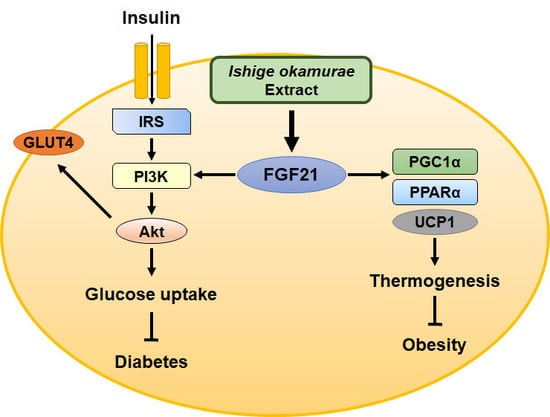
Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21
Abstract
:1. Introduction
2. Results
2.1. Treatment with IOE Improves Glucose Tolerance and Insulin Sensitivity in Mice
2.2. IOE Administration Ameliorates the Hyperglycemia and Dyslipidemia of db/db Mice
2.3. IOE Administration Increases GLUT4 Expression and Activates the Insulin Signaling Pathway in Skeletal Muscle
2.4. IOE Administration Also Increases GLUT4 expression and Activates the Insulin Signaling Pathway in WAT
2.5. IOE Ameliorates Obesity in db/db Mice
2.6. IOE Administration Increases the Expression of Proteins Involved in Adipocyte Browning, Perhaps by Upregulating FGF21 Secretion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of IOE
4.3. Animals and Treatments
4.4. Oral Glucose and Intraperitoneal Insulin Tolerance Testing
4.5. Measurement of Blood Glucose, Body Mass, and Water Intake
4.6. Serum Lipid Profile Analyses
4.7. Histologic Analysis of WAT
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gavrilova, O.; Marcus-Samuels, B.; Graham, D.; Kim, J.K.; Shulman, G.I.; Castle, A.L.; Vinson, C.; Eckhaus, M.; Reitman, M.L. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J. Clin. Invest. 2000, 105, 271–278. [Google Scholar] [CrossRef]
- Walley, A.J.; Blakemore, A.I.; Froguel, P. Genetics of obesity and the prediction of risk for health. Hum. Mol. Genet. 2006, 15, R124–R130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. Ccr2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Honka, M.J.; Latva-Rasku, A.; Bucci, M.; Virtanen, K.A.; Hannukainen, J.C.; Kalliokoski, K.K.; Nuutila, P. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: A positron emission tomography study. Eur. J. Endocrinol. 2018, 178, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef]
- Buchkovich, N.J.; Yu, Y.; Zampieri, C.A.; Alwine, J.C. The torrid affairs of viruses: Effects of mammalian DNA viruses on the pi3k-akt-mtor signalling pathway. Nat. Rev. Microbiol. 2008, 6, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. Akt/pkb signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the glut4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel fgf, fgf-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. Fgf-21 as a novel metabolic regulator. J. Clin. Invest. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the ampk-sirt1-pgc-1alpha pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Warfel, J.D.; Wicks, S.E.; Ghosh, S.; Salbaum, J.M.; Burk, D.; Dubuisson, O.S.; Mendoza, T.M.; Zhang, J.; Noland, R.C.; et al. Impaired mitochondrial fat oxidation induces fgf21 in muscle. Cell Rep. 2016, 15, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. Fgf21 regulates pgc-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Rajapakse, N.; Kim, S.K. Anti-inflammatory effect of ishige okamurae ethanolic extract via inhibition of nf-kappab transcription factor in raw 264.7 cells. Phytother. Res. 2009, 23, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Radical scavenging capacity and cytoprotective effect of enzymatic digests of ishige okamurae. J. App. Phycol. 2008, 20, 1087–1095. [Google Scholar] [CrossRef]
- Davidson, M.B.; Peters, A.L. An overview of metformin in the treatment of type 2 diabetes mellitus. Am. J. Med. 1997, 102, 99–110. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Reichelt, A.J.; Branchtein, L.; Matos, M.C.; Costa E Forti, A.; Spichler, E.R.; Pousada, J.M.; Teixeira, M.M.; Yamashita, T.; et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care 2001, 24, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M.J.J.o.e. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, J.-I.; Heo, S.-J.; Park, M.-H.; Park, P.-J.; Jeon, B.-T.; Kim, S.-K.; Han, J.-S.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Pae (Ishige okamurae) protects high glucose-induced damage in rinm5f pancreatic β cells via its antioxidant effects. Food Sci. Biotechnol. 2012, 21, 239–246. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.-J.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Antioxidant effects of phlorotannins isolated from ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Lee, K.; Song, J.-H.; Chei, S.; Lee, B.-Y. Ishige okamurae extract suppresses obesity and hepatic steatosis in high fat diet-induced obese mice. Nutrients 2018, 10, 1802. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, H.J.; Jeon, Y.J.; Han, J.S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in c57bl/ksj- db/db mice. Diabetes Res. Clin. Pract 2011, 93, 70–76. [Google Scholar] [CrossRef]
- Ryu, B.; Jiang, Y.; Kim, H.-S.; Hyun, J.-M.; Lim, S.-B.; Li, Y.; Jeon, Y.-J. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar. Drugs 2018, 16, 436. [Google Scholar] [CrossRef]
- Magnusson, I.; Rothman, D.L.; Katz, L.D.; Shulman, R.G.; Shulman, G.I. Increased rate of gluconeogenesis in type ii diabetes mellitus. A 13c nuclear magnetic resonance study. J. Clin. Invest. 1992, 90, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am. J. Med. 2003, 115, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Augustine, R.A.; Steger, J.; Ganjam, G.K.; Benzler, J.; Pracht, C.; Lowe, C.; Schwartz, M.W.; Shepherd, P.R.; Anderson, G.M.; et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J. Neurosci. 2010, 30, 16180–16187. [Google Scholar] [CrossRef] [PubMed]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-talk between mitogenic ras/mapk and survival pi3k/akt pathways: A fine balance. Biochem. Soc. Trans. 2012, 40, 139–146. [Google Scholar] [CrossRef]
- Deepa, S.S.; Walsh, M.E.; Hamilton, R.T.; Pulliam, D.; Shi, Y.; Hill, S.; Li, Y.; Van Remmen, H. Rapamycin modulates markers of mitochondrial biogenesis and fatty acid oxidation in the adipose tissue of db/db mice. J. Biochem. Pharmacol. Res. 2013, 1, 114–123. [Google Scholar]
- Derosa, G.; Tinelli, C.; Maffioli, P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes. Metab. 2009, 11, 1091–1099. [Google Scholar] [CrossRef]
- Mashili, F.L.; Austin, R.L.; Deshmukh, A.S.; Fritz, T.; Caidahl, K.; Bergdahl, K.; Zierath, J.R.; Chibalin, A.V.; Moller, D.E.; Kharitonenkov, A.; et al. Direct effects of fgf21 on glucose uptake in human skeletal muscle: Implications for type 2 diabetes and obesity. Diabetes Metab. Res. Rev. 2011, 27, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheung, B.M.; Tso, A.W.; Wang, Y.; Law, L.S.; Ong, K.L.; Wat, N.M.; Xu, A.; Lam, K.S. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes: A 5.4-year population-based prospective study in chinese subjects. Diabetes Care 2011, 34, 2113–2115. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.J.F. Defining “fgf21 resistance” during obesity: Controversy, criteria and unresolved questions. F1000Res. 2018, 7, 289. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Bina, H.A.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. Fgf21 is an akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, J.E.; Cha, J.J.; Hyun, Y.Y.; Kim, J.E.; Lee, M.H.; Song, H.K.; Nam, D.H.; Han, J.Y.; Han, S.Y.; et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013, 154, 3366–3376. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by pparalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by pparα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef]
- Fernando, K.H.N.; Yang, H.W.; Jiang, Y.; Jeon, Y.J.; Ryu, B. Diphlorethohydroxycarmalol isolated from Ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-J.; Lee, K.; Chei, S.; Jeon, Y.-J.; Lee, B.-Y. Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21. Mar. Drugs 2019, 17, 407. https://doi.org/10.3390/md17070407
Seo Y-J, Lee K, Chei S, Jeon Y-J, Lee B-Y. Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21. Marine Drugs. 2019; 17(7):407. https://doi.org/10.3390/md17070407
Chicago/Turabian StyleSeo, Young-Jin, Kippeum Lee, Sungwoo Chei, You-Jin Jeon, and Boo-Yong Lee. 2019. "Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21" Marine Drugs 17, no. 7: 407. https://doi.org/10.3390/md17070407
APA StyleSeo, Y. -J., Lee, K., Chei, S., Jeon, Y. -J., & Lee, B. -Y. (2019). Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21. Marine Drugs, 17(7), 407. https://doi.org/10.3390/md17070407