Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
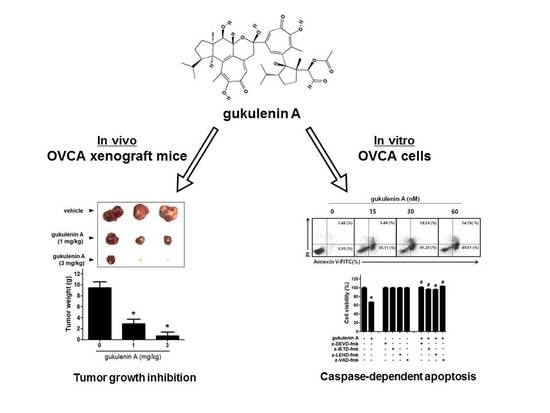
2.1. Gukulenin A Inhibited Tumor Growth in an Ovarian Cancer Xenograft Mouse Model
2.2. Gukulenin A Decreased Human Ovarian Cancer Cell Viability
2.3. Gukulenin A-Induced Apoptotic Cell Death in Human Ovarian Cancer Cells
2.4. Caspases Are Involved in Gukulenin A-Induced Apoptosis in Human Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Isolation of Gukulenin A
4.2. Materials
4.3. Cell Culture
4.4. Animal Study
4.5. Cell Viability
4.6. Flow Cytometry Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McClung, E.C.; Wenham, R.M. Profile of bevacizumab in the treatment of platinum-resistant ovarian cancer: Current perspectives. Int. J. Womens Health 2016, 8, 59–75. [Google Scholar] [PubMed]
- Marsh, S. Pharmacogenomics of Taxane/Platinum Therapy in Ovarian Cancer. Int. J. Gynecol. Cancer 2009, 19, S30–S34. [Google Scholar] [CrossRef]
- Laport, M.; Santos, O.; Muricy, G. Marine Sponges: Potential Sources of New Antimicrobial Drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Choi, H.; Hwang, H.; Kang, H.; Rho, J.-R. Gukulenins A and B, Cytotoxic Tetraterpenoids from the Marine Sponge Phorbas gukulensis. J. Nat. Prod. 2010, 73, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-E.; Kim, H.; Sim, C.J.; Oh, K.-B.; Liao, L.; Oh, D.-C.; Shin, J. Cytotoxic Diterpenoid Pseudodimers from the Korean Sponge Phorbas gukhulensis. J. Nat. Prod. 2013, 76, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, J.; Paciolla, I.; Cecere, S.; Pisano, C.; Di Napoli, M.; Arenare, L.; Setola, S.; Losito, N.; Califano, D.; Orditura, M.; et al. Trabectedin in Ovarian Cancer: Is it now a Standard of Care? Clin. Oncol. 2018, 30, 498–503. [Google Scholar] [CrossRef]
- Leisch, M.; Egle, A.; Greil, R. Plitidepsin: A potential new treatment for relapsed/refractory multiple myeloma. Future Oncol. 2019, 15, 109–120. [Google Scholar] [CrossRef]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essack, M.; Bajic, V.B.; Archer, J.A. Recently Confirmed Apoptosis-Inducing Lead Compounds Isolated from Marine Sponge of Potential Relevance in Cancer Treatment. Mar. Drugs 2011, 9, 1580–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhao, D.; He, W.; Zhang, H.; Li, Z.; Luan, Y. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. J. Colloid Interface Sci. 2017, 487, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, S.L. Eribulin Mesylate (E7389): Review of Efficacy and Tolerability in Breast, Pancreatic, Head and Neck, and Non–Small Cell Lung Cancer. Clin. Ther. 2012, 34, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.J.; Giannakakou, P.; Gunasekera, S.P.; Longley, R.E.; Day, B.W.; Hamel, E. The Microtubule-Stabilizing Agent Discodermolide Competitively Inhibits the Binding of Paclitaxel (Taxol) to Tubulin Polymers, Enhances Tubulin Nucleation Reactions More Potently than Paclitaxel, and Inhibits the Growth of Paclitaxel-Resistant Cells. Mol. Pharmacol. 1997, 52, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, A.; Stuhldreier, F.; Wex, K.W.; Berscheid, A.; Hartmann, R.; Rehberg, N.; Sureechatchaiyan, P.; Chaidir, C.; Kassack, M.U.; Kalscheuer, R.; et al. Cyclic Cystine-Bridged Peptides from the Marine Sponge Clathria basilana Induce Apoptosis in Tumor Cells and Depolarize the Bacterial Cytoplasmic Membrane. J. Nat. Prod. 2017, 80, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Holland, I.P.; McCluskey, A.; Sakoff, J.A.; Gilbert, J.; Chau, N.; Robinson, P.J.; Motti, C.A.; Wright, A.D.; Van Altena, I.A. Steroids from an Australian Sponge Psammoclema sp. J. Nat. Prod. 2009, 72, 102–106. [Google Scholar] [CrossRef]
- Huang, G.S.; Lopez-Barcons, L.; Freeze, B.S.; Smith, A.B.; Goldberg, G.L.; McDaid, H.M.; Horwitz, S.B.; McDaid, H.M. Potentiation of Taxol Efficacy by Discodermolide in Ovarian Carcinoma Xenograft-Bearing Mice. Clin. Cancer Res. 2006, 12, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.H.; Jeon, J.-E.; Ryu, S.; Lee, H.-S.; Oh, K.-B.; Shin, J. Polyoxygenated Diterpenes from the Sponge Phorbas sp. J. Nat. Prod. 2008, 71, 1701–1707. [Google Scholar] [CrossRef]
- Rho, J.-R.; Hwang, B.S.; Sim, C.J.; Joung, S.; Lee, H.-Y.; Kim, H.-J. Phorbaketals A, B, and C, Sesterterpenoids with a Spiroketal of Hydrobenzopyran Moiety Isolated from the Marine Sponge Phorbas sp. Org. Lett. 2009, 11, 5590–5593. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Chen, Y.-C.; El-Shazly, M.; Du, Y.-C.; Su, C.-W.; Tsao, C.-W.; Liu, L.-L.; Chou, Y.; Chang, W.-B.; Su, Y.-D.; et al. Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway. Mar. Drugs 2013, 11, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Uranchimeg, B.; Cardellina, J.H.; Meragelman, T.L.; Matsunaga, S.; Fusetani, N.; Del Bufalo, D.; Shoemaker, R.H.; Melillo, G. Induction of Apoptosis in Human Cancer Cells by Candidaspongiolide, a Novel Sponge Polyketide. JNCI J. Natl. Cancer Inst. 2008, 100, 1233–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Incalci, M.; Badri, N.; Galmarini, C.M.; Allavena, P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br. J. Cancer 2014, 111, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valdes, N.; Basagoiti, M.; Dotor, J.; Aranda, F.; Monreal, I.; Riezu-Boj, J.I.; Borras-Cuesta, F.; Sarobe, P.; Feijoo, E. Induction of monocyte chemoattractant protein-1 and interleukin-10 by tgfbeta1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Res. 2011, 71, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Edsparr, K.; Basse, P.H.; Goldfarb, R.H.; Albertsson, P. Matrix Metalloproteinases in Cytotoxic Lymphocytes Impact on Tumour Infiltration and Immunomodulation. Cancer Microenviron. 2010, 4, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Germano, G.; Frapolli, R.; Simone, M.; Tavecchio, M.; Erba, E.; Pesce, S.; Pasqualini, F.; Grosso, F.; Sanfilippo, R.; Casali, P.G.; et al. Antitumor and Anti-inflammatory Effects of Trabectedin on Human Myxoid Liposarcoma Cells. Cancer Res. 2010, 70, 2235–2244. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-H.; Woo, J.-H.; Rho, J.-R.; Choi, J.-H. Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo. Mar. Drugs 2019, 17, 126. https://doi.org/10.3390/md17020126
Ahn J-H, Woo J-H, Rho J-R, Choi J-H. Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo. Marine Drugs. 2019; 17(2):126. https://doi.org/10.3390/md17020126
Chicago/Turabian StyleAhn, Ji-Hye, Jeong-Hwa Woo, Jung-Rae Rho, and Jung-Hye Choi. 2019. "Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo" Marine Drugs 17, no. 2: 126. https://doi.org/10.3390/md17020126
APA StyleAhn, J. -H., Woo, J. -H., Rho, J. -R., & Choi, J. -H. (2019). Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo. Marine Drugs, 17(2), 126. https://doi.org/10.3390/md17020126