Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro
Abstract
:1. Introduction
2. Results
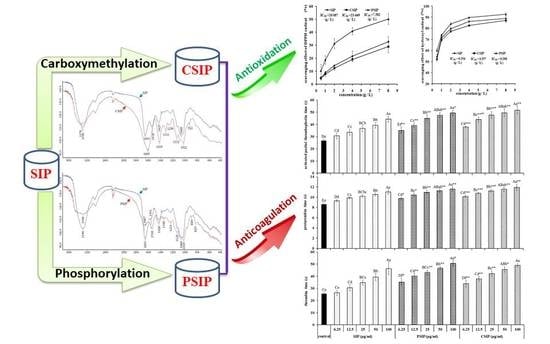
2.1. Determination of Carboxymethylation Parameters and Molecular Characteristics of CSIP
2.2. Determination of the Phosphorylation Parameters and Molecular Characteristics of PSIP
2.3. Antioxidation Activity of CSIP and PSIP
2.4. Anticoagulation Activity of CSIP and PSIP
3. Discussion
4. Materials and Methods
4.1. Preparation of SIP
4.2. Preparation of CSIP
4.3. Preparation of PSIP
4.4. Infrared Spectral Analysis
4.5. Scavenging Activity of Hydroxyl Radical
4.6. Scavenging Activity of DPPH
4.7. Determination of the Anticoagulation Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.P.; Luo, P.; Liu, H.Z. A potential adjuvant agent of chemotherapy: sepia ink polysaccharide. Mar. Drugs 2018, 16, 106. [Google Scholar]
- Liu, H.Z.; Zhang, Y.B.; Li, M.W.; Luo, P. Beneficial effect of Sepia esculenta ink polysaccharide on cyclophosphamide-induced immunosuppression and ovarian failure in mice. Int. J. Biol. Macromol. 2019, 140, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Z.; Tao, Y.X.; Luo, P.; Deng, C.M.; Gu, Y.P.; Yang, L.; Zhong, J.P. Preventive effects of a novel polysaccharide from Sepia esculenta ink on ovarian failure and its action mechanisms in cyclophosphamide-treated mice. J. Agric. Food Chem. 2016, 64, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Li, Z.G.; Wang, Y.M.; Li, G.Y.; Sun, B.B.; Xue, C.H. Sulfation of a squid ink polysaccharide and its anticoagulant activities. Chem. J. Chin. Univ. 2010, 31, 2407–2412. [Google Scholar]
- Bohn, J.A.; Be Miller, J.N. (1→3)-β-D-Glucan as biological response modifiers: A review of structure–functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Chen, J.; Huang, G. Antioxidant activities of garlic polysaccharide and its phosphorylated derivative. Int. J. Biol. Macromol. 2019, 125, 432–435. [Google Scholar] [CrossRef]
- Chen, S.G.; Wang, J.F.; Xue, C.H.; Li, H.; Sun, B.B.; Xue, Y.; Chai, W.G. Sulfation of a squid ink polysaccharide and its inhibitory effect on tumor cell metastasis. Carbohydr. Polym. 2010, 81, 560–566. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Y.; Wang, F.; Sun, L.; Liu, C.; Chen, G.; Li, Y.; Ward, S.G.; Qu, X. Inhibition activity of sulfated polysaccharide of Sepiella maindroni ink on matrix metalloproteinase (MMP)-2. Biomed. Pharmacother. 2008, 62, 297–302. [Google Scholar] [CrossRef]
- Zong, A.Z.; Zhao, T.; Zhang, Y.; Song, X.L.; Shi, Y.K.; Cao, H.Z.; Liu, C.H.; Cheng, Y.N.; Qu, X.J.; Cao, J.C.; et al. Anti-metastatic and anti-angiogenic activities of sulfated polysaccharide of Sepiella maindroni ink. Carbohydr. Polym. 2013, 91, 403–409. [Google Scholar] [CrossRef]
- Zong, A.Z.; Liu, Y.H.; Zhang, Y.; Song, X.; Shi, Y.K.; Cao, H.Z.; Liu, C.H.; Cheng, Y.; Jiang, W.J.; Du, F.L.; et al. Anti-tumor activity and the mechanism of SIP-S: a sulfated polysaccharide with anti-metastatic effect. Carbohydr. Polym. 2015, 129, 50–54. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Shen, M.Y.; Tang, W.; Wang, H.; Nie, S.P.; Xie, M.Y. Carboxymethylation of polysaccharide from Cyclocarya paliurus and their characterization and antioxidant properties evaluation. Carbohydr. Polym. 2016, 136, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Kagimura, F.Y.; da Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.H.; Malfatti, C.R.M. Biological activities of derivatized D-glucans: a review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, K.; Lee, K.G.; Ochi, H.; Shibamoto, T. Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. J. Agric. Food Chem. 2002, 50, 5480–5484. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, W.; Yao, W.; Pang, X.; Yin, D.; Gao, X. Carboxymethylation of a polysaccharide extracted from Ganoderma lucidum enhances its antioxidant activities in vitro. Carbohydr. Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, G.; Wu, X. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2011, 49, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhao, M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol. 2015, 72, 526–530. [Google Scholar] [CrossRef]
- Li, J.; Shang, W.; Si, X.; Bu, D.; Strappe, P.; Zhou, Z.; Blanchard, C. Carboxymethylation of corn bran polysaccharide and its bioactive property. Int. J. Food Sci. Tech. 2017, 52, 1176–1184. [Google Scholar] [CrossRef]
- Wei, D.; Cheng, W.; Wei, Y.; Zhang, L. Phosphorylated modification and in vitro antioxidant activity of Radix Hedysari polysaccharide. Glycoconj. J. 2012, 29, 167–172. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, L.; Liu, X.; Li, Y.; Zhao, C.; Xu, Z.; Yan, W.; Zhang, H. Synthesis and antioxidant activity of phosphorylated polysaccharide from Portulaca oleracea L. with H3PW12O40 immobilized on polyamine functionalized polystyrene bead as catalyst. J. Mol. Catal. Chem. 2011, 342, 74–82. [Google Scholar] [CrossRef]
- Liu, X.X.; Wan, Z.J.; Shu, L.; Lu, X.X. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- He, Y.; Ye, M.; Jiang, L.; Du, Z.; Surahio, M.; Xu, H.; Li, J. Preparation characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum. Carbohydr. Polym. 2015, 117, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Du, H.; Yang, J.; Ming, K.; Song, M.; Liu, J. Comparison of the anti-duck hepatitis A virus activities of phosphorylated and sulfated Astragalus polysaccharides. Exp. Biol. Med. 2017, 242, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.; Xu, J.; Shang, J.; Cheng, Y. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2015, 72, 894–899. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Warrier, D.U.; Gaikwad, S.R.; Shevate, P.M. Phosphorylation of psyllium seed polysaccharide and its characterization. Int. J. Biol. Macromol. 2016, 85, 317–326. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Q.; Zhao, M.; Qi, H. Phosphorylation of low-molecular-weight polysaccharide from Enteromorpha linza with antioxidant activity. Carbohydr. Polym. 2013, 96, 371–375. [Google Scholar] [CrossRef]
- Song, Y.; Ni, Y.; Hu, X.; Li, Q. Effect of phosphorylation on antioxidant activities of pumpkin (Cucurbita pepo, Lady godiva) polysaccharide. Int. J. Biol. Macromol. 2015, 81, 41–48. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, G.; Huang, H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int. J. Biol. Macromol. 2019, 126, 842–845. [Google Scholar] [CrossRef]
- Dace, R.; McBride, E.; Brooks, K.; Gander, J.; Buszko, M.; Doctor, V.M. Comparison of the anticoagulant action of sulfated and phosphorylated polysaccharides. Thromb. Res. 1997, 1, 113–121. [Google Scholar] [CrossRef]
- Church, F.C.; Pratt, C.W.; Treavor, R.E.; Whina, H.C. Anticoagulant action of phosvitin and other phosphate containing polyanions is mediated by heparin cofactor II. FEBS Let. 1988, 237, 26–30. [Google Scholar] [CrossRef]
- Lindahl, V.; Thunberg, L.; Backstrom, G.; Risenfeld, T.; Nordling, K.; Bjork, I. Extension and structural variability of the antithrombin-binding sequence in heparin. J. Biol. Chem. 1984, 259, 12368–12374. [Google Scholar] [PubMed]
- Gu, Y.P.; Yang, X.M.; Luo, P.; Li, Y.Q.; Tao, Y.X.; Duan, Z.H.; Xiao, W.; Zhang, D.Y.; Liu, H.Z. Inhibition of acrolein-induced autophagy and apoptosis by a glycosaminoglycan from Sepia esculenta ink in mouse Leydig cells. Carbohydr. Polym. 2017, 163, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.H.; Duan, W.W.; Li, F.P.; Li, Y.Q.; Luo, P.; Liu, H.Z. Effect of carboxymethylation on properties of fucoidan from Laminaria japonica: antioxidant activity and preservative effect on strawberry during cold storage. Postharvest Biol. Tech. 2019, 151, 127–133. [Google Scholar] [CrossRef]
- Zhang, M.; Su, N.; Huang, Q.; Zhang, Q.; Wang, Y.; Li, J.; Ye, M. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. J. Food Drug Anal. 2017, 25, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pan, D.D.; Zeng, X.Q.; Cao, J.X. Phosphorylation modification of polysaccharides from Enteromorpha. Food Sci. 2011, 24, 73–77. [Google Scholar]







| Items | Chloroacetic Acid (g) | NaOH (mol/L) | Temperature (℃) | Time (h) | DS |
|---|---|---|---|---|---|
| 1 | 1.89 | 1.0 | 30 | 2.5 | 0.43 |
| 2 | 1.89 | 3.0 | 40 | 3.0 | 0.60 |
| 3 | 1.89 | 5.0 | 50 | 3.5 | 0.58 |
| 4 | 2.37 | 1.0 | 40 | 3.5 | 0.45 |
| 5 | 2.37 | 3.0 | 50 | 2.5 | 0.75 |
| 6 | 2.37 | 5.0 | 30 | 3.0 | 0.75 |
| 7 | 2.84 | 1.0 | 50 | 3.0 | 0.61 |
| 8 | 2.84 | 3.0 | 30 | 3.5 | 0.67 |
| 9 | 2.84 | 5.0 | 40 | 2.5 | 0.84 |
| K1 | 0.49 | 0.53 | 0.61 | 0.67 | |
| K2 | 0.67 | 0.65 | 0.63 | 0.65 | |
| K3 | 0.72 | 0.70 | 0.64 | 0.56 | |
| R | 0.23 | 0.17 | 0.03 | 0.11 |
| Items | STPP (g/L) | STMP (g/L) | Phosphate Radical Content (%) |
|---|---|---|---|
| 1 | 0 | 0 | 0.305 |
| 2 | 70 | 0 | 1.451 |
| 3 | 60 | 10 | 2.095 |
| 4 | 50 | 20 | 3.818 |
| 5 | 40 | 30 | 3.661 |
| 6 | 30 | 40 | 2.826 |
| 7 | 20 | 50 | 3.081 |
| 8 | 10 | 60 | 1.921 |
| 9 | 0 | 70 | 2.269 |
| Items | STPP/STMP (g/L) | Temperature (°C) | Time (h) | pH | Phosphate Content (%) |
|---|---|---|---|---|---|
| 1 | 40/30 | 55 | 3 | 5.0 | 1.52 |
| 2 | 40/30 | 65 | 4 | 6.0 | 0.33 |
| 3 | 40/30 | 75 | 5 | 7.0 | 0.63 |
| 4 | 50/20 | 55 | 4 | 7.0 | 0.20 |
| 5 | 50/20 | 65 | 5 | 5.0 | 0.76 |
| 6 | 50/20 | 75 | 3 | 6.0 | 0.39 |
| 7 | 60/10 | 55 | 5 | 6.0 | 0.52 |
| 8 | 60/10 | 65 | 3 | 7.0 | 0.55 |
| 9 | 60/10 | 75 | 4 | 5.0 | 0.25 |
| K1 | 0.82 | 0.74 | 0.82 | 0.84 | |
| K2 | 0.45 | 0.54 | 0.26 | 0.41 | |
| K3 | 0.44 | 0.42 | 0.63 | 0.46 | |
| R | 0.38 | 0.32 | 0.56 | 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, F.; Luo, P. Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro. Mar. Drugs 2019, 17, 626. https://doi.org/10.3390/md17110626
Liu H, Li F, Luo P. Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro. Marine Drugs. 2019; 17(11):626. https://doi.org/10.3390/md17110626
Chicago/Turabian StyleLiu, Huazhong, Fangping Li, and Ping Luo. 2019. "Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro" Marine Drugs 17, no. 11: 626. https://doi.org/10.3390/md17110626
APA StyleLiu, H., Li, F., & Luo, P. (2019). Effect of Carboxymethylation and Phosphorylation on the Properties of Polysaccharides from Sepia esculenta Ink: Antioxidation and Anticoagulation in Vitro. Marine Drugs, 17(11), 626. https://doi.org/10.3390/md17110626