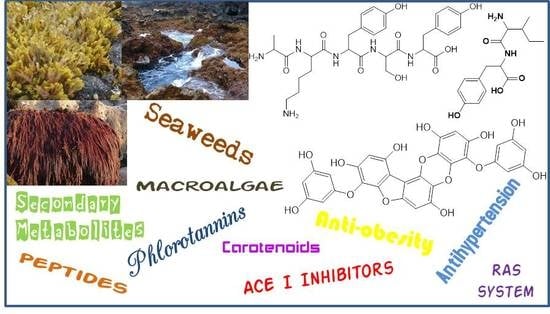
Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds
Abstract
:1. Introduction
2. Anti-Hypertensive Seaweed Compounds
2.1. Peptides
2.2. Phlorotannins
2.3. Polysacharides
3. Anti-Obesity Seaweed Compounds
3.1. Phlorotannins
3.2. Sterols
3.3. Indole Derivatives
3.4. Caretonoids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Peterson, E.D.; McCullough, P.A. Impact of obesity on cardiovascular disease. Med. Clin. North Am. 2011, 95, 919–937. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Leggio, M.; Lombardi, M.; Caldarone, E.; Severi, P.; D'Emidio, S.; Armeni, M.; Bravi, V.; Bendini, M.G.; Mazza, A. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens. Res. 2017, 40, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B. Hypertension in obesity and the impact of weight loss. Curr. Cardiol. Rep. 2017, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Schellack, N.; Naicker, P. Hypertension: A review of antihypertensive medication, past and present. South Afr. Pharm. J. 2015, 82, 17–25. [Google Scholar]
- Faulkner, J.L.; Belin de Chantemèle, E.J. Sex differences in mechanisms of hypertension associated with obesity. Hypertension 2018, 71, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Jarari, N.; Rao, N.; Peela, J.R.; Ellafi, K.A.; Shakila, S.; Said, A.R.; Nelapalli, N.K.; Min, Y.; Tun, K.D.; Jamallulail, S.I.; et al. A review on prescribing patterns of antihypertensive drugs. Clin. Hypertens. 2016, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO) Fisheries Technical Paper 441: A Guide to the Seaweed Industry. Available online: http://www.fao.org/docrep/006/y4765e/y4765e0b.htm (accessed on 30 April 2018).
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.; Medema, M.H.; van der Oost, J.; Sipkem, D. Exploration and exploitation of the environment for novel specialized metabolites. Curr. Opin. Biotechnol. 2018, 50, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sustain. Energy Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Verma, P.; Kumar, M.; Mishra, G.; Sahoo, D. Multivariate analysis of fatty acid and biochemical constitutes of seaweeds to characterize their potential as bioresource for biofuel and fine chemicals. Bioresour. Technol. 2017, 226, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl celulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Zao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food. Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Magnusson, M.; Ward, L.C.; Paul, N.A.; Brown, L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs 2015, 13, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Sabirin, F.; Soo, K.K.; Ziau, H.S.; Kuen, L.S. Antihypertensive effects of edible brown seaweeds in rats. Int. J. Adv. Appl. Sci. 2016, 3, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sadiq, F.A.; Fu, L.; Zhu, H.; Zhong, M.; Sohail, M. Identification of angiotensin I-converting enzyme inhibitory peptides derived from enzymatic hydrolysates of razor clam Sinonovacula constricta. Mar. Drugs 2016, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Nasri, M. Protein Hydrolisates and Biopeptides: Production, Biological Activities and Application in Foods and Health Benefits. A Review. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: London, UK, 2017; Volume 81, pp. 109–159. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2018. Available online: http://www.algaebase.org (accessed on 30 April 2018).
- Hayes, M.; Tiwari, B.K. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Croft, A.K.; Hayes, M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010, 53, 387–408. [Google Scholar] [CrossRef]
- Saito, M.; Hagino, H. Antihypertensive effect of oligopeptides derived from nori (Porphyra yezoensis) and Ala-Lys-Tyr-Ser-Tyr in rats. J. Jpn. Soc. Nutr. Food Sci. 2005, 58, 177–184. [Google Scholar] [CrossRef]
- Saito, M.; Kawai, M.; Hagino, H.; Okada, J.; Yamamoto, K.; Hayashida, M.; Ikeda, T. Antihypertensive effect of Nori-peptides derived from red alga Porphyra yezoensis in hypertensive patients. Am. J. Hypertens. 2002, 15, 210A. [Google Scholar] [CrossRef]
- Suetsuna, K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar] [PubMed]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; Ishii, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.-J.; Ikemura, K.; Takaoka, M.; Moriguchi, S.; et al. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hayden, H.S.; Blomster, J.; Maggs, C.A.; Silva, P.C.; Stanhope, M.J.; Waaland, J.R. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 2003, 38, 277–294. [Google Scholar] [CrossRef]
- Gradman, A.H.; Kad, R. Renin inhibition in hypertension. J. Am. Coll. Cardiol. 2008, 51, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.S.; Lima, E.M.C.; Neto, A.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Cha, S.H.; Ahn, G.N.; Heo, S.J.; Kim, K.N.; Lee, K.W.; Song, C.B. Screening of extracts from marine green and brown algae in Jeju for potential marine angiotensin-I converting enzyme (ACE) inhibitory activity. J. Korean Soc. Food Sci. Nutr. 2006, 35, 307–314. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Ancona, D.B.; Guerrero, L.C.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure−activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Design. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- He, H.; Liu, D.; Ma, C. Review on the angiotensin-I-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Mullick, A.E. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol. Res. 2017, 125, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Aluko, R.E. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J. Agric. Food. Chem. 2010, 58, 11471–11476. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Youn, J.-Y.; Cai, H. Mechanisms and consequences of endothelial nitric oxide synthase dysfunction in hypertension. J. Hypert. 2015, 33, 1128–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandana, T.; Venkatesh, Y.P. Occurrence, functions and biological significance of arginine-rich proteins. Curr. Protein Peptide Sci. 2016, 17, 507–516. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods. 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins and peptides from macroalgae, fish, shellfish and marine processing waste. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S.-K., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp. 5–39. ISBN 978-1-118-37506-8. [Google Scholar]
- Cian, R.E.; Alaiz, M.; Vioque, J.; Drago, S.R. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbina residual cake. J. Appl. Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Olivares-Molina, A.; Fernández, K. Comparison of different extraction techniques for obtaining extracts from brown seaweeds and their potential effects as angiotensin I-converting enzyme (ACE) inhibitors. J. Appl. Phycol. 2016, 28, 1295–1302. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.-K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Proc. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B: Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ko, S.-C.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fisheries Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, M.C.; Kang, N.; Kim, H.-S.; Lee, S.-H.; Ahn, G.; Jung, W.-K.; Jeon, Y.-J. Effect of angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO) production of 6,6′-bieckol, a marine algal polyphenol and its antihypertensive effect in spontaneously hypertensive rats. Proc. Biochem. 2017, 58, 326–332. [Google Scholar] [CrossRef]
- Shibata, T.; Yamaguchi, K.; Nagayama, K.; Kawagushi, S.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against glycosidases from the viscera of the turban shell Turbo cornutus. Eur. J. Phycol. 2002, 37, 493–500. [Google Scholar] [CrossRef]
- Nasser, S.A.; El-Mas, M.M. Endothelin ETA receptor antagonism in cardiovascular disease. Eur. J. Pharmacol. 2014, 737, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.B.; Basra, S.S. Are there any new pharmacologic therapies on the horizon to better treat hypertension? A state-of-the-art paper. J. Cardiov. Pharmacol. Ther. 2014, 19, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Planes, N.; Caballero-George, C. Marine and soil derived natural products: A new source of novel cardiovascular protective agents targeting the endothelin system. Planta Med. 2015, 81, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-B.; Geng, M.-Y.; Guan, H.-S.; Zhang, J.T. Antihypertensive effects of D-polymannuronic sulfate and its related mechanisms in renovascular hypertensive rats. Acta Pharmacol. Sin. 2000, 21, 727–732. [Google Scholar]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and cardiovascular disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Tong, Q. An update on the regulation of adipogenesis. Drug Discov. Today: Dis. Mech. 2013, 10, e15–e19. [Google Scholar] [CrossRef]
- Choi, K.-M.; Jeon, Y.S.; Kim, W.; Lee, A.; Kim, Y.-G.; Lee, J.H.; Kang, Y.E.; Jung, J.-C.; Lee, J.; Min, B.; et al. Xanthigen attenuates high-fat diet-induced obesity through down-regulation of PPARγ and activation of the AMPK pathway. Food Sci. Biotechnol. 2014, 23, 931–935. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Awang, A.N.; Ng, J.L.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H. Anti-obesity property of the brown seaweed, Sargassum polycystum using an in vivo animal model. J. Appl. Phycol. 2014, 26, 1043–1048. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, J.; Lee, Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr. Res. Pract. 2016, 10, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kang, N.; Ko, S.-C.; Kim, Y.-B.; Jeon, Y.-J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Nakazono, S.; Cho, K.; Isaka, S.; Abu, R.; Yokose, T.; Murata, M.; Ueno, M.; Tachibana, K.; Hirasaka, K.; Kim, D.; et al. Anti-obesity effects of enzymatically-digested alginate oligomer in mice model fed a high-fat-diet. Bioactive Carboh. Diet. Fibre 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Gao, Y.; Xue, C.-h.; Li, R.W.; Tang, Q.-j. Transcriptome analysis revealed anti-obesity effects of the sodium alginate in high-fat diet-induced obese mice. Int. J. Biol. Macromol. 2018, 115, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lee, P.; Chisholm, D.J.; James, D.E. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front. Endocrinol. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-C.; Hsieh, P.-H.; Pan, M.-H.; Ho, C.-T. Cellular models for the evaluation of the antiobesity effect of selected phytochemicals from food and herbs. J. Food Drug Anal. 2017, 25, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-H.; Wu, Y.-X.; Kim, J.-S.; Woo, J.-H.; Park, K.T.; Kwon, O.J.; Seo, H.-J.; Kim, T.; Park, N.-H. 6,6′-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food Agric. 2015, 95, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.-S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharm. Res. 2014, 37, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Jung, H.A.; Kang, M.J.; Choi, J.S.; Kim, G.-D. Fucosterol, isolated from Ecklonia stolonifera, inhibits adipogenesis through modulation of FoxO1 pathway in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2017, 69, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Ding, Y.; Kim, A.-A.; Choi, Y.K.; Araujo, T.D.; Heo, S.-J.; Lee, S.-H. Indole derivatives isolated from brown alga Sargassum thunbergii inhibit adipogenesis through AMPK activation in 3T3-L1 preadipocytes. Mar. Drugs 2017, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Muradian, K.; Vaiserman, A.; Min, K.-J.; Fraifeld, V.E. Fucoxanthin and lipid metabolism: A minireview. Nutr. Metab. Card. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: A review. J. Oleo Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-I.; Ko, H.-C.; Shin, H.-S.; Kim, H.-M.; Hong, Y.-S.; Lee, N.-H.; Kim, S.-J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Yim, M.-J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive effects of amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPAR𝛾 and C/EBP𝛼 mRNA expression. J. Agric. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Shih, P.-H.; Wang, W.; Wu, C.-H.; Hsia, S.-M.; Wang, H.-J.; Hwang, P.-A.; Wang, C.-Y.; Chen, S.-H.; Kuo, Y.-T. Inhibitory effects of high stability fucoxanthin on palmitic acid-induced lipid accumulation in human adipose-derived stem cells through modulation of long non-coding RNA. Food Funct. 2015, 6, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; Arunkumar, R.; Baskaran, V. Chitosan-glycolipid nanogels loaded with anti-obese marine carotenoid fucoxanthin: Acute and sub-acute toxicity evaluation in rodent model. J. Biomater. Appl. 2015, 30, 420–434. [Google Scholar] [CrossRef] [PubMed]









| Secondary metabolite (No) | Effects 1 and mechanism 2 IC50 3 | Reference |
|---|---|---|
| ACE I inhibitors | ||
| Peptide IPP (1) | Positive inhibition; ↓ Blood pressure | [32] |
| Peptide VPP (2) | Positive inhibition; ↓ Blood pressure | [32] |
| Peptide FY (3) | Positive inhibition; ↓ Blood pressure | [33] |
| Peptide VY (4) | Positive inhibition; ↓ Blood pressure | [33] |
| Peptide IY (5) | IC50 2.96 µM; ↓ Blood pressure | [33,39] |
| Peptide AKYSY (6) | IC50 1.52 µM; ↓ Blood pressure | [29,32,34,39] |
| Peptide MKY (7) | IC50 7.26 µM; ↓ Blood pressure | [39] |
| Peptide LRY (8) | IC50 5.06 µM; ↓ Blood pressure | [39,40] |
| Peptide YH (9) | IC50 5.1 µM; ↓ Blood pressure | [35,42] |
| Peptide KY (10) | IC50 7.7 µM; ↓ Blood pressure | [35,42] |
| Peptide PAFG (11) | IC50 35.9 µM; ↓ Blood pressure | [43] |
| Peptide IP (12) | IC50 87.6 µM | [46] |
| Peptide AFL (13) | IC50 65.9 µM | [46] |
| Peptide PAFG (14) | IC50 16.0 µM | [46] |
| Peptide VYRT (15) | Positive inhibition | [40] |
| Peptide QVEY (16) | IC50 474.36 µM | [47] |
| Dieckol (18) | IC50 1470 µM; ↑ production of NO in EAhy926 cells | [67] |
| Phlorofucofuroeckol A (19) | IC50 12.74 µM; | [68] |
| 6,6′-Bieckol (20) | IC50 0.42 mM; interact with the S1, S′1 and S′2 pockets of ACE; ↑ eNOS-mediated NO in HUVEC cells; ↓ Systolic blood pressure | [69] |
| D-Polymannuronic sulphate (21) | Positive inhibition; ↑ production of NO; ↓ concentrations of Ang II; ↓ concentrations of ET 1; ↓Blood pressure | [74] |
| RAS inhibitors | ||
| Peptide QVEY (17) | Positive inhibition | [55] |
| Secondary metabolite (No) | Target and activity | Reference |
|---|---|---|
| Dieckol (18) | Reduced lipid accumulation in 3T3-L1cells; ↓ C/EBPα and PPARγ expression | [88] |
| Phlorofucofuroeckol A (19) | Reduced lipid accumulation in 3T3-L1 cells (IC50 17.86 μM); ↓ C/EBPα and PPARγ expression | [88] |
| 6,6′-Bieckol (20) | Suppressed lipid accumulation in 3T3-L1 adipocytes; inhibition of lipogenic enzymes; ↓ mRNA expression | [89] |
| Phloroglucinol (22) | Reduced lipid accumulation in 3T3-L1 cells; potent inhibitory activities on adipocyte differentiation; ↓ C/EBPα and PPARγ expression | [88] |
| Dioxinodehydroeckol (23) | Reduced lipid accumulation in 3T3-L1; potent inhibitory activities on adipocyte differentiation; ↓ C/EBPα and PPARγ expression | [88] |
| Eckol (24) | Reduced lipid accumulation in 3T3-L1 cells; potent inhibitory activities on adipocyte differentiation; ↓ C/EBPα and PPARγ expression | [88] |
| Fucosterol (25) | ↓ C/EBPα and PPARγ expression; inhibited adipogenesis of 3T3-L1; ↓ SREBP; modulation of PI3K/Akt- and ERK-dependent FoxO signalling pathways | [90,91] |
| 1H-Indole-2-carbaldehyde (26) | inhibition of the 3T3-L1 cells adipocyte differentiation; ↑AMPK signal pathway | [92] |
| 1H-Indole-6-carbaldehyde (27) | inhibition of the 3T3-L1 cells adipocyte differentiation; ↑AMPK signal pathway | [92] |
| Fucoxanthin (28) | inhibit the intercellular lipid accumulation; ↓ C/EBPα and PPARγ expression; ↓ SREBP; ↑ uncoupling protein-1 (UCP-1); ↑ β3-adrenergic receptor expression | [95,96,97] |
| Fucoxanthinol (29) | ↓ PPARγ expression; ↓ adipocyte differentiation in 3T3-L1 cells | [98,99] |
| Amarouciaxanthin A (30) | ↓ PPARγ expression; ↓ adipocyte differentiation in 3T3-L1 cells | [98,99] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seca, A.M.L.; Pinto, D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. https://doi.org/10.3390/md16070237
Seca AML, Pinto DCGA. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Marine Drugs. 2018; 16(7):237. https://doi.org/10.3390/md16070237
Chicago/Turabian StyleSeca, Ana M. L., and Diana C. G. A. Pinto. 2018. "Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds" Marine Drugs 16, no. 7: 237. https://doi.org/10.3390/md16070237
APA StyleSeca, A. M. L., & Pinto, D. C. G. A. (2018). Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Marine Drugs, 16(7), 237. https://doi.org/10.3390/md16070237