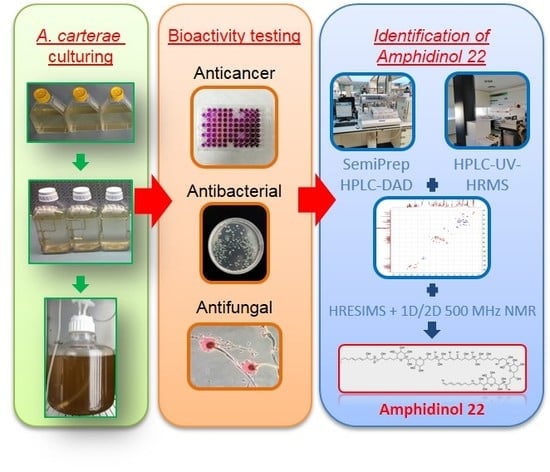
Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Culturing and Harvesting
2.2. Chemical Extraction
2.3. Anticancer Assays
2.4. Antifungal Assays
2.5. Antibacterial Assays
2.6. General Chemical Analysis Procedures
2.7. Fractionation
2.8. Amphidinol 22
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Bioactivity Screening of Raw Extract and HPLC-UV-MS Analysis
3.2. Structure Identification
3.3. Amphidinol 22 Bioactivities
3.3.1. Anticancer Activity
3.3.2. Antifungal Activity
3.3.3. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobayashi, J.; Kubota, T. Bioactive Metabolites from Marine Dinoflagellates. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Lui, H.-W., Eds.; Elsevier Science: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- Kathiresan, K.; Nabeel, M.A.; Manivannan, S. Bioprospecting of marine organisms for novel bioactive compounds. Sci. Trans. Environ. Technovation 2017, 1, 107–120. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Seabra, R.; Ferreira, A.C.S.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Ghate, N.B.; Deb, S.; Panja, S.; Sarkar, R.; Rout, J.; Mandal, N. Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol. Res. 2014, 47, 24. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Shah, M.M.R.; Lee, J.H.; Kang, M.C.; O-Nam, K.; Lee, J.B.; Jeon, Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 2013, 28, 111–119. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Kubota, T. Bioactive Macrolides and Polyketides from Marine Dinoflagellates of the Genus Amphidinium. J. Nat. Prod. 2007, 70, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Baudelet, P.H.; Gagez, A.L.; Bérard, J.B.; Juin, C.; Bridiau, N.; Kaas, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. Antiproliferative activity of Cyanophora paradoxa pigments in melanoma, breast and lung cancer cells. Mar. Drugs 2013, 11, 4390–4406. [Google Scholar] [CrossRef] [PubMed]
- Castrec, J.; Soudant, P.; Payton, L.; Tran, D.; Miner, P.; Lambert, C.; Le Goïc, N.; Huvet, A.; Quillien, V.; Boullot, F.; et al. Bioactive extracellular compounds produced by the dinoflagellate Alexandrium minutum are highly detrimental for oysters. Aquat. Toxicol. 2018, 199, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Sanmukh, S. Bioactive Compounds Derived from Microalgae Showing Antimicrobial Activities. J. Aquac. Res. Dev. 2014, 5, 3. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine microalgae with anti-cancer properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine Natural Products from Microalgae: An Omics Overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C.R.; Hasle, G.R.; Syvertsen, E.E.; Steidinger, K.A.; Tangen, K. Identifying Marine Diatoms and Dinoflagellates; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Samarakoon, K.W.; Ko, J.; Lakmal, H.C.; Lee, J.; An, S.; Jeon, Y. Potentiality of benthic dinoflagellate cultures and screening of their bioactivities in Jeju Island, Korea. Afr. J. Biotechnol. 2014, 13, 792–805. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and its related macrolides from marine dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Minamida, M.; Akakabe, M.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-2, a novel linear polyketide with potent cytotoxic activity from a marine dinoflagellate Amphidinium species. Biorg. Med. Chem. Lett. 2015, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Echigoya, R.; Rhodes, L.; Oshima, Y.; Satake, M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae 2005, 4, 383–389. [Google Scholar] [CrossRef]
- Satake, M.; Cornelio, K.; Hanashima, S.; Malabed, R.; Murata, M.; Matsumori, N.; Zhang, H.; Hayashi, F.; Mori, S.; Kim, J.S.; et al. Structures of the Largest Amphidinol Homologues from the Dinoflagellate Amphidinium carterae and Structure-Activity Relationships. J. Nat. Prod. 2017, 80, 2883–2888. [Google Scholar] [CrossRef]
- Morsy, N.; Konoki, K.; Houdai, T.; Matsumori, N.; Oishi, T.; Murata, M.; Aimoto, S. Roles of integral protein in membrane permeabilization by amphidinols. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Washida, K.; Koyama, T.; Yamada, K.; Kita, M.; Uemura, D. Karatungiols A and B, two novel antimicrobial polyol compounds, from the symbiotic marine dinoflagellate Amphidinium sp. Tetrahedron Lett. 2006, 47, 2521–2525. [Google Scholar] [CrossRef]
- Kellmann, R.; Stüken, A.; Orr, R.J.S.; Svendsen, H.M.; Jakobsen, K.S. Biosynthesis and molecular genetics of polyketides in marine dinoflagellates. Mar. Drugs 2010, 8, 1011–1048. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; John, U.; Van Dolah, F.M.; Murray, S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016, 10, 1877–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; De Luca, D.; Ferrarini, A.; Avanzato, C.; Minio, A.; Esposito, F.; Ianora, A. De novo transcriptome of the cosmopolitan dinoflagellate Amphidinium carterae to identify enzymes with biotechnological potential. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a Polyhydroxypolyene Antifungal Agent with an Unprecedented Structure, from a Marine Dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Sardo, A.; Fontana, A. The Missing piece in biosynthesis of amphidinols: First evidence of glycolate as a starter unit in New Polyketides from Amphidinium carterae. Mar. Drugs 2017, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.A.; Tan, M.S.; Oyong, G.G. Evaluation of the anti-cancer potential of amphidinol 2, a polyketide metabolite from the marine dinoflagellate Amphidinium klebsii. Jordan, J. Biol. Sci. 2017, 10, 297–302. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Escalera, L.; Reguera, B.; Moita, T.; Pazos, Y.; Cerejo, M.; Cabanas, J.M.; Ruiz-Villarreal, M. Bloom dynamics of Dinophysis acuta in an upwelling system: In situ growth versus transport. Harmful Algae 2010, 9, 312–322. [Google Scholar] [CrossRef]
- Audoin, C.; Bonhomme, D.; Ivanisevic, J.; De La Cruz, M.; Cautain, B.; Monteiro, M.C.; Reyes, F.; Rios, L.; Perez, T.; Thomas, O.P. Balibalosides, an original family of glucosylated sesterterpenes produced by the Mediterranean sponge Oscarella balibaloi. Mar. Drugs 2013, 11, 1477–1489. [Google Scholar] [CrossRef]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin Microtiter Assay Plate: Simple and Inexpensive Method for Detection of Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an antiplasmodial betaine lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Perez-Victoria, I.; Martin, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor & Francis Group Dictionary of Marine Natural Products. Available online: http://dmnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 22 May 2019).
- National Center for Biotechnology Information PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 22 May 2019).
- Karafas, S.; Teng, S.T.; Leaw, C.P.; Alves-de-Souza, C. An evaluation of the genus Amphidinium (Dinophyceae) combining evidence from morphology, phylogenetics, and toxin production, with the introduction of six novel species. Harmful Algae 2017, 68, 128–151. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.K.; Matsumori, N.; Murata, M.; Tachibana, K. Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetrahedron Lett. 1995, 36, 6279–6282. [Google Scholar] [CrossRef]
- Kobayashi, J.; Hideyuki, S.; Masami, I.; Terufumi, Y.; Hiroshi, H.; Takuma, S. Amphidinolides G and H: New Potent Cytotoxic Macrolides from the Cultured Symbiotic Dinoflagellate Amphidinium sp. J. Org. Chem. 1991, 56, 5221–5224. [Google Scholar] [CrossRef]
- Usui, T.; Kazami, S.; Dohmae, N.; Mashimo, Y.; Kondo, H.; Tsuda, M.; Terasaki, A.G.; Ohashi, K.; Kobayashi, J.; Osada, H. A potent cytotoxic macrolide, covalently binds on actin subdomain 4 and stabilizes actin filament. Chem. Biol. 2004, 11, 1269–1277. [Google Scholar] [CrossRef]
- Lauritano, C.; Martín, J.; De La Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. (Thessalon.) 2014, 21, 6. [Google Scholar] [CrossRef]
- Assunção, J.; Guedes, A.; Malcata, F. Biotechnological and Pharmacological Applications of Biotoxins and Other Bioactive Molecules from Dinoflagellates. Mar. Drugs 2017, 15, 393. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological applications of bioactive peptides from marine sources. Adv. Microb. Physiol. 2018, 73, 171–220. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine microorganisms as a promising and sustainable source of bioactive molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]





| Carbon | δ13C | δ1H, mult, J (Hz) | Carbon | δ13C | δ1H, mult, J (Hz) |
|---|---|---|---|---|---|
| 1 | 14.09 | 0.91, t, 7.4 | 43 | 41.90 | 1.97, m; 1.51, m |
| 2 | 23.58 | 1.42, m, 2H | 44 | 71.79 | 3.85, m |
| 3 | 35.83 | 2.06, m | 45 | 36.68 | 1.67, m; 1.58, m |
| 4 | 136.10 | 5.69, m | 46 | 36.68 | 2.20, m; 2.11, m |
| 5 | 131.23 | 6.05, dd, 15.2, 10.5 | 47 | 139.30 | Null |
| 6 | 132.41 | 6.22, dd, 15.7, 10.4 | 48 | 126.24 | 5.48, br d, 8.7 |
| 7 | 134.08 | 5.61, dd, 15.2, 8.6 | 49 | 67.97 | 4.56, dd, 8.9, 1.7 |
| 8 | 71.39 | 4.26, ddd, 6.6, 6.6, 6.6 | 50 | 72.50 | 3.68, dd, 9.5, 1.9 |
| 9 | 40.90 | 1.75, m, 2H | 51 | 79.21 | 3.95, m |
| 11 | 62.32 | 2.72, dd, 5.2, 2.1 | 53 | 67.48 | 3.97, m |
| 12 | 71.91 | 3.41, m | 54 | 30.41 | 1.76, m |
| 13 | 35.35 | 1.62, m; 1.48, m | 55 | 75.71 | 3.48, m |
| 14 | 22.89 | 1.77, m; 1.44, m | 56 | 74.63 | 3.60, m |
| 15 | 32.90 | 1.89, m; 1.44, m | 57 | 32.44 | 1.96, m; 1.55, m |
| 16 | 81.54 | 3.07, m | 58 | 27.89 | 2.41, m; 2.09, m |
| 17 | 73.10 | 3.38, m | 59 | 151.35 | Null |
| 18 | 76.77 | 3.39, m | 60 | 76.77 | 4.18, d, 8.9 |
| 19 | 69.89 | 4.08, m | 61 | 75.23 | 3.35, m |
| 20 | 82.36 | 3.14, br d, 8.9 | 62 | 70.41 | 4.04, m |
| 21 | 68.63 | 3.87, m | 63 | 31.55 | 2.08, m; 1.55, m |
| 22 | 35.14 | 2.05, m; 1.74, m | 64 | 67.36 | 4.05, m |
| 23 | 77.92 | 4.22, m | 65 | 68.73 | 4.04, m |
| 24 | 71.63 | 3.64, m | 66 | 80.58 | 3.74, br d, 9.9 |
| 25 | 67.32 | 3.92, m | 67 | 72.01 | 3.97, m |
| 26 | 30.41 | 1.76, m | 68 | 74.22 | 4.37, dd, 7.6, 2.9 |
| 27 | 74.63 | 3.54, m | 69 | 129.15 | 5.63, dd, 16.5, 8.0 |
| 28 | 72.59 | 3.71, m | 70 | 134.97 | 5.80, m |
| 29 | 36.76 | 1.70, m; 1.38, m | 71 | 33.59 | 2.19, m |
| 30 | 33.59 | 1.97, m | 72 | 33.65 | 2.21, m |
| 31 | 79.41 | 3.12, dd, 7.6, 2.8 | 73 | 135.94 | 5.78, m |
| 32 | 70.50 | 3.85, m | 74 | 131.97 | 6.10, dd, 15.2, 10.4 |
| 33 | 46.06 | 2.29, m | 75 | 134.75 | 6.21, dd, 15.7, 10.2 |
| 34 | 136.85 | null | 76 | 132.68 | 6.13, dd, 15.7, 10.2 |
| 35 | 129.54 | 5.72, br s | 77 | 138.61 | 6.35, ddd, 16.9, 10.2, 10.2 |
| 36 | 144.65 | null | 78 | 116.68 | 5.15, dd, 17.0, 1.0; 5.01, dd, 10.2, 1.0 |
| 37 | 47.72 | 2.27, m; 2.21, m | 79 | 17.25 | 0.97, d, 6.8 |
| 38 | 68.68 | 3.80, m | 80 | 18.51 | 1.84, br s |
| 39 | 42.50 | 1.63, m; 1.27 m | 81 | 115.77 | 5.05, br s; 4.85, br s |
| 40 | 31.15 | 2.12, m | 82 | 13.91 | 0.90, d, 7.4 |
| 41 | 77.13 | 3.33, m | 83 | 17.39 | 1.74, br s |
| 42 | 72.68 | 3.64, m | 84 | 113.21 | 5.07, br s; 4.98 br s |
| Anticancer screening a | |||||
| Cancer cell line | A549 | A2058 | HepG2 | MCF7 | MiaPaca-2 |
| % Growth Inhibition | 100 | 100 | 100 | 100 | 100 |
| Antibacterial screening b | |||||
| Bacteria | E. coli | K. pneumoniae | MRSA | MSSA | M. tuberculosis |
| % Growth Inhibition | 37 | 19 | 95 | 88 | 83 |
| Antifungal screening b | |||||
| Fungus | A. fumigatus | ||||
| % Growth Inhibition | 100 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. https://doi.org/10.3390/md17070385
Martínez KA, Lauritano C, Druka D, Romano G, Grohmann T, Jaspars M, Martín J, Díaz C, Cautain B, de la Cruz M, et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Marine Drugs. 2019; 17(7):385. https://doi.org/10.3390/md17070385
Chicago/Turabian StyleMartínez, Kevin A., Chiara Lauritano, Dana Druka, Giovanna Romano, Teresa Grohmann, Marcel Jaspars, Jesús Martín, Caridad Díaz, Bastien Cautain, Mercedes de la Cruz, and et al. 2019. "Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae" Marine Drugs 17, no. 7: 385. https://doi.org/10.3390/md17070385
APA StyleMartínez, K. A., Lauritano, C., Druka, D., Romano, G., Grohmann, T., Jaspars, M., Martín, J., Díaz, C., Cautain, B., de la Cruz, M., Ianora, A., & Reyes, F. (2019). Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Marine Drugs, 17(7), 385. https://doi.org/10.3390/md17070385