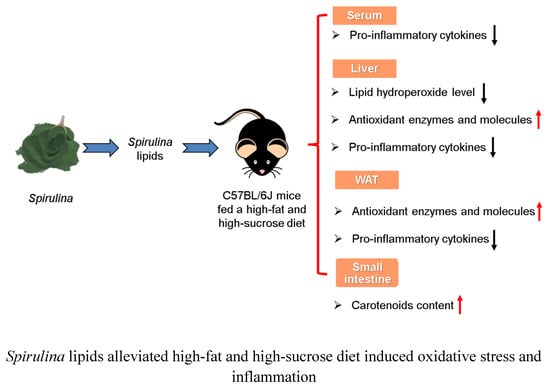
Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet
Abstract
:1. Introduction
2. Results
2.1. Chloroform/Methanol (SLC) and Ethanol (SLE) Analysis and Fatty Acid Composition of Dietary Lipids
2.2. Lipid Hydroperoxide Levels in the Liver
2.3. Antioxidant Enzyme Activity and GSSG/GSH Ratio in the Liver and Epididymal WAT
2.4. mRNA Expression of Antioxidant Enzymes in the Liver and eWAT.
2.5. Serum Pro-Inflammatory Cytokines Levels
2.6. mRNA Expression of Pro-Inflammatory Cytokines in the Liver and eWAT
2.7. Carotenoids Accumulation in the Liver, eWAT, and Small Intestine
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Spirulina lipids
4.3. Analysis of SLC and SLE
4.4. Animals and Diets
4.5. Hydroperoxide Analysis of Hepatic Lipids
4.6. Analysis of Antioxidant Enzymes and Reduced/Oxidized Glutathione Levels
4.7. Analysis of Serum Pro-Inflammatory Cytokines Levels
4.8. Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Carotenoid Contents in the Liver, Epididymal WAT, and Small Intestine
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.A.; Dai, Y.Y.; Nawaz, W.; Hong, Z.; Chen, X.Z.; Talal, B.; Taleb, A.; Mais, E.; Ding, Q.L. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef]
- Jernås, M.; Palming, J.; Sjöholm, K.; Jennische, E.; Svensson, P.A.; Gabrielsson, B.G.; Levin, M.; Sjögren, A.; Rudemo, M.; Lystig, T.C.; et al. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB J. 2006, 20, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Agustsson, T.; Rydén, M.; Hoffstedt, J.; van Harmelen, V.; Dicker, A.; Laurencikiene, J.; Isaksson, B.; Permert, J.; Arner, P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007, 67, 5531–5537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustsson, T.; Wikrantz, P.; Rydén, M.; Brismar, T.; Isaksson, B. Adipose tissue volume is decreased in recently diagnosed cancer patients with cachexia. Nutrition 2012, 28, 851–855. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.; Shargill, N.; Spiegelman, B. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The role of oxidative stress and hormones in controlling obesity. Front. Endocrinol. Lausanne 2019, 10, 540. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, J.V.P.; Rigolon, T.C.B.; Souza, M.S.D.S.; Alvarez-Leite, J.I.; Lucia, C.M.D.; Martino, H.S.D.; Rosa, C.O.B. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Blizniewska, H.; Sitarek, P.; Merecz-Sadowska, A.; Malinowska, K.; Zajdel, K.; Jablonska, M.; Sliwinski, T.; Zajdel, R. Plant extracts and reactive oxygen species as two counteracting agents with anti- and pro-obesity properties. Int. J. Mol. Sci. 2019, 20, 4556. [Google Scholar] [CrossRef] [Green Version]
- Hursel, R.; Westerterp-Plantenga, M.S. Thermogenic ingredients and body weight regulation. Int. J. Obes. 2010, 34, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lim, Y.; Kim, E.K. Therapeutic phytogenic compounds for obesity and diabetes. Int. J. Mol. Sci. 2014, 15, 21505–21537. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, G.E.; Arcidiacono, B.; De Rose, R.F.; Lepore, S.M.; Costa, N.; Montalcini, T.; Brunetti, A.; Russo, D.; De Sarro, G.; Celano, M. Normocaloric diet restores weight gain and insulin sensitivity in obese mice. Front. Endocrinol. Lausanne 2016, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, G.E.; Lepore, S.M.; Morittu, V.M.; Arcidiacono, B.; Colica, C.; Procopio, A.; Maggisano, V.; Bulotta, S.; Costa, N.; Mignogna, C.; et al. Effects of oleacein on high-fat diet-dependent steatosis, weight gain, and insulin resistance in mice. Front. Endocrinol. Lausanne 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.X.; Lee, Y.; Bae, M.; Hu, S.; Kang, H.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Spirulina supplementation in a mouse model of diet-induced liver fibrosis reduced the pro-inflammatory response of splenocytes. Br. J. Nutr. 2019, 121, 748–755. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjun, G.K.G.; Udaya, S.K.; Sarada, R.; Ravishankar, G.A. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J. Food Sci. Technol. 2015, 52, 3627–3633. [Google Scholar]
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical carbon dioxide and microwave-assisted extraction of functional lipophilic compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of supercritical carbon dioxide extraction parameters on the biological activities and metabolites present in extracts from Arthrospira platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [PubMed]
- Ku, C.S.; Yang, Y.; Park, Y.; Lee, J. Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease. J. Med. Food 2013, 16, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coué, M.; Tesse, A.; Falewée, J.; Aguesse, A.; Croyal, M.; Fizanne, L.; Chaigneau, J.; Boursier, J.; Ouguerram, K. Spirulina liquid extract protects against fibrosis related to non-alcoholic steatohepatitis and increases ursodeoxycholic acid. Nutrients 2019, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T.; Fujita, H. Dietary gamma-linolenic acid in the form of borage oil causes less body fat accumulation accompanying an increase in uncoupling protein 1 mRNA level in brown adipose tissue. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2000, 127, 213–222. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Thao, N.P.; Tai, B.H.; Lim, J.Y.; Ki, H.H.; Kim, D.K.; Lee, Y.M.; Kim, Y.H. Chemical constituents of Triticum aestivum and their effects on adipogenic differentiation of 3T3-L1 preadipocytes. Arch. Pharm. Res. 2015, 38, 1011–1018. [Google Scholar] [CrossRef]
- Ma, A.C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.C.; Guan, H.S.; Liu, H.B. Isolation of the molecular species of monogalactosyldiacylglycerols from brown edible seaweed Sargassum horneri and their inhibitory effects on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina lipids on high-fat and high-sucrose diet induced obesity and hepatic lipid accumulation in C57BL/6J mice. J. Funct. Foods 2020, 65, 103741. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Cho, S.Y.; Miyashita, K.; Miyazawa, T.; Fujimoto, K.; Kaneda, T. Autoxidation of ethyl eicosapentaenoate and docosahexaenoate. J. Am. Oil Chem. Soc. 1987, 64, 876–879. [Google Scholar] [CrossRef]
- Cosgrove, J.P.; Church, D.F.; Pryor, W.A. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 1987, 22, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, B.; He, J.; Han, L.; Zhan, Y.; Wang, Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011, 136, 309–315. [Google Scholar] [CrossRef]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Varela, A.T.; Rolo, A.P.; Palmeira, C.M. High-fat and obesogenic diets: Current and future strategies to fight obesity and diabetes. Genes Nutr. 2014, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Waldon, D.; Teffera, Y.; Roberts, J.; Wells, M.; Langley, M.; Zhao, Z. Ratios of biliary glutathione disulfide (GSSG) to glutathione (GSH): A potential index to screen drug-induced hepatic oxidative stress in rats and mice. Anal. Bioanal. Chem. 2013, 405, 2635–2642. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Luo, J.; Zang, W.; Chen, D.; Xu, H.; Shi, H.; Jing, X. Gamma-linolenic acid suppresses NF-κB signaling via CD36 in the lipopolysaccharide-induced inflammatory response in primary goat mammary gland epithelial cells. Inflammation 2016, 39, 1225–1237. [Google Scholar] [PubMed]
- Chang, C.S.; Sun, H.L.; Lii, C.K.; Chen, H.W.; Chen, P.Y.; Liu, K.L. Gamma-linolenic acid inhibits inflammatory responses by regulating NF-κB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoo, T.H.; Lee, S.H.; Kang, H.Y.; Nam, B.Y.; Kwak, S.J.; Kim, J.K.; Park, J.T.; Han, S.H.; Kang, S.W. Gamma linolenic acid exerts anti-inflammatory and anti-fibrotic effects in diabetic nephropathy. Yonsei Med. J. 2012, 53, 1165–1175. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- El-Habit, O.H.; Saada, H.; Azab, K.S.; Abdel-Rahman, M.; El-Malah, D. The modifying effect of β-carotene on gamma radiation-induced elevation of oxidative reactions and genotoxicity in male rats. Mutat. Res. Toxicol. Environ. Mutagen. 2000, 466, 179–186. [Google Scholar] [CrossRef]
- Lin, W.T.; Huang, C.C.; Lin, T.J.; Chen, J.R.; Shieh, M.J.; Peng, H.C.; Yang, S.C.; Huang, C.Y. Effects of beta-carotene on antioxidant status in rats with chronic alcohol consumption. Cell Biochem. Funct. 2009, 27, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Huang, C.C.; Chu, J.S.; Chen, J.R. Effects of beta-carotene on cell viability and antioxidant status of hepatocytes from chronically ethanol-fed rats. Br. J. Nutr. 2004, 92, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.K.; Lee, S.J.; Na, H.J.; Ha, K.S.; Han, J.A.; Lee, H.; Kwon, Y.G.; Chung, C.K.; Kim, Y.M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Prevot, A.F.; Mordret, F.X. Utilisation des colonnes capillaries de verre pour l’analyse des corp gras par chromatographie en phase gazeuse. Rev. Fse. Corps. Gras. 1976, 23, 409–423. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, K.; Sasaki, I.; Ohrui, H.; Meguro, H. A simple fluorometry of hydroperoxides in oils and foods. Biosci. Biotechnol. Biochem. 1992, 56, 605–607. [Google Scholar] [CrossRef] [Green Version]







| LFD | HFHSD | SLC | SLE | |
|---|---|---|---|---|
| Ingredient (g/kg) | ||||
| Soybean oil | 70.00 | 70.00 | 30.00 | 30.00 |
| SLC | - | - | 40.00 | 40.00 |
| SLE | - | - | - | 40.00 |
| Lard | - | 230.00 | 230.00 | 230.00 |
| β-Cornstarch | 397.49 | 110.62 | 110.62 | 110.62 |
| α-(dextrinized) Cornstarch | 132.00 | 36.87 | 36.87 | 36.87 |
| Casein | 200.00 | 250.00 | 250.00 | 250.00 |
| Sucrose | 100.00 | 200.00 | 200.00 | 200.00 |
| AIN-93 mineral mixture | 35.00 | 35.00 | 35.00 | 35.00 |
| AIN-93 vitamin mixture | 10.00 | 10.00 | 10.00 | 10.00 |
| l-Cystine | 3.00 | 3.75 | 3.75 | 3.75 |
| Choline bitatrate | 2.50 | 2.50 | 2.50 | 2.50 |
| Cellulose | 50.00 | 50.00 | 50.00 | 50.00 |
| tert-Butylhydroquinone | 0.01 | 0.06 | 0.06 | 0.06 |
| Cholesterol | - | 1.20 | 1.20 | 1.20 |
| Fatty acid (wt%) | SLC | SLE | Experimental diets | |||
|---|---|---|---|---|---|---|
| LFD | HFHSD | SLC | SLE | |||
| C16:0 | 46.79 | 45.69 | 10.43 | 18.97 | 25.66 | 23.13 |
| C16:1 | 2.14 | 2.69 | 0.11 | 1.74 | 2.76 | 2.55 |
| C18:0 | 0.71 | 0.86 | 4.06 | 14.90 | 13.01 | 13.16 |
| C18:1† | 2.39 | 2.62 | 23.82 | 46.12 | 39.38 | 43.56 |
| C18:2n-6 | 16.78 | 17.24 | 53.16 | 14.76 | 13.28 | 13.09 |
| C18:3n-3 | - | - | 5.07 | - | - | - |
| C18:3n-6 | 20.28 | 22.06 | - | - | 1.96 | 2.28 |
| Spirulina Lipids | Liver | eWAT | Small Intestine | |||||
|---|---|---|---|---|---|---|---|---|
| SLC | SLE | SLC Group | SLE Group | SLC Group | SLE Group | SLC Group | SLE Group | |
| β-carotene | 9.29 ± 0.55 | 8.57 ± 0.67 | a N.D. | N.D. | N.D. | N.D. | 0.09 ± 0.02 | 0.06 ± 0.01 |
| Zeaxanthin | 6.18 ± 0.42 | 8.69 ± 0.86 | N.D. | N.D | N.D. | N.D. | 0.07 ± 0.02 | 0.10 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet. Mar. Drugs 2020, 18, 148. https://doi.org/10.3390/md18030148
Yang Y, Du L, Hosokawa M, Miyashita K. Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet. Marine Drugs. 2020; 18(3):148. https://doi.org/10.3390/md18030148
Chicago/Turabian StyleYang, Yuhong, Lei Du, Masashi Hosokawa, and Kazuo Miyashita. 2020. "Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet" Marine Drugs 18, no. 3: 148. https://doi.org/10.3390/md18030148
APA StyleYang, Y., Du, L., Hosokawa, M., & Miyashita, K. (2020). Spirulina Lipids Alleviate Oxidative Stress and Inflammation in Mice Fed a High-Fat and High-Sucrose Diet. Marine Drugs, 18(3), 148. https://doi.org/10.3390/md18030148