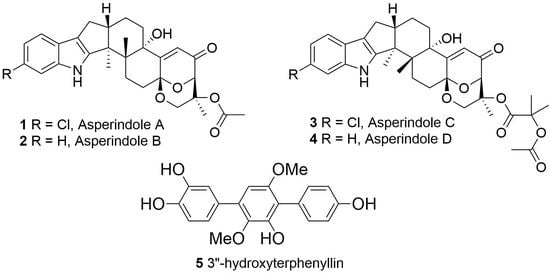
Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain
3.3. Cultivation of Fungus
3.4. Extraction and Isolation
3.5. Cell Culture
3.6. Cytotoxicity Assay
3.7. Cell Cycle and Apoptosis Induction Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Marchelli, R.; Vining, L.C. Terphenyllin, a novel p terphenyl metabolite from Aspergillus candidus. J. Antibiot. 1975, 28, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Kurobane, I.; Vining, L.C.; McInnes, A.G.; Smith, D.G. 3-Hydroxyterphenyllin, a new metabolite of Aspergillus candidus. Structure elucidation by 1H and 13C nuclear magnetic resonance spectroscopy. J. Antibiot. 1979, 32, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Kamigauchi, T.; Sakazaki, R.; Nagashima, K.; Kawamura, Y.; Yasuda, Y.; Matsushima, K.; Tani, H.; Takahashi, Y.; Ishii, K.; Suzuki, R.; et al. Terprenins, novel immunosuppressants produced by Aspergillus candidus. J. Antibiot. 1998, 51, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rahbæk, L.; Frisvad, J.C.; Christophersen, C. An amendment of Aspergillus section Candidi based on chemotaxonomical evidence. Phytochemistry 2000, 53, 581–586. [Google Scholar] [CrossRef]
- Cai, S.; Sun, S.; Zhou, H.; Kong, X.; Zhu, T.; Li, D.; Gu, Q. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. J. Nat. Prod. 2011, 74, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.E.; Marshall, A.C. Structure of chlorflavonin. J. Chem. Soc. C 1969, 18, 2418–2420. [Google Scholar] [CrossRef]
- Yan, T.; Guo, Z.K.; Jiang, R.; Wei, W.; Wang, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. New Flavonol and Diterpenoids from the Endophytic Fungus Aspergillus sp. YXf3. Planta Med. 2013, 79, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, E.; Surup, F.; Herrmann, J.; Huch, V.; Müller, R.; Stadler, M.; Rickenyls, A.-E. antioxidative terphenyls from the fungus Hypoxylon rickii (Xylariaceae, Ascomycota). Phytochemistry 2015, 118, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Valeria Calì, C.S.; Tringali, C. Polyhydroxy-P-Terphenyls and Related P-Terphenylquinones From Fungi: Overview and Biological Properties. Stud. Nat. Prod. Chem. 2003, 29, 263–307. [Google Scholar]
- Kobayashi, A.; Takemoto, A.; Koshimizu, K.; Kawazu, K. p-Terphenyls with cytotoxic activity toward sea urchin embryos. Agric. Biol. Chem. 1985, 49, 867–868. [Google Scholar] [CrossRef]
- Buttachon, S.; Ramos, A.A.; Inácio, Â.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef] [PubMed]
- Munday-Finch, S.C.; Miles, C.O.; Wilkins, A.L.; Hawkes, A.D. Isolation and Structure Elucidation of Lolitrem A, a Tremorgenic Mycotoxin from Perennial Ryegrass Infected with Acremonium lolii. J. Agric. Food Chem. 1995, 43, 1283–1288. [Google Scholar] [CrossRef]
- Gallagher, R.T.; White, E.P.; Mortimer, P.H. Ryegrass staggers: Isolation of potent neurotoxins lolitrem a and lolitrem b from staggers-producing pastures. N. Z. Vet. J. 1981, 29, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Munday-Finch, S.C.; Wilkins, A.L.; Miles, C.O.; Tomoda, H.; Omura, S. Isolation and Structure Elucidation of Lolilline, a Possible Biosynthetic Precursor of the Lolitrem Family of Tremorgenic Mycotoxins. J. Agric. Food Chem. 1997, 45, 199–204. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Finer, J.; Clardy, J.; Leutwiler, A.; Weibel, F.; Acklin, W.; Arigoni, D. Paspalinine, a Tremorgenic Metabolite from Claviceps paspali Stevens Et Hall. Tetrahedron Lett. 1980, 21, 235–238. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Liu, P.; Fu, P.; Zhu, T.; Wang, W.; Zhu, W. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J. Nat. Prod. 2013, 76, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.N.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Antiinsectan alkaloids: Shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron 1995, 51, 3959–3968. [Google Scholar] [CrossRef]
- Harms, H.; Rempel, V.; Kehraus, S.; Kaiser, M.; Hufendiek, P.; Müller, C.E.; König, G.M. Indoloditerpenes from a marine-derived fungal strain of Dichotomomyces cejpii with antagonistic activity at GPR18 and cannabinoid receptors. J. Nat. Prod. 2014, 77, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Sallam, A.A.; Houssen, W.E.; Gissendanner, C.R.; Orabi, K.Y.; Foudah, A.I.; El Sayed, K.A. Bioguided discovery and pharmacophore modeling of the mycotoxic indole diterpene alkaloids penitrems as breast cancer proliferation, migration, and invasion inhibitors. MedChemComm 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, A.E.; Steyn, P.S.; van Heerden, F.R.; Vleggaar, R.; Wessels, P.L.; Hull, W.E. Tremorgenic mycotoxins from Penicillium crustosum. Structure elucidation and absolute configuration of penitrems B–F. J. Chem. Soc. Perkin Trans. 1 1983, 41, 1857–1861. [Google Scholar] [CrossRef]
- De Jesus, A.E.; Steyn, P.S.; Van Heerden, F.R.; Vleggaar, R.; Wessels, P.L.; Hull, W.E. Tremorgenic mycotoxins from Penicillium crustosum: Isolation of penitrems A–F and the structure elucidation and absolute configuration of penitrem A. J. Chem. Soc. Perkin Trans. 1 1983, 14, 1847–1856. [Google Scholar] [CrossRef]
- Penn, J.; Biddle, J.R.; Mantle, P.G.; Bilton, J.N.; Sheppard, R.N. Pennigritrem, a naturally-occurring penitrem A analogue with novel cyclisation in the diterpenoid moiety. J. Chem. Soc. Perkin Trans. 1 1992, 23, 23–26. [Google Scholar] [CrossRef]
- Cutler, H.G.; Cole, R.J.; Cox, R.H.; Wells, J.M. Fungal metabolites: Interesting new plant growth inhibitors. Proc. Plant Growth Regul. Work. Group 1979, 6, 87–91. [Google Scholar]
- Cutler, H.G.; LeFiles, J.H.; Crumley, F.G.; Cox, R.H. Hydroxyterphenyllin: A novel fungal metabolite with plant growth inhibiting properties. J. Agric. Food Chem. 1978, 26, 632–635. [Google Scholar] [CrossRef]
- Guo, Z.K.; Yan, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. P-terphenyl and diterpenoid metabolites from endophytic Aspergillus sp. YXf3. J. Nat. Prod. 2012, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.G.; Weedon, C.M. Biosynthesis and transformation of tremorgenic indole-diterpenoids by Penicillium paxilli and Acremonium lolii. Phytochemistry 1994, 36, 1209–1217. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; et al. The marine triterpene glycoside frondoside A exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyshlovoy, S.A.; Venz, S.; Shubina, L.K.; Fedorov, S.N.; Walther, R.; Jacobsen, C.; Stonik, V.A.; Bokemeyer, C.; Balabanov, S.; Honecker, F. Activity of aaptamine and two derivatives, demethyloxyaaptamine and isoaaptamine, in cisplatin-resistant germ cell cancer. J. Proteom. 2014, 96, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Hauschild, J.; Amann, K.; Tabakmakher, K.M.; Venz, S.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; et al. Marine alkaloid monanchocidin a overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget 2015, 6, 17328–17341. [Google Scholar] [CrossRef] [PubMed]








| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 2 | 154.0, C | 152.8, C | 154.1, C | 152.8, C |
| 3 | 51.4, C | 51.2, C | 51.4, C | 51.2, C |
| 4 | 38.5, C | 38.6, C | 38.5, C | 38.6, C |
| 5 | 26.3, CH2 | 26.2, CH2 | 26.3, CH2 | 26.2, CH2 |
| 6 | 30.0, CH2 | 30.1, CH2 | 30.0, CH2 | 30.0, CH2 |
| 7 | 93.6, C | 93.6, C | 93.6, C | 93.5, C |
| 9 | 78.3, CH | 78.3, CH | 78.3, CH | 78.6, CH |
| 10 | 195.9, C | 195.9, C | 195.9, C | 195.7, C |
| 11 | 119.9, CH | 119.9, CH | 119.9, CH | 119.8, CH |
| 12 | 159.1, C | 159.2, C | 159.1, C | 159.2, C |
| 13 | 77.0, C | 77.0, C | 77.0, C | 77.0, C |
| 14 | 31.5, CH2 | 31.6, CH2 | 31.5, CH2 | 31.5, CH2 |
| 15 | 20.8, CH2 | 20.9, CH2 | 20.82, CH2 | 20.9, CH2 |
| 16 | 48.3, CH | 48.3, CH | 48.3, CH | 48.4, CH |
| 17 | 26.9, CH2 | 27.1, CH2 | 26.9, CH2 | 27.1, CH2 |
| 18 | 115.0, C | 114.8, C | 115.0, C | 114.8, C |
| 19 | 123.3, C | 124.6, C | 123.3, C | 124.6, C |
| 20 | 118.6, CH | 117.5, CH | 118.6, CH | 117.5, CH |
| 21 | 118.7, CH | 118.3, CH | 118.7, CH | 118.3, CH |
| 22 | 123.7, C | 119.1, CH | 123.7, C | 119.1, CH |
| 23 | 111.3, CH | 111.8, CH | 111.3, CH | 111.8, CH |
| 24 | 140.2, C | 139.9, C | 140.2, C | 139.9, C |
| 25 | 16.1, CH3 | 16.2, CH3 | 16.1, CH3 | 16.2, CH3 |
| 26 | 23.4, CH3 | 23.4, CH3 | 23.4, CH3 | 23.3, CH3 |
| 27 | 75.0, C | 75.0, C | 75.8, C | 75.8, C |
| 28 | 64.8, CH2 | 64.8, CH2 | 64.1, CH2 | 64.1, CH2 |
| 29 | 17.0, CH3 | 17.0, CH3 | 16.4, CH3 | 16.4, CH3 |
| 1′ | 170.2, C | 170.2, C | 171.1, C | 171.1, C |
| 2′ | 21.8, CH3 | 21.8, CH3 | 77.9, C | 77.9, C |
| 3′ | 23.9, CH3 | 23.9, CH3 | ||
| 4′ | 24.2, CH3 | 24.2, CH3 | ||
| 1″ | 169.3, C | 169.3, C | ||
| 2″ | 20.75, CH3 | 20.8, CH3 |
| Position | 1 * | 2 ** | 3 ** | 4 ** |
|---|---|---|---|---|
| NH | 10.73, brs | 10.54, brs | 10.73, s | 10.52, s |
| 5α 5β | 1.93, m 2.40, dd (13.4, 10.0) | 1.96, m 2.41, t (12.3) | 1.95, m 2.39, dd (13.4,10.0) | 1.96, m 2.41, t (12.3) |
| 6α 6β | 2.00, dd (12.9, 8.6) 2.55, m | 1.99, m 2.55, m | 1.95, dd (12.9, 8.6) 2.55, m | 1.95, dd (12.9, 8.6) 2.55, m |
| 9 | 4.74, d (2.3) | 4.74, d (2.1) | 4.74, d (2.3) | 4.63, d (2.4) |
| 11 | 6.11, s | 6.11, s | 6.11, s | 6.12, s |
| 14α 14β | 2.11, dt (13.6, 2.8) 1.77, td (13.2, 4.5) | 2.12, brd (13.4) 1.78, brt (13.4) | 2.12, dt (13.6, 2.8) 1.76, td (13.2, 4.5) | |
| 15α 15β | 1.91, m 1.66, m | 1.91, m 1.66, m | 1.91, m 1.65, m | 1.91, m 1.65, m |
| 16 | 2.72, m | 2.72, m | 2.72, m | 2.63, m |
| 17α 17β | 2.30, dd (13.0, 10.9) 2.60, dd (13.0, 6.4) | 2.30, t (12.3) 2.60, dd (12.3, 6.6) | 2.30, dd (13.0, 10.9) 2.60, dd (13.0, 6.4) | 2.31, dd (13.0, 10.9) 2.60, dd (13.0, 6.2) |
| 20 | 7.26, d (8.6) | 7.25, d (7.6) | 7.26, d (8.3) | 7.25, d (7.5) |
| 21 | 6.91, dd (8.3, 1.9) | 6.89, t (7.6) | 6.91, dd (8.3, 2.0) | 6.88, brt (7.2) |
| 22 | 6.93, t (7.6) | 6.92, brt (7.1) | ||
| 23 | 7.27, d (2.2) | 7.27, d (7.6) | 7.25, d (2.0) | 7.27, d (6.9) |
| 25 | 1.31, s | 1.30, s | 1.31, s | 1.30, s |
| 26 | 1.02, s | 1.03, s | 1.02, s | 1.03, s |
| 28α 28β | 4.04, dd (13.4, 2.5) 3.67, d (13.4) | 4.05, dd (13.3, 2.1) 3.68, d (13.3) | 4.11, dd (13.2, 2.5) 3.68, d (13.2) | 4.11, dd (13.4, 2.4) 3.69, d (13.4) |
| 29 | 1.21, s | 1.21, s | 1.17, s | 1.18, s |
| 3′ | 1.52, s | 1.52, s | ||
| 4′ | 1.54, s | 1.54, s | ||
| 2″ | 2.07, s | 2.07, s | 2.04, s | 2.04, s |
| 13-OH | 5.10, s | 5.08, s | 5.11, s | 5.08, s |
| Position | δС, mult | δH (J in Hz) | HMBC | ROESY |
|---|---|---|---|---|
| 1 | 126.8, C | |||
| 2 | 133.7, CH | 7.25, d (8.4) | 4, 6, 4′ | |
| 3 | 115.9, CH | 6.85, d (8.5) | 1, 5 | |
| 4 | 157.6, C | |||
| 5 | 115.9, CH | 6.85, d (8.5) | 1, 3 | |
| 6 | 133.7, CH | 7.25, d (8.4) | 2, 4, 4′ | 5′-OMe |
| 1′ | 134.2, C | |||
| 2′ | 140.8, C | |||
| 3′ | 149.8, C | |||
| 4′ | 118.3, C | |||
| 5′ | 155.1, C | |||
| 6′ | 104.8, CH | 6.47, s | 2′, 4′, 1″ | 2″, 6″ |
| 1″ | 131.9, C | |||
| 2″ | 117.5, CH | 7.19, d (2.1) | 1′, 4″, 6″ | 6′, 2′-OMe |
| 3″ | 146.4, C | |||
| 4″ | 146.3, C | |||
| 5″ | 116.8, CH | 6.91, d (8.1) | 1″, 3″ | |
| 6″ | 122.0, CH | 7.02, dd (8.1, 2.1) | 1′, 2″, 4″ | 6′, 2′-OMe |
| 2′-OMe | 61.4, CH3 | 3.41, s | 2′ | 2″, 6” |
| 5′-OMe | 56.8, CH3 | 3.71, s | 5′ | 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; Von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. https://doi.org/10.3390/md16070232
Ivanets EV, Yurchenko AN, Smetanina OF, Rasin AB, Zhuravleva OI, Pivkin MV, Popov RS, Von Amsberg G, Afiyatullov SS, Dyshlovoy SA. Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Marine Drugs. 2018; 16(7):232. https://doi.org/10.3390/md16070232
Chicago/Turabian StyleIvanets, Elena V., Anton N. Yurchenko, Olga F. Smetanina, Anton B. Rasin, Olesya I. Zhuravleva, Mikhail V. Pivkin, Roman S. Popov, Gunhild Von Amsberg, Shamil Sh. Afiyatullov, and Sergey A. Dyshlovoy. 2018. "Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676" Marine Drugs 16, no. 7: 232. https://doi.org/10.3390/md16070232
APA StyleIvanets, E. V., Yurchenko, A. N., Smetanina, O. F., Rasin, A. B., Zhuravleva, O. I., Pivkin, M. V., Popov, R. S., Von Amsberg, G., Afiyatullov, S. S., & Dyshlovoy, S. A. (2018). Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Marine Drugs, 16(7), 232. https://doi.org/10.3390/md16070232