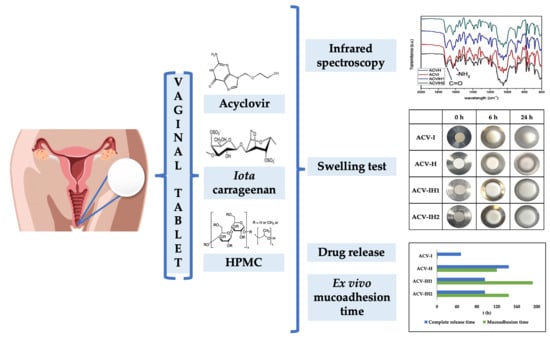
Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of the Tablets
2.2. Infrared Spectroscopy
2.3. Swelling Tests
2.4. Microstructure of Swelling Witnesses
2.5. Drug Release
2.6. Mucoadhesion Assessment
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Tablets and Infrared Spectroscopy
3.2.2. Swelling Tests
3.2.3. Preparation and Characterization of Swelling Witnesses. SEM Microscopy and Hg Porosimetry
3.2.4. Drug Release
3.2.5. Mucoadhesion Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowley, J.; Hoorn, S.V.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef]
- Garland, S.M.; Steben, M.; Sauerbrei, A.; Garland, S.M.; Steben, M.; Sauerbrei, A.; Eundem, A.; Fellow, R.; Steben, M.; Advisor, M.; et al. Genital herpes. Diagn. Pathog. Sex. Transm. Infect. 2018, 28, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 2015, 10, e114989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Guidelines for the Treatment of Genital Herpes Simplex Virus; World Health Organization: Geneva, Switzerland, 2016; Volume 8, ISBN 9789241549875. [Google Scholar]
- Freeman, E.; Weiss, H.; Glynn, J.R.; Cross, P.L.; Whitworth, J.; Hayes, R. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 2006, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Magaret, A.S.; Celum, C.; Wald, A.; Longini, I.M.; Self, S.G.; Corey, L. Genital Herpes Has Played a More Important Role than Any Other Sexually Transmitted Infection in Driving HIV Prevalence in Africa. PLoS ONE 2008, 3, e2230. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, S.J.; Risbud, A.; Shepherd, M.; Zenilman, J.M.; Brookmeyer, R.S.; Paranjape, R.S.; Divekar, A.D.; Gangakhedkar, R.; Ghate, M.; Bollinger, R.C.; et al. Recent Herpes Simplex Virus Type 2 Infection and the Risk of Human Immunodeficiency Virus Type 1 Acquisition in India. J. Infect. Dis. 2003, 187, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Tobian, A.A.; Charvat, B.; Ssempijja, V.; Kigozi, G.; Serwadda, D.; Makumbi, F.; Iga, B.; Laeyendecker, O.; Riedesel, M.; Oliver, A.; et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J. Infect. Dis. 2009, 199, 945–949. [Google Scholar] [CrossRef]
- Weber, J.; Desai, K.; Darbyshire, J. The Development of Vaginal Microbicides for the Prevention of HIV Transmission. PLoS Med. 2005, 2, e142. [Google Scholar] [CrossRef] [Green Version]
- Acartürk, F. Mucoadhesive vaginal drug delivery systems. Recent Pat. Drug Deliv. Formul. 2009, 3, 193–205. [Google Scholar] [CrossRef]
- Omar, R.F.; Bergeron, M.G. The future of microbicides. Int. J. Infect. Dis. 2011, 15, e656–e660. [Google Scholar] [CrossRef] [Green Version]
- Notario-Pérez, F.; Ruiz-Caro, R.; Veiga, M.-D. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des. Dev. Ther. 2017, 11, 1767–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, J.J.; Campoli-Richards, D.M. Acyclovir: An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1989, 37, 233–309. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.-P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.J.; Carro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and Kappa-Carrageenan Vaginal Acyclovir Formulations for Prevention of Genital Herpes. In Vitro and Ex Vivo Evaluation. Mar. Drugs 2015, 13, 5976–5992. [Google Scholar] [CrossRef] [Green Version]
- Corey, L.; Benedetti, J.K.; Critchlow, C.W.; Remington, M.R.; Winter, C.A.; Fahnlander, A.L.; Smith, K.; Salter, D.L.; Keeney, R.E.; Davis, L.; et al. Double-blind controlled trial of topical acyclovir in genital herpes simplex virus infections. Am. J. Med. 1982, 73, 326–334. [Google Scholar] [CrossRef]
- Corey, L.; Nahmias, A.J.; Guinan, M.E.; Benedetti, J.K.; Critchlow, C.W.; Holmes, K.K. A Trial of Topical Acyclovir in Genital Herpes Simplex Virus Infections. N. Engl. J. Med. 1982, 306, 1313–1319. [Google Scholar] [CrossRef]
- McConville, C.; Major, I.; Devlin, B.; Brimer, A. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Eur. J. Pharm. Biopharm. 2016, 104, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Shankar, G.; Alt, C. Prophylactic treatment with a novel bioadhesive gel formulation containing aciclovir and tenofovir protects from HSV-2 infection. J. Antimicrob. Chemother. 2014, 69, 3282–3293. [Google Scholar] [CrossRef]
- Moss, J.A.; Malone, A.M.; Smith, T.J.; Kennedy, S.; Kopin, E.; Nguyen, C.; Gilman, J.; Butkyavichene, I.; Vincent, K.L.; Motamedi, M.; et al. Simultaneous Delivery of Tenofovir and Acyclovir via an Intravaginal Ring. Antimicrob. Agents Chemother. 2011, 56, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Asvadi, N.H.; Dang, N.T.T.; Davis-Poynter, N.; Coombes, A.G. Evaluation of microporous polycaprolactone matrices for controlled delivery of antiviral microbicides to the female genital tract. J. Mater. Sci. Mater. Electron. 2013, 24, 2719–2727. [Google Scholar] [CrossRef]
- Ensign, L.M.; Tang, B.C.; Wang, Y.-Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect Against Herpes Simplex Virus. Sci. Transl. Med. 2012, 4, 138ra79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, O.; Garg, T.; Rath, G.; Goyal, A.K. Microbicides for the Treatment of Sexually Transmitted HIV Infections. J. Pharm. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavelić, Ž.; Skalko-Basnet, N.; Filipović-Grčić, J.; Martinac, A.; Jalšenjak, I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. J. Control. Release 2005, 106, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, Ž.; Skalko-Basnet, N.; Jalšenjak, I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int. J. Pharm. 2005, 301, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, V.; Deveswaran, R.; Bharath, S.; Basavaraj, B.V.; Madhavan, V. Design and optimization of bioadhesive vaginal tablets of acyclovir. Indian J. Pharm. Educ. Res. 2013, 47, 140–147. [Google Scholar]
- Duan, J.; Zhang, L. Robust and smart hydrogels based on natural polymers. Chin. J. Polym. Sci. 2017, 35, 1165–1180. [Google Scholar] [CrossRef]
- Fink, J.K. Marine, Waterborne and Water-Resistant Polymers: Chemistry and Applications; Wiley Blackwell: Hoboken, NJ, USA, 2015; ISBN 9781119185000. [Google Scholar]
- Xiong, Z.; Wang, J.-F.; Hao, Y.-Y.; Wang, Y. Recent Advances in the Discovery and Development of Marine Microbial Natural Products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.I.; Coutinho, A.J.; Lima, S.A.C.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [Green Version]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Maciel, D.J.; Ferreira, I.; Da Costa, G.M.; Da Silva, M.R. Nanocomposite hydrogels based on iota-carrageenan and maghemite: Morphological, thermal and magnetic properties. Eur. Polym. J. 2016, 76, 147–155. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Boil. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Raju, K.M. Iota-Carrageenan-based biodegradable Ag0 nanocomposite hydrogels for the inactivation of bacteria. Carbohydr. Polym. 2013, 95, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Novetsky, A.; Keller, M.J.; Gradissimo, A.; Chen, Z.; Morgan, S.L.; Xue, X.; Strickler, H.D.; Fernández-Romero, J.A.; Burk, R.; Einstein, M.H. In vitro inhibition of human papillomavirus following use of a carrageenan-containing vaginal gel. Gynecol. Oncol. 2016, 143, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.N.; Buck, C.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, U.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Buck, C.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, U.R.; Schiller, J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and Carrageenan Combination To Target Herpes Simplex Virus 2 and Human Papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [Green Version]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef]
- Fernández-Romero, J.A.; Abraham, C.J.; Rodriguez, A.; Kizima, L.; Jean-Pierre, N.; Menon, R.; Begay, O.; Seidor, S.; Ford, B.; Gil, P.I.; et al. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob. Agents Chemother. 2011, 56, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Calenda, G.; Villegas, G.; Barnable, P.; Litterst, C.; Levendosky, K.; Gettie, A.; Cooney, M.L.; Blanchard, J.; Fernández-Romero, J.A.; Zydowsky, T.M.; et al. MZC Gel Inhibits SHIV-RT and HSV-2 in Macaque Vaginal Mucosa and SHIV-RT in Rectal Mucosa. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 74, e67–e74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizima, L.; Rodriguez, A.; Kenney, J.; Derby, N.; Mizenina, O.; Menon, R.; Seidor, S.; Zhang, S.; Levendosky, K.; Jean-Pierre, N.; et al. A Potent Combination Microbicide that Targets SHIV-RT, HSV-2 and HPV. PLoS ONE 2014, 9, e94547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.-Y.; Wei, G.; Lu, W. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.K.; Rao, S.R.M.; Sudhakar, M. HPMC a biomedical polymer in pharmaceutical dosage forms. J. Chem. Pharm. Sci. 2015, 8, 875–881. [Google Scholar]
- Ghosal, K.; Hazra, B.T.; Bhowmik, B.B.; Thomas, S. Formulation Development, Physicochemical Characterization and In Vitro-in vivo drug release of vaginal Films. Curr. HIV Res. 2015, 14, 295–306. [Google Scholar] [CrossRef]
- Grammen, C.; Mooter, G.V.D.; Appeltans, B.; Michiels, J.; Crucitti, T.; Ariën, K.K.; Augustyns, K.; Augustijns, P.; Brouwers, J. Development and characterization of a solid dispersion film for the vaginal application of the anti-HIV microbicide UAMC01398. Int. J. Pharm. 2014, 475, 238–244. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Massetti, E.; Rossi, C. New solid mucoadhesive systems for benzydamine vaginal administration. Colloids Surf. B Biointerfaces 2011, 84, 413–420. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F. Vaginal Delivery of Benzydamine Hydrochloride through Liposomes Dispersed in Mucoadhesive Gels. Chem. Pharm. Bull. 2017, 65, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Peña, J.; Veiga, M.-D. Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV. Int. J. Pharm. 2019, 570, 118643. [Google Scholar] [CrossRef]
- Khan, A.B.; Thakur, R.S. Formulation and evaluation of mucoadhesive vaginal tablets of tenofovir disoproxil fumarate. Der Pharm. Lett. 2014, 6, 184–197. [Google Scholar]
- Genç, L.; Oğuzlar, C.; Güler, E. Studies on vaginal bioadhesive tablets of acyclovir. Die Pharm. 2000, 55, 297–299. [Google Scholar]
- Gafitanu, C.A.; Filip, D.; Rusu, D.; Macocinschi, D.; Zaltariov, M.-F.; Cernatescu, C.; Tuchilus, C.G. Design, Preparation and Evaluation of HPMC-Based PAA or SA Freeze-Dried Scaffolds for Vaginal Delivery of Fluconazole. Pharm. Res. 2017, 34, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Amish, D. Formulation and Evaluation Once Daily Mucoadhesive Vaginal Tablet of Clotrimazole Using Natural and Synthetic Polymers. Asian J. Pharm. Health Sci. 2011, 1, 176–182. [Google Scholar]
- Ghani, N.A.A.; Othaman, R.; Ahmad, A.; Anuar, F.H.; Hassan, N.H. Impact of purification on iota carrageenan as solid polymer electrolyte. Arab. J. Chem. 2019, 12, 370–376. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Filho, G.R.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and Characterization of Methylcellulose Produced from Bacterial Cellulose under Heterogeneous Condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U.; Yaqoob, A. Formulation and In Vitro Evaluation of Acyclovir Loaded Polymeric Microparticles: A Solubility Enhancement Study. Acta Pol. Pharm. Drug Res. 2016, 73, 1311–1324. [Google Scholar]
- Tako, M.; Nakamura, S.; Kohda, Y. Indicative evidence for a conformational transition in ι-carrageenan. Carbohydr. Res. 1987, 161, 247–255. [Google Scholar] [CrossRef]
- Thrimawithana, T.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef]
- Kulinowski, P.; Dorożyński, P.; Młynarczyk, A.; Węglarz, W.P. Magnetic Resonance Imaging and Image Analysis for Assessment of HPMC Matrix Tablets Structural Evolution in USP Apparatus 4. Pharm. Res. 2010, 28, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Odeniyi, M.; Khan, N.H.; Peh, K.K. Release and mucoadhesion properties of diclofenac matrix tablets from natural and synthetic polymer blends. Acta Pol. Pharm. Drug Res. 2015, 72, 559–567. [Google Scholar]
- Costa, P.C.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuğcu-Demiröz, F.; Acartürk, F.; Erdogan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Andrews, G.P.; Jones, D.S. Mucoadhesion and characterization of mucoadhesive properties. In Mucosal Delivery of Biopharmaceuticals: Biology, Challenges and Strategies; Springer: Boston, MA, USA, 2014; pp. 35–58. ISBN 9781461495246. [Google Scholar]
- Andersen, T.; Vanić, Ž.; Flaten, G.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Pectosomes and Chitosomes as Delivery Systems for Metronidazole: The One-Pot Preparation Method. Pharmaceutics 2013, 5, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Berginc, K.; Suljaković, S.; Skalko-Basnet, N.; Kristl, A. Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. Eur. J. Pharm. Biopharm. 2014, 87, 40–46. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Lalan, M.; Misra, A. Applications of polymers in vaginal drug delivery. In Applications of Polymers in Drug Delivery; Smithers Rapra Technology: Shropshire, UK, 2014; pp. 351–377. ISBN 978-1859574799. [Google Scholar]
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and Dissolution Study of Chitosan Freeze-Dried Systems for Drug Controlled Release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Zioni, T.; Gati, I.; Kleinstern, J.; Rubinstein, A. Luminal delivery and dosing considerations of local celecoxib administration to colorectal cancer. Eur. J. Pharm. Sci. 2006, 28, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga-Ochoa, M.D. Matrix Tablets: The Effect of Hydroxypropyl Methylcellulose/Anhydrous Dibasic Calcium Phosphate Ratio on the Release Rate of a Water-Soluble Drug Through the Gastrointestinal Tract I. In Vitro Tests. AAPS Pharm. Sci. Tech. 2012, 13, 1073–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hixson, A.W.; Crowell, J.H. Dependence of Reaction Velocity upon Surface and Agitation. Ind. Eng. Chem. 1931, 23, 1160–1168. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profile. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]








| Batch | Iota-Carrageenan | Hydroxypropyl Methylcellulose | Magnesium Stearate | ACV |
|---|---|---|---|---|
| I | 225 | 3 | ||
| H | 225 | 3 | ||
| IH1 | 135 | 90 | 3 | |
| IH2 | 90 | 135 | 3 | |
| ACV-I | 225 | 3 | 100 | |
| ACV-H | 225 | 3 | 100 | |
| ACV-IH1 | 135 | 90 | 3 | 100 |
| ACV-IH2 | 90 | 135 | 3 | 100 |
| Batch | Korsmeyer-Peppas | Hixson-Crowell | Hopfenberg | ||||
|---|---|---|---|---|---|---|---|
| R2 | KKP | n | R2 | KHC | R2 | KHF | |
| ACV-I | 0.9931 | 0.1127 | 0.9633 | 0.9789 | 0.0513 | 0.9917 | 0.0655 |
| ACV-H | 0.9900 | 0.0279 | 0.7410 | 0.9994 | 0.0045 | 0.9972 | 0.0060 |
| ACV-IH1 | 0.9830 | 0.0326 | 0.6474 | 0.9860 | 0.0063 | 0.9919 | 0.0083 |
| ACV-IH2 | 0.9960 | 0.0368 | 0.6822 | 0.9961 | 0.0061 | 0.9970 | 0.0081 |
| Batch | ACV-I | ACV-H | ACV-IH1 | ACV-IH2 |
|---|---|---|---|---|
| ACV-I | 23.57 | 23.62 | 24.73 | |
| ACV-H | 60.27 | 59.99 | ||
| ACV-IH1 | 79.11 | |||
| ACV-IH2 | ------ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.-D. Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Mar. Drugs 2020, 18, 249. https://doi.org/10.3390/md18050249
Pacheco-Quito E-M, Ruiz-Caro R, Rubio J, Tamayo A, Veiga M-D. Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Marine Drugs. 2020; 18(5):249. https://doi.org/10.3390/md18050249
Chicago/Turabian StylePacheco-Quito, Edisson-Mauricio, Roberto Ruiz-Caro, Juan Rubio, Aitana Tamayo, and María-Dolores Veiga. 2020. "Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes" Marine Drugs 18, no. 5: 249. https://doi.org/10.3390/md18050249
APA StylePacheco-Quito, E. -M., Ruiz-Caro, R., Rubio, J., Tamayo, A., & Veiga, M. -D. (2020). Carrageenan-Based Acyclovir Mucoadhesive Vaginal Tablets for Prevention of Genital Herpes. Marine Drugs, 18(5), 249. https://doi.org/10.3390/md18050249