial | 20 |
| Gombaspiroketal A–C (176–178) | Sesterterpene | C. gombawuiensis | Korea | Antibacterial, enzyme inhibitors | 85 |
| 179 and 181 | Norterpene/triterpene | C. gombawuiensis | Korea | Antibacterial | 86 |
| Rotalins (182–183) | Diterepene | M. rotalis | Mediterranean | Nr | 87 |
| Mycgranol (184) | Diterepene | M. aff. graveleyi | Kenya | Nr | 88 |
| 185–189 | Cyclic norterpenoid peroxide | M. ancorina | Australia | Nr | 89 |
| 190 and 191 | Cyclic norterpenoid peroxide | M. (carmia) cf. spongiosa | Australia | Antimicrobial | 90 |
| 192 and 193 | Cyclic norterpenoid peroxide | Mycale sp. | Thailand | Cytotoxic, antiviral | 91 |
| 194–201 | Cyclic peroxide/norditerepene | Mycale sp. | Australia | Nr | 92 |
| 202–204 | Cyclic norterpenoid peroxide | Mycale sp. | Australia | Nr | 93 |
| 205 | Cyclic norterpenoid peroxide | Mycale sp. | Thailand | Cytotoxic | 94 |
| 206 and 207 | Tetraterpene | C. frondifera (=C. (Thalysias vulpina) | Japan | Nr | 95–96 |
| Contignasterol (208) | Steroid | C. (Clathria) lissosclera | New Zealand | Histamine inhibitory | 17–18 |
| Clathriols A&B (209&210) | Steroid | C. (Clathria) lissosclera | New Zealand | Anti-inflammatory, histamine inhibitory | 17–18 |
| Clathsterol (211) | Sulphated sterol | Clathria sp. | Red Sea | Anti-HIV-1 | 16 |
| Biemansterol (212) | Sterol | Biemna sp. | Okinawa, Japan | Cytotoxic | 97 |
| 213 | Sterol | Biemna sp. | Okinawa, Japan | Cytotoxic | 97 |
| Foristerol (214) | Sterol | B. fortis | China | Nr | 98 |
| 215 | Sterol | B. triraphis | Madagascar | Nr | 99 |
| 216–224 | Sterol | B. fortis | China | Lymphocytes and hPTP1B inhibition | 100 |
| 225 and 226 | Sterol | B. ehrenbergi | Red Sea | Cytotoxic, antibacterial | 101 |
| 227–235 | Sterol | M. laxissima | Caribbean | Fertilized eggs inhibitors | 102–103 |
| Mycapolyols A–F (184–189) | Mixed PKS/NPRS | M. izuensis | Japan | Cytotoxic | 104 |
| 242 | Thio-sugar | C. (Dendrocia) pyramida | Australia | Nr | 105 |
| 243 | Glycol | C.reinwardtti | India | Nr | 106 |
| 244 | 1,5-Diamine | Mycale sp. | Kenya | Cytotoxic | 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Demerdash, A.; Tammam, M.A.; Atanasov, A.G.; Hooper, J.N.A.; Al-Mourabit, A.; Kijjoa, A. Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria. Mar. Drugs 2018, 16, 214. https://doi.org/10.3390/md16060214
El-Demerdash A, Tammam MA, Atanasov AG, Hooper JNA, Al-Mourabit A, Kijjoa A. Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria. Marine Drugs. 2018; 16(6):214. https://doi.org/10.3390/md16060214
Chicago/Turabian StyleEl-Demerdash, Amr, Mohamed A. Tammam, Atanas G. Atanasov, John N. A. Hooper, Ali Al-Mourabit, and Anake Kijjoa. 2018. "Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria" Marine Drugs 16, no. 6: 214. https://doi.org/10.3390/md16060214
APA StyleEl-Demerdash, A., Tammam, M. A., Atanasov, A. G., Hooper, J. N. A., Al-Mourabit, A., & Kijjoa, A. (2018). Chemistry and Biological Activities of the Marine Sponges of the Genera Mycale (Arenochalina), Biemna and Clathria. Marine Drugs, 16(6), 214. https://doi.org/10.3390/md16060214