Physicochemical and Functional Properties of Type I Collagens in Red Stingray (Dasyatis akajei) Skin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield
2.2. Structural Characterization
2.2.1. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.2.2. Secondary Structure
2.3. Physicochemical Properties
2.3.1. Zeta Potential
2.3.2. Water Contact Angle (WCA)
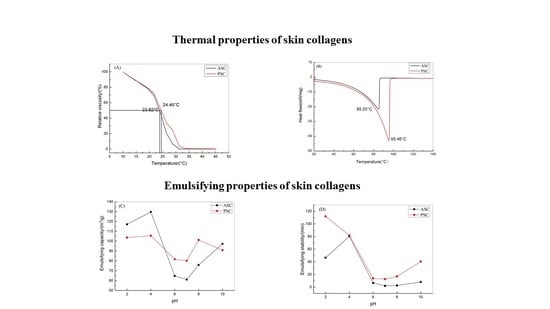
2.3.3. Thermal Properties
2.3.4. Rheological Characterization of Collagen Solutions
2.4. Functional Properties
2.4.1. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
2.4.2. Foaming and Emulsifying Properties
2.5. Cell Proliferation
3. Materials and Methods
3.1. Materials
3.2. Extraction of Collagens
3.2.1. Extraction of ASC
3.2.2. Extraction of PSC
3.2.3. Yield
3.3. Structural Characterization
3.3.1. SDS-PAGE Analysis
3.3.2. Spectroscopy Analysis
3.4. Physicochemical Properties
3.4.1. Zeta Potential
3.4.2. WCA
3.4.3. Thermal Properties
3.4.4. Rheological Properties
3.5. Functional Properties
3.5.1. WAC and OAC
3.5.2. FC and FS
3.5.3. Emulsifying Properties
3.6. Cell Proliferation
3.7. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foegeding, E.A.; Laneir, T.C.; Hultin, H.O. Characteristics of Edible Muscle Tissues; Fennema, O.R., Ed.; Food chemistry, Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 902–906. [Google Scholar]
- Gara, S.K.; Grumati, P.; Urciuolo, A.; Bonaldo, P.; Kobbe, B.; Koch, M.; Paulsson, M.; Wagener, R. Three novel collagen VI chains with high homology to the α3 chain. J. Biol. Chem. 2008, 283, 10658–10670. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Li, L.; Yi, R.Z.; Gao, R.; He, J.L. Release kinetics of Tilapia scale I collagen peptides during tryptic hydrolysis. Food Hydrocoll. 2018, 77, 931–936. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Henrickson, R.L. Chemical, Biochemical, Functional and Nutritional Characteristics of Collagen in Food Systems; Chichester, C.O., Mrata, E.M., Schweigert, B.S., Eds.; Advances in food research, Academic Press: London, UK, 1982; pp. 232–372. [Google Scholar]
- Nalinanon, S.; Benjakul, S.; Kishimura, H. Collagens from the skin of arabesque greenling (Pleurogrammus azonus) solubilized with the aid of acetic acid and pepsin from albacore tuna (Thunnus alalunga) stomach. J. Sci. Food Agr. 2010, 90, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.M.; Montero, M.P.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Mørkøre, T.; Borderías, J. Collagen characteristics of farmed Atlantic salmon with firm and soft fillet texture. Food Chem. 2012, 134, 678–685. [Google Scholar] [CrossRef]
- Sun, L.L.; Li, B.F.; Song, W.K.; Si, L.L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, M.; Yi, R.Z.; Bai, K.K.; Wang, G.Y.; Tan, R.; Sun, S.S.; Xu, N.H. Electrodialysis extraction of pufferfish skin (Takifugu flavidus): a promising source of collagen. Mar. drugs 2019, 17, 25. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structure and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Tamilmozhi, S.; Anguchamy, V.; Arumugam, M. Isolation and characterization of acid and pepsin-solubilized collagen from the skin of sailfish (Istiophorus platypterus). Food Res. Int. 2013, 54, 1499–1505. [Google Scholar] [CrossRef]
- Yu, F.M.; Zong, C.H.; Jin, S.J.; Zheng, J.W.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.F.; Yang, Z.S.; Tang, Y.P.; et al. Optimization of extraction conditions and characterization of pepsin-solubilised collagen from skin of giant croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef]
- Li, N.; Song, N.; Cheng, G.P.; Gao, T.X. Genetic diversity and population structure of the red stingray, Dasyatis akajei inferred by AFLP marker. Biochem. Syst Ecol. 2013, 51, 130–137. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Z.H.; Hou, H.; Zhao, X.; Li, B.F.; Zhao, T.F.; Liu, L.Y. Characterization of acid-and pepsin-soluble collagens from the cuticle of Perinereis Nuntia (Savigny). Food Biophys. 2018, 13, 274–283. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.L.; Liu, H.Y.; Zhou, D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. Biol. Macromol. 2018, 106, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Shiau, C.Y.; Chen, H.H.; Huang, B.C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocoll. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Huda, N.; Seow, N.H.; Normawati, M.N.; Aisyah, N.M.N. Preliminary study on physicochemical properties of duck feet collagen. Int.J. Poult. Sci. 2013, 12, 615–621. [Google Scholar] [CrossRef]
- Iswariya, S.; Velswamy, P.; Uma, T.S. Isolation and characterization of biocompatible collagen from the skin of puffer fish (Lagocephalus inermis). J. Polym. Environ. 2018, 26, 2086–2095. [Google Scholar] [CrossRef]
- Liu, D.S.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterization of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Chen, J.D.; Li, L.; Yi, R.Z.; Xu, N.H.; Gao, R.; Hong, B.H. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT-Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Exposito, J.Y.; Larroux, C.; Cluzel, C.; Valcourt, U.; Lethias, C.; Degnan, B.M. Demosponge and sea anemone fibrillar collagen diversity reveals the early emergence of A/C clades and the maintenance of the modular structure of type V/XI collagens from sponge to human. J. Biol. Chem. 2008, 283, 28226–28235. [Google Scholar] [CrossRef]
- Jeevithan, E.; Wu, W.H.; Wang, N.P.; Lan, H.; Bao, B. Isolation purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus) skeletal and head bone. Process Biochem. 2014, 49, 1767–1777. [Google Scholar] [CrossRef]
- Doyl, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Plepis, A.M.D.G.; Goissis, G.; Das-Gupta, D.K. Dielectric and pyroelectric characterization of anionic and native collagen. Polym. Eng. Sci. 1996, 36, 2932–2938. [Google Scholar] [CrossRef]
- Usha, R.; Ramasami, T. Structure and conformation of intramolecularly cross-linked collagen. Colloid. Surface. B. 2005, 41, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.B.; Melacini, G.; Taulane, J.P.; Goodan, M. Acetyl-terminated and template-assembled collagen-based polypeptides composed of Gly-Pro-Hyp sequence. 2. Synthesis and conformational analysis by circular dichroism ultraviolet absorbance, and optical rotation. J. Am. Chem. Soc. 1996, 118, 10351–10358. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.T.; Li, G.Y.; Shi, B.; Miao, Y.Q.; Wu, X.H. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterization of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Cui, W.G.; Cheng, L.Y.; Li, H.Y.; Zhou, Y.; Zhang, Y.G.; Chang, J. Preparation of hydrophilic poly (L-Lactide) electrospun fibrous scaffolds modified with chitosan for enhanced cell biocompatibility. Polymer 2012, 53, 2298–2305. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhou, X.; Zhong, J.Z.; Tan, L.; Liu, C.M. Effect of pH on emulsification performance of a new functional protein from jackfruit seeds. Food Hydrocoll. 2019, 93, 325–334. [Google Scholar] [CrossRef]
- Zhao, W.H.; Chi, C.F.; Zhao, Y.Q.; Wang, B. Preparation, physicochemical and antioxidant properties of acid-and pepsin-soluble collagens from the swim bladders of miiuy croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef]
- Wang, S.S.; Yu, Y.; Sun, Y.; Liu, N.; Zhou, D.Q. Comparison of physicochemical characteristics and fibril formation ability of collagens extracted from the skin of farmed river puffer (Takifugu obscurus) and tiger puffer (Takifugu rubripes). Mar. drugs 2019, 17, 462. [Google Scholar] [CrossRef]
- Tang, Y.P.; Jin, S.J.; Li, X.Y.; Li, X.J.; Hu, X.Y.; Chen, Y.; Huang, F.F.; Yang, Z.S.; Yu, F.M.; Ding, G.F. Physicochemical properties and biocompatibility evaluation of collagen from the skin of giant croaker (Nibea japonica). Mar. drugs 2018, 16, 222. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Cai, P.P.; Li, P.P.; Zhang, M.H.; Sun, Z.L.; Sun, C.; Xu, W.M.; Wang, D.Y. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2017, 105, 1602–1610. [Google Scholar] [CrossRef]
- Hadian, M.; Corcoran, B.M.; Bradshaw, J.P. Molecular changes in fibrillar collagen in myxomatous mitral valve disease. Cardiovasc. Pathol. 2010, 19, 141–148. [Google Scholar] [CrossRef]
- Kim, H.W.; Yeo, I.J.; Hwang, K.E.; Song, D.H.; Kim, Y.J.; Ham, Y.K.; Jeong, T.J.; Choi, Y.S.; Kim, C.J. Isolation and characterization of pepsin-soluble collagens from bones, skins, and tendons in duck feet. Korean J. Food Sci. An. 2016, 36, 665–670. [Google Scholar] [CrossRef]
- Yu, W.J.; Xu, D.; Li, D.D.; Guo, L.N.; Su, X.Q.; Zhang, Y.; Wu, F.F.; Xu, X.M. Effect of pigskin-originated gelatin on properties of wheat flour dough and bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Sai, K.P.; Babu, M. Studies on rana tigerina skin collagen. Comp. Biochem. Phys B. 2001, 128, 81–90. [Google Scholar]
- Ju, H.Y.; Liu, M.; Dan, W.H.; Hu, Y.; Lin, H.; Dan, N.H. Dynamic rheological property of type I collagen fibrils. J. Mech. Med. Biol. 2013, 13, 1340015. [Google Scholar] [CrossRef]
- Han, J.R.; Tang, Y.; Li, Y.; Shang, W.H.; Yan, J.N.; Du, Y.N.; Wu, H.T.; Zhu, B.W.; Xiong, Y.L.L. Physiochemical properties and functional characteristics of protein isolates from the scallop (Patinopecten yessoensis) gonad. J. Food Sci. 2019, 84, 1023–1034. [Google Scholar] [CrossRef]
- Pham, T.T.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Effects of pH and salt concentration on functional properties of pumpkin seed protein fractions. J. Food Process. Pres. 2016, 41, e13073. [Google Scholar] [CrossRef]
- Ren, Z.Y.; Chen, Z.Z.; Zhang, Y.Y.; Zhao, T.; Ye, X.G.; Gao, X.; Lin, X.R.; Li, B. Functional properties and structural profiles of water-insoluble proteins from three types of tea residues. LWT-Food Sci. Technol. 2019, 110, 324–331. [Google Scholar] [CrossRef]
- Wu, W.; Hua, Y.F.; Lin, Q.L.; Xiao, H.X. Effects of oxidative modification on thernal aggergation and gel properties of soy protein by peroxyl radicals. Int. J. Food Sci. Tech. 2011, 46, 1891–1897. [Google Scholar] [CrossRef]
- Jitngarmkusol, S.; Hongsuwankul, J.; Tananuwong, K. Chemical compositions functional properties and microstructure of defatted macadamia flours. Food Chem. 2008, 110, 23–30. [Google Scholar] [CrossRef]
- Maruyama, N.; Katsube, T.; Wada, Y.; Oh, M.H.; De La Rosa, A.P.B.; Okuda, E.; Nakagawa, S.; Utsumi, S. The roles of the N - Linked glycans and extension regions of soybean β - conglycinin in folding, assembly and structural features. Eur. J. Biochem. 1998, 258, 854–862. [Google Scholar] [CrossRef]
- Chandi, G.K.; Sogi, D.S. Functional properties of rice bran protein concentrates. J. Food Eng. 2007, 79, 592–597. [Google Scholar] [CrossRef]
- Deng, Y.J.; Huang, L.X.; Zhang, C.H.; Xie, P.J.; Cheng, J.; Wang, X.J.; Li, S.H. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, Y.L.; Pan, J.F.; Long, H.; Huang, L.S.; Zhang, X.K. Compare study between icephobicity and superhydrophobicity. Physica B. 2019, 556, 118–130. [Google Scholar] [CrossRef]
- Yi, X.Z.; Zheng, Q.H.; Pan, M.-H.; Chiou, Y.-S.; Li, Z.S.; Li, L.; Chen, Y.; Hu, J.; Duan, S.Z.; Wei, S.D.; et al. Liposomal vesicles-protein interaction: Influences of iron liposomes on emulsifying properties of whey protein. Food Hydrocoll. 2019, 89, 602–612. [Google Scholar] [CrossRef]
- Xue, S.W.; Yu, X.B.; Lin, X.; Zhao, X.; Han, M.Y.; Xu, X.L.; Zhou, G.H. Structural changes and emulsion properties of goose liver proteins obtained by isoelectric solubilization/precipitation processes. LWT-Food Sci. Technol. 2019, 102, 190–196. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: The sea urchin Paracentrotus lividus. Mar. drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Jeevithan, E.; Bao, B.; Wang, S.J.; Gao, K.L.; Zhang, C.Y.; Wu, W.H. Structural characterization, in-vivo acute systemic toxicity assessment and in-vitro intestinal absorption properties of tilapia (Oreochromis niloticus) skin acid and pepsin solublilized type I collagen. Process Biochem. 2016, 51, 2017–2025. [Google Scholar] [CrossRef]
- Deng, L.L.; Zhang, X.; Yang, L.; Oue, F.; Kang, X.F.; Liu, Y.Y.; Feng, F.Q. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, J.M.; Wu, S.J.; Tsai, H.T. Isolation and characterization of fish scale collagen from tilapia (Oreochromis Sp.) by a novel extrusion-hydro-extraction process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, X.; Zhao, W.P.; Gao, G.X.; Zhang, X.W.; Wang, Y.B.; Wang, Y.N. Impact of pork collagen superfine powder on rheological and texture properties of Harbin red sausage. J. Texture Stud. 2018, 49, 300–308. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physico-chemical and functional properties of native and hydrolysed protein isolates from Indian black gram (Phaseolus mungo L.) cultivars. LWT-Food Sci. Technol. 2015, 60, 848–854. [Google Scholar] [CrossRef]
- Celik, M.; Güzel, M.; Yildirim, M. Effect of pH on protein extraction from sour cherry kernels and functional properties of resulting protein concentrate and functional properties of resulting protein concentrate. J. Food Sci. Tech. 2019, 56, 3023–3032. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, J.; Li, Z.; Yi, R.; Shi, S.; Wu, K.; Li, Y.; Wu, S. Physicochemical and Functional Properties of Type I Collagens in Red Stingray (Dasyatis akajei) Skin. Mar. Drugs 2019, 17, 558. https://doi.org/10.3390/md17100558
Chen J, Li J, Li Z, Yi R, Shi S, Wu K, Li Y, Wu S. Physicochemical and Functional Properties of Type I Collagens in Red Stingray (Dasyatis akajei) Skin. Marine Drugs. 2019; 17(10):558. https://doi.org/10.3390/md17100558
Chicago/Turabian StyleChen, Junde, Jianying Li, Zhongbao Li, Ruizao Yi, Shenjia Shi, Kunyuan Wu, Yushuang Li, and Sijia Wu. 2019. "Physicochemical and Functional Properties of Type I Collagens in Red Stingray (Dasyatis akajei) Skin" Marine Drugs 17, no. 10: 558. https://doi.org/10.3390/md17100558
APA StyleChen, J., Li, J., Li, Z., Yi, R., Shi, S., Wu, K., Li, Y., & Wu, S. (2019). Physicochemical and Functional Properties of Type I Collagens in Red Stingray (Dasyatis akajei) Skin. Marine Drugs, 17(10), 558. https://doi.org/10.3390/md17100558