Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids
Abstract
:1. Introduction
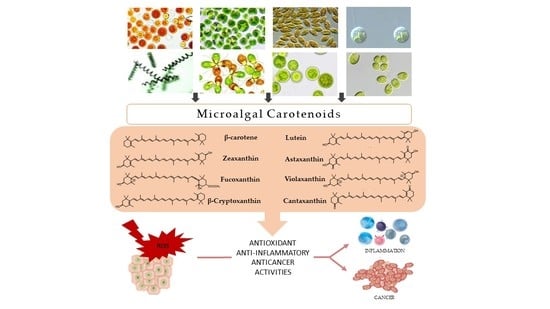
2. Microalgal Carotenoids
2.1. β-Carotene
2.2. Lutein
2.3. Zeaxanthin
2.4. Astaxanthin
2.5. Fucoxanthin
2.6. Violaxanthin
2.7. β-Cryptoxanthin
2.8. Canthaxanthin
3. Inflammation and Cancer
4. Anti-Inflammatory Activity of Carotenoids
4.1. β-Carotene
4.1.1. In Vitro Studies
4.1.2. In Vivo Studies
4.1.3. Human Studies
4.2. Lutein
4.2.1. In Vitro Studies
4.2.2. In Vivo Studies
4.2.3. Human Studies
4.3. Zeaxanthin
4.3.1. In Vitro Studies
4.3.2. In Vivo Studies
4.3.3. Human Studies
4.4. Astaxanthin
4.4.1. In Vitro Studies
4.4.2. In Vivo Studies
4.4.3. Human Studies
4.5. Fucoxanthin
4.5.1. In Vitro Studies
4.5.2. In Vivo Studies
4.5.3. Human Studies
4.6. β-Cryptoxanthin
4.6.1. In Vivo Studies
4.6.2. Human Studies
| Carotenoid | Source | Bioactivity | References |
|---|---|---|---|
| β-Carotene | Dunaliella salina Chlamydomonas reinhardtii Isochrysis galbana Tetraselmis suecica | Inflammation | |
| Colitis | [130,131,132] | ||
| Hepatic fibrosis | [133] | ||
| Non-alcoholic fatty liver | [134] | ||
| Atherosclerosis | [135,136] | ||
| Atopic dermatitis | [137,138] | ||
| Neurogenic inflammation | [139] | ||
| Acute spinal cord injury | [140] | ||
| Arthritis | [127] | ||
| Asthma | [141] | ||
| Irritable bowel syndrome | [142] | ||
| Type 2 diabetes mellitus | [143] | ||
| Skin photoaging | [144,145] | ||
| Cancer | |||
| Colon cancer | [311,312] | ||
| Liver cancer | [313,314] | ||
| Gastric cancer | [315,316] | ||
| Esophageal squamous cell | [317,318] | ||
| carcinoma | |||
| Prostate cancer | [319] | ||
| Neuroblastoma | [320] | ||
| Breast cancer | [321,322,323] | ||
| Pancreatic cancer | [324] | ||
| Non-Hodgkin lymphoma | [325] | ||
| Lutein | Chlorella sorokiniana Chromochloris zoofingiensis Auxenochlorella protothecoides Dunaliella salina Chlamydomonas sp. Tetraselmis suecica | Inflammation | |
| Age-related macular | [165,184,185,186,187,188] | ||
| degeneration | |||
| Diabetic retinopathy | [166,167,168] | ||
| Uveitis | [171,172] | ||
| Dry eye syndrome | [173] | ||
| Atherosclerosis | [174,175] | ||
| Hepatic injury | [176] | ||
| Pain | [139,177,178,179] | ||
| Osteoporosis | [181] | ||
| Alcohol-induced hepatic | [182] | ||
| damage | |||
| Ischemia/Reperfusion | [183] | ||
| Photoprotective/ | [191,192,193] | ||
| Antiaging effects | |||
| Cancer | |||
| Colon cancer | [326,327] | ||
| Hepatocellular carcinoma | [328] | ||
| Breast cancer | [329,330] | ||
| Bladder cancer | [331] | ||
| Renal cell carcinoma | [332] | ||
| Neck cancer | [333] | ||
| Non-Hodgkin lymphoma | [325] | ||
| Pharyngeal cancer | [334] | ||
| Esophageal cancer | [318] | ||
| Pancreatic cancer | [335] | ||
| Zeaxanthin | Synechocystis sp. Microcystis aeruginosa Nannochloropsis oculata Chloroidium saccharophilum Dunaliella sp. Porphyridium purpureum Heterosigma akashiwo | Inflammation | |
| Age-related macular | [184,185,196,336] | ||
| degeneration | |||
| Traumatic brain injury | [198] | ||
| Colitis | [199] | ||
| Edema | [200] | ||
| Alcoholic fatty liver | [201] | ||
| Depression/Anxiety | [202] | ||
| Eye dry syndrome | [205] | ||
| Cancer | |||
| Uveal melanoma | [337] | ||
| Pancreatic cancer | [338] | ||
| Ovarian cancer | [339] | ||
| Bladder cancer | [331] | ||
| Breast cancer | [330] | ||
| Non-Hodgkin lymphoma | [325] | ||
| Pharyngeal cancer | [334] | ||
| Esophageal cancer | [318] | ||
| Colon cancer | [340] | ||
| Pancreatic cancer | [335] | ||
| Astaxanthin | Haematococcus lacustris Chromochloris zofingiensis Chlorococcum sp. Dunaliella salina Tetraselmis suecica | Inflammation | |
| Non-alcoholic fatty liver | [221,222,223] | ||
| Liver inflammation | [224,225,226,227,228,229,230] | ||
| Kidney inflammation | [231] | ||
| Cardiac dysfunction | [232] | ||
| Diabetes mellitus | [233,234,235,270,271] | ||
| Diabetes-related disorders | [168,236,237,238] | ||
| Depression | [239,240,341] | ||
| Epilepsy-induced | [241,242,243] | ||
| neuroinflammation | |||
| Acute cerebral infarction | [248] | ||
| Arthritis | [215,249,250,251,342] | ||
| Colitis | [254,255] | ||
| Asthma | [257] | ||
| Acute lung injury | [258,259,343] | ||
| Contact dermatitis | [344] | ||
| Atopic dermatitis | [260,261,262,263] | ||
| Dry eye | [217] | ||
| Photoprotective/ | [266] | ||
| Antiaging effects | |||
| Cognitive function | [268] | ||
| Cancer | |||
| Hepatocellular carcinoma | [345,346,347] | ||
| Mammary tumor | [348] | ||
| Colon cancer | [349] | ||
| Esophageal cancer | [350] | ||
| Oral cancer | [351,352] | ||
| Prostate cancer | [353] | ||
| Lung metastatic melanoma | [354] | ||
| Fucoxanthin | Isochrysis sp. Odontella aurita Chaetoceros neogracilis Chrysotila carterae Phaeodactylum tricornutum Pavlova sp. | Inflammation | |
| Psoriasis/Acute erythema | [275] | ||
| Atopic dermatitis | [288] | ||
| Edema | [289] | ||
| Colitis | [290] | ||
| Arthritis | [291] | ||
| Depression/Anxiety | [292] | ||
| Lung injury | [278,293] | ||
| Asthma | [294,295] | ||
| Obesity | [296,297,298,299] | ||
| Diabetes | [300] | ||
| Non-alcoholic fatty liver | [301,302,303] | ||
| Cancer | |||
| Colon cancer | [355,356,357,358,359] | ||
| Lung cancer | [360,361,362] | ||
| Hepatocellular carcinoma | [363] | ||
| Glioblastoma | [364] | ||
| Cervical cancer | [365] | ||
| Melanoma | [366] | ||
| Sarcoma | [367] | ||
| β-Cryptoxanthin | Phaeodactylum tricornutum Auxenochlorella pyrenoidosa Porphyridium purpureum | Inflammation | |
| Obesity | [305,306] | ||
| Ischemia/Reperfusion | [307] | ||
| Osteoarthritis | [308] | ||
| Lung inflammation | [309] | ||
| Non-alcoholic fatty liver | [310] | ||
| Cancer | |||
| Gastric cancer | [368,369] | ||
| Hepatocellular carcinoma | [370] | ||
| Lung cancer | [371,372,373] | ||
| Non-Hodgkin lymphoma | [374] | ||
| Colon cancer | [375] | ||
| Head/Neck cancer | [333] | ||
| Breast cancer | [376] | ||
| Renal cell carcinoma | [377] |
5. Anticancer Activity of Carotenoids
5.1. β-Carotene
5.1.1. In Vitro Studies
5.1.2. In Vivo Studies
5.1.3. Human Studies
5.2. Lutein
5.2.1. In Vitro Studies
5.2.2. In Vivo Studies
5.2.3. Human Studies
5.3. Zeaxanthin
5.3.1. In Vitro and Animal Studies
5.3.2. Human Studies
5.4. Astaxanthin
5.4.1. In Vitro Studies
5.4.2. In Vivo Studies
5.4.3. Human Studies
5.5. Fucoxanthin
5.5.1. In Vitro Studies
5.5.2. In Vivo Studies
5.6. β-Cryptoxanthin
5.6.1. In Vitro Studies
5.6.2. In Vivo Studies
5.6.3. Human Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irigoien, X.; Hulsman, J.; Harris, R.P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 2004, 429, 863–867. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Posten, C.; Schaub, G. Microalgae and terrestrial biomass as source for fuels—A process view. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef]
- Zhu, L.D.; Li, Z.H.; Hiltunen, E. Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H.; Sturm, B.S.M.; deNoyelles, F.J.; Billings, S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2010, 25, 301–309. [Google Scholar] [CrossRef]
- Popp, J.; Harangi-Rákos, M.; Gabnai, Z.; Balogh, P.; Antal, G.; Bai, A. Biofuels and their co-products as livestock feed: Global economic and environmental implications. Molecules 2016, 21, 285. [Google Scholar] [CrossRef] [Green Version]
- Rumin, J.; Nicolau, E.; de Oliveira, R.G.; Fuentes-Grünewald, C.; Picot, L. Analysis of scientific research driving microalgae market opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Raff, J.D.; Njegic, B.; Chang, W.L.; Gordon, M.S.; Dabdub, D.; Gerber, R.B.; Finlayson-Pitts, B.J. Chlorine activation indoors and outdoors via surface-mediated reactions of nitrogen oxides with hydrogen chloride. Proc. Natl. Acad. Sci. USA 2009, 106, 13647–13654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine diterpenoids as potential anti-inflammatory agents. Mediat. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [Green Version]
- Eseberri, I.; Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskibar, I.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Anti-obesity effects of microalgae. Int. J. Mol. Sci. 2020, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and chlorophyll-derived molecules from the diatom Cylindrotheca closterium with anti-inflammatory activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Iconomou, D.; Sotiroudis, T.; Israilides, C.; Muylaert, K. Exploration of using stripped ammonia and ash from poultry litter for the cultivation of the cyanobacterium Arthrospira platensis and the green microalga Chlorella vulgaris. Bioresour. Technol. 2015, 196, 459–468. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Roy, K.; Goyal, A.; Moholkar, V.S. Investigations in ultrasonic enhancement of β-carotene production by isolated microalgal strain Tetradesmus obliquus SGM19. Ultrason. Sonochem. 2019, 58, 104697. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Sharma, A.K.; Daniell, H.; Kumar, S. Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2. Plant Biotechnol. J. 2015, 13, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Venkata Mohan, S.; Hemalatha, M.; Chakraborty, D.; Chatterjee, S.; Ranadheer, P.; Kona, R. Algal biorefinery models with self-sustainable closed loop approach: Trends and prospective for blue-bioeconomy. Bioresour. Technol. 2020, 295, 122128. [Google Scholar] [CrossRef] [PubMed]
- PatentScope Database. World Intelectual Propiety Organization. Available online: https://patentscope.wipo.int/search/es/search.jsf (accessed on 6 February 2021).
- Magri, M. Una Nueva Microalga Chlorella Para la Producción de Aceite Vegetal Para Biodiésel y Unidades de Energía de Cogeneración. Spanish Patent No. ES2755158, 21 April 2020. [Google Scholar]
- Fernández Acién, G.F.; Fernández Sevilla, J.M.; Molina Grima, E.; Gómez Serrano, C. Sistema de Eliminación de Metales Pesados en Aguas Mediante Microalgas. Spanish Patent No. ES2642462, 16 November 2017. [Google Scholar]
- Frazao de Andrade, A.; Figueiredo Porto, A.L.; De Araujo Viana Marques, D.; De Lima Filho, J.L.; Madruga Lima Ribeiro, M.H.; Nunes Herculano, P.; Pedrosa Bezerra, R.; Goncalves De Melo, R.; Pedrosa Brandão Costa, R.M.; Da Silva, V.A. Formulação Tópica em Gel Com Atividade Cicatrizante Contendo Extrato de Microalga. British Patent No. BR102018077212, 7 July 2020. [Google Scholar]
- Leclere-Bienfait, S.; Bredif, S. Extract of Chlamydomonas Acidophila, Method for Preparing Same and Cosmetic Compositions and Dermatological Compositions Comprising Same. French Patent No. WO2020136283, 2 July 2020. [Google Scholar]
- Herrera Valencia, V.A.; Peraza Echeverría, S.; Beltrán Aguilar, A.G. Inducible Crgpdh3 Promoter of Chlamydomonas Reinhardtii and the Ese Thereof for the Expression of Recombinant Proteins. Mexican Patent No. WO2020130772, 25 June 2020. [Google Scholar]
- Riquelme Salamanca, C.E.; Silva Aciares, F.R.; Gonzalez Cortes, L.A.; Marticorena de la Rosa, P.A. Método de Cultivo al Exterior u “Outdoor” de la Microalga Muriellopsis sp. para Producir Biomasa Con Alto Contenido en Luteína y Bajo Contenido en Metales Que Tiene Buenas Propiedades Antioxidantes y Util para Preparar Alimento Animal o de Consumo Humano. Chile Patent No. WO2019071364, 18 April 2019. [Google Scholar]
- Yueming, L.; Jianchun, X.; Lina, X.; Xiuluan, X.; Bingzheng, X. Method for Comprehensively Extracting EPA and Fucoxanthin from Phaeodactylum Tricornutum. Chinese Patent No. CN111205179, 9 January 2020. [Google Scholar]
- Nakashima, A.; Suzuki, K.; Sugawara, T.; Manabe, Y. Agent for Suppressing Increment of Blood Glucose Level, Diabetes Preventing Agent, and Food Composition. Japanese Patent No. WO2020045647, 5 March 2020. [Google Scholar]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana dietary supplementation increases antioxidant capacities and reduces ros release in mitochondria of hyperthyroid rat liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [Green Version]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.C.; Ferreira, I.C.; Dias, M.; Barreiro, M.F. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-I.; Kim, S.; Lee, C.; Choi, Y.E. Blue-red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J. Microbiol. 2019, 57, 101–106. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rathod, J.P.; Vira, C.; Lali, A.M.; Prakash, G. Metabolic engineering of Chlamydomonas reinhardtii for enhanced β-carotene and lutein production. Appl. Biochem. Biotechnol. 2020, 190, 1457–1469. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Low, K.L.; Idris, A.; Mohd Yusof, N. Novel protocol optimized for microalgae lutein used as food additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef]
- Jalali Jivan, M.; Abbasi, S. Nano based lutein extraction from marigold petals: Optimization using different surfactants and co-surfactants. Heliyon 2019, 5, e01572. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, M.M.; Sun, Z.; Liu, S.F.; Qin, Z.H.; Mou, J.H.; Zhou, Z.G.; Lin, C.S.K. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef]
- Saha, S.K.; Kazipet, N.; Murray, P. The carotenogenic Dunaliella salina CCAP 19/20 produces enhanced levels of carotenoid under specific nutrients limitation. Biomed. Res. Int. 2018, 2018, 7532897. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Lu, K.; Zhao, X.; Ma, R.; Chen, J.; Ho, S.H. Manipulating nutritional conditions and salinity-gradient stress for enhanced lutein production in marine microalga Chlamydomonas sp. Biotechnol. J. 2019, 14, e1800380. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Schenk, P.M. Induced carotenoid accumulation in Dunaliella salina and Tetraselmis suecica by plant hormones and UV-C radiation. Appl. Microbiol. Biotechnol. 2015, 99, 9407–9416. [Google Scholar] [CrossRef] [PubMed]
- Wojtasiewicz, B.; Stoń-Egiert, J. Bio-optical characterization of selected cyanobacteria strains present in marine and freshwater ecosystems. J. Appl. Phycol. 2016, 28, 2299–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-Y.; Min, B.-S.; Chang, C.-S.; Jin, E. Isolation and characterization of a xanthophyll aberrant mutant of the green alga Nannochloropsis oculata. Mar. Biotechnol. 2006, 8, 238–245. [Google Scholar] [CrossRef]
- Singh, D.; Puri, M.; Wilkens, S.; Mathur, A.S.; Tuli, D.K.; Barrow, C.J. Characterization of a new zeaxanthin producing strain of Chlorella saccharophila isolated from New Zealand marine waters. Bioresour. Technol. 2013, 143, 308–314. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, F.K.; Hussein, R.A.; Saleh, D.O.; Jaleel, G.A.R.A. Zeaxanthin isolated from Dunaliella salina microalgae ameliorates age associated cardiac dysfunction in rats through stimulation of retinoid receptors. Mar. Drugs 2019, 17, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfellotto, F.; Stella, G.R.; Ferrante, M.I.; Falciatore, A.; Brunet, C. Engineering the unicellular alga Phaeodactylum tricornutum for enhancing carotenoid production. Antioxidants 2020, 9, 757. [Google Scholar] [CrossRef]
- Sun, K.M.; Gao, C.; Zhang, J.; Tang, X.; Wang, Z.; Zhang, X.; Li, Y. Rapid formation of antheraxanthin and zeaxanthin in seconds in microalgae and its relation to non-photochemical quenching. Photosynth. Res. 2020, 144, 317–326. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other nutrients from Haematococcus pluvialis—Multifunctional applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Mao, X.; Lao, Y.; Sun, H.; Li, X.; Yu, J.; Chen, F. Time-resolved transcriptome analysis during transitions of sulfur nutritional status provides insight into triacylglycerol (TAG) and astaxanthin accumulation in the green alga Chromochloris zofingiensis. Biotechnol. Biofuels 2020, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Janchot, K.; Rauytanapanit, M.; Honda, M.; Hibino, T.; Sirisattha, S.; Praneenararat, T.; Kageyama, H.; Waditee-Sirisattha, R. Effects of potassium chloride-induced stress on the carotenoids canthaxanthin, astaxanthin, and lipid accumulations in the green Chlorococcal microalga strain TISTR 9500. J. Eukaryot. Microbiol. 2019, 66, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1.40. under optimized culture conditions. J. Basic Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méresse, S.; Fodil, M.; Fleury, F.; Chénais, B. Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 2020, 21, 9273. [Google Scholar] [CrossRef]
- Bustamam, M.S.A.; Pantami, H.A.; Azizan, A.; Shaari, K.; Min, C.C.; Abas, F.; Nagao, N.; Maulidiani, M.; Banerjee, S.; Sulaiman, F.; et al. Complementary analytical platforms of NMR spectroscopy and LCMS analysis in the metabolite profiling of Isochrysis galbana. Mar. Drugs 2021, 19, 139. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.W.; Chen, F.; Liu, B. A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef] [Green Version]
- Dogdu Okcu, G.; Eustance, E.; Lai, Y.J.S.; Rittmann, B.E. Evaluation of co-culturing a diatom and a coccolithophore using different silicate concentrations. Sci. Total Environ. 2021, 769. [Google Scholar] [CrossRef]
- Kanamoto, A.; Kato, Y.; Yoshida, E.; Hasunuma, T.; Kondo, A. Development of a method for fucoxanthin production using the Haptophyte marine microalga Pavlova sp. OPMS 30543. Mar. Biotechnol. 2021, 23, 331–341. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B.; Yun, J.H.; Ryu, A.J.; Yun, J.; Kim, J.W.; Lee, S.; Choi, S.; Cho, D.H.; Choi, D.Y.; Lee, Y.J.; et al. Development of a novel Nannochloropsis strain with enhanced violaxanthin yield for large-scale production. Microb. Cell Fact. 2021, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Mounsef, J.R.; Lteif, R. A simple and fast experimental protocol for the extraction of xanthophylls from microalga Chlorella luteoviridis. Prep. Biochem. Biotechnol. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schüler, L.M.; Bombo, G.; Duarte, P.; Santos, T.F.; Maia, I.B.; Pinheiro, F.; Marques, J.; Jacinto, R.; Schulze, P.S.C.; Pereira, H.; et al. Carotenoid biosynthetic gene expression, pigment and n-3 fatty acid contents in carotenoid-rich Tetraselmis striata CTP4 strains under heat stress combined with high light. Bioresour. Technol. 2021, 337, 125385. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.B.; Ferreira, O.; Rosado, T.; Gallardo, E.; Silvestre, S.; Santos, L.M.A. Eustigmatophyte strains with potential interest in cancer prevention and treatment: Partial chemical characterization and evaluation of cytotoxic and antioxidant activity. Biotechnol. Lett. 2021, 43, 1487–1502. [Google Scholar] [CrossRef]
- Lohr, M.; Wilhelm, C. Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta 2001, 212, 382–391. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Markina, Z.V.; Orlova, T.Y.; Vasyanovich, Y.A.; Vardavas, A.I.; Stivaktakis, P.D.; Vardavas, C.I.; Kokkinakis, M.N.; Rezaee, R.; Ozcagli, E.; Golokhvast, K.S. Porphyridium purpureum microalga physiological and ultrastructural changes under copper intoxication. Toxicol. Rep. 2021, 8, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; Bonnet, A.; Nicolau, E.; Bérard, J.B.; Devillers, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. UPLC-MSE profiling of phytoplankton metabolites: Application to the identification of pigments and structural analysis of metabolites in Porphyridium purpureum. Mar. Drugs 2015, 13, 2541–2558. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a red-hot carotenoid: Applications, synthesis, and biosynthetic evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Lotan, T.; Hirschberg, J. Cloning and expression in Escherichia coli of the gene encoding beta-C-4-oxygenase, that converts beta-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 1995, 364, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua-Bin, L.; Fan, K.W.; Chen, F. Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 699–703. [Google Scholar] [CrossRef]
- Anila, N.; Simon, D.P.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Metabolic engineering of Dunaliella salina for production of ketocarotenoids. Photosynth. Res. 2016, 127, 321–333. [Google Scholar] [CrossRef]
- Kumar, T.S.; Josephine, A.; Sreelatha, T.; Azger Dusthackeer, V.N.; Mahizhaveni, B.; Dharani, G.; Kirubagaran, R.; Raja Kumar, S. Fatty acids-carotenoid complex: An effective anti-TB agent from the chlorella growth factor-extracted spent biomass of Chlorella vulgaris. J. Ethnopharmacol. 2020, 249, 112392. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Custódio, L.; Rodrigues, M.J.; De Sousa, C.B.; Oliveira, M.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological activities and chemical composition of methanolic extracts of selected autochthonous microalgae strains from the Red Sea. Mar. Drugs 2015, 13, 3531–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grama, B.S.; Chader, S.; Khelifi, D.; Agathos, S.N.; Jeffryes, C. Induction of canthaxanthin production in a Dactylococcus microalga isolated from the Algerian Sahara. Bioresour. Technol. 2014, 151, 297–305. [Google Scholar] [CrossRef]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. Methods Mol. Biol. 2018, 1803, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Doyle, R.; Sadlier, D.M.; Godson, C. Pro-resolving lipid mediators: Agents of anti-ageing? Semin. Immunol. 2018, 40, 36–48. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H.L. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Mu, X.; Li, Y.; Fan, G.-C. Tissue-resident macrophages in the control of infection and resolution of inflammation. Shock 2021, 55, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [Green Version]
- Adams, N.M.; Grassmann, S.; Sun, J.C. Clonal expansion of innate and adaptive lymphocytes. Nat. Rev. Immunol. 2020, 20, 694–707. [Google Scholar] [CrossRef]
- Golstein, P.; Griffiths, G.M. An early history of T cell-mediated cytotoxicity. Nat. Rev. Immunol. 2018, 18, 527–535. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Obata-Ninomiya, K.; Domeier, P.P.; Ziegler, S.F. Basophils and eosinophils in nematode infections. Front. Immunol. 2020, 11, 583824. [Google Scholar] [CrossRef]
- Xia, M.; Wang, B.; Wang, Z.; Zhang, X.; Wang, X. Epigenetic regulation of NK cell-mediated antitumor immunity. Front. Immunol. 2021, 12, 672328. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Edin, M.L.; Maeyer, R.P.H.D.; Bystrom, J.; Newson, J.; Lih, F.B.; Stables, M.; Zeldin, D.C.; Bishop-Bailey, D. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA 2016, 113, E3240–E3249. [Google Scholar] [CrossRef] [Green Version]
- Jaén, R.I.; Sánchez-García, S.; Fernández-Velasco, M.; Boscá, L.; Prieto, P. Resolution-based therapies: The potential of lipoxins to treat human diseases. Front. Immunol. 2021, 12, 658840. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Immuno-resolving ability of resolvins, protectins, and maresins derived from omega-3 fatty acids in metabolic syndrome. Mol. Nutr. Food Res. 2020, 64, e1900824. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gupta, R. Nutraceutical status and scientific strategies for enhancing production of omega-3 fatty acids from microalgae and their role in healthcare. Curr. Pharm. Biotechnol. 2020, 21, 1616–1631. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Valenzuela, F.; Escobar, E.; Pérez-Tomás, R.; Montecinos, V.P. The inflammatory profile of the tumor microenvironment, orchestrated by cyclooxygenase-2, promotes epithelial-mesenchymal transition. Front. Oncol. 2021, 11, 686792. [Google Scholar] [CrossRef]
- Cortese, N.; Carriero, R.; Laghi, L.; Mantovani, A.; Marchesi, F. Prognostic significance of tumor-associated macrophages: Past, present and future. Semin. Immunol. 2020, 48, 101408. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet. Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.M.; Ries, C.H.; Rüttinger, D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- Cortese, N.; Donadon, M.; Rigamonti, A.; Marchesi, F. Macrophages at the crossroads of anticancer strategies. Front. Biosci. Landmark 2019, 24, 1271–1283. [Google Scholar] [CrossRef]
- Vonderheide, R.H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 blockade by Hu5F9-G4 and rituximab in Non-Hodgkin’s lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, K.; Shen, C.; Wang, X.; Wang, L.; Huang, Y.Y. Astaxanthin promotes M2 macrophages and attenuates cardiac remodeling after myocardial infarction by suppression inflammation in rats. Chin. Med. J. 2020, 133, 1786–1797. [Google Scholar] [CrossRef]
- Zbakh, H.; Zubía, E.; de Los Reyes, C.; Calderón-Montaño, J.M.; Motilva, V. Anticancer activities of meroterpenoids isolated from the brown alga Cystoseira usneoides against the human colon cancer cells HT-29. Foods 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Román, J.; Talero, E.; de los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-derived oxylipins decrease inflammatory mediators by regulating the subcellular location of NFκB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Asp. Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Holley, C.L.; Schroder, K. The rOX-stars of inflammation: Links between the inflammasome and mitochondrial meltdown. Clin. Transl. Immunol. 2020, 9, e01109. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Pourbagher-Shahri, A.M.; Azimi-Nezhad, M.; Forouzanfar, F.; Brockmueller, A.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Roles of Nrf2 in gastric cancer: Targeting for therapeutic strategies. Molecules 2021, 26, 3157. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The role of Nrf2 activity in cancer development and progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [Green Version]
- Carambia, A.; Schuran, F.A. The aryl hydrocarbon receptor in liver inflammation. Semin. Immunopathol. 2021, 43, 563–575. [Google Scholar] [CrossRef]
- Bock, K.W. Aryl hydrocarbon receptor (AHR) functions: Balancing opposing processes including inflammatory reactions. Biochem. Pharmacol. 2020, 178, 114093. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Z.; Zeng, Y.; Liu, Y.; Zhou, L.; Yang, S.; Wang, D. Role of necroptosis in infection-related, immune-mediated, and autoimmune skin diseases. J. Dermatol. 2021, 48, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ding, Y.; Dai, G.L.; Zhang, Y.; Xu, M.T.; Shen, J.R.; Chen, T.T.; Chen, Y.; Meng, G.L. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol. Sin. 2020, 42, 230–241. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.Y.; Song, M.Y.; Kim, E.H. Role of oxidative stress and Nrf2/keap1 signaling in colorectal cancer: Mechanisms and therapeutic perspectives with phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef]
- Tarafdar, A.; Pula, G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Song, M.H.; Oh, J.W.; Keum, Y.S.; Saini, R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: A review of emerging evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The benefits and risks of certain dietary carotenoids that exhibit both anti-and pro-oxidative mechanisms—A comprehensive review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Ucci, M.; Di Tomo, P.; Tritschler, F.; Cordone, V.G.P.; Lanuti, P.; Bologna, G.; Di Silvestre, S.; Di Pietro, N.; Pipino, C.; Mandatori, D.; et al. Anti-inflammatory role of carotenoids in endothelial cells derived from umbilical cord of women affected by gestational diabetes mellitus. Oxid. Med. Cell. Longev. 2019, 2019, 8184656. [Google Scholar] [CrossRef]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. β-Carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef]
- Cho, S.O.; Kim, M.-H.; Kim, H. β-Carotene inhibits activation of NF-κB, activator protein-1, and STAT3 and regulates abnormal expression of some adipokines in 3T3-L1 adipocytes. J. Cancer Prev. 2018, 23, 37–43. [Google Scholar] [CrossRef]
- Lesmana, R.; Felia Yusuf, I.; Goenawan, H.; Achadiyani, A.; Khairani, A.F.; Nur Fatimah, S.; Supratman, U. Low dose of β-carotene regulates inflammation, reduces caspase signaling, and correlates with autophagy activation in cardiomyoblast cell lines. Med. Sci. Monit. Basic Res. 2020, 26, e928648. [Google Scholar] [CrossRef]
- Yang, G.; Lee, H.E.; Moon, S.; Ko, K.M.; Koh, J.H.; Seok, J.K.; Min, J.; Heo, T.; Kang, H.C.; Cho, Y.; et al. Direct binding to NLRP3 pyrin domain is a novel strategy to prevent NLRP3-driven inflammation and gouty arthritis. Arthritis Rheumatol. 2020, 72, 1192–1202. [Google Scholar] [CrossRef]
- Li, R.; Hong, P.; Zheng, X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef]
- Lin, H.W.; Chang, T.J.; Yang, D.J.; Chen, Y.C.; Wang, M.; Chang, Y.Y. Regulation of virus-induced inflammatory response by β-carotene in RAW264.7 cells. Food Chem. 2012, 134, 2169–2175. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Jena, G.B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. Eur. J. Nutr. 2015, 54, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Hong, P.; Lang, W.; Hui, J.; Yang, Y.; Zheng, X. β-Carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Anim. Biosci. 2021, 34, 1221–1234. [Google Scholar] [CrossRef] [Green Version]
- Latief, U.; Ahmad, R. β-Carotene inhibits NF-κB and restrains diethylnitrosamine-induced hepatic inflammation in Wistar rats. Int. J. Vitam. Nutr. Res. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- El-Din, S.H.S.; El-Lakkany, N.M.; El-Naggar, A.A.; Hammam, O.A.; El-Latif, H.A.A.; Ain-Shoka, A.A.; Ebeid, F.A. Effects of rosuvastatin and/or β-carotene on non-alcoholic fatty liver in rats. Res. Pharm. Sci. 2015, 10, 275–287. [Google Scholar]
- Relevy, N.Z.; Harats, D.; Harari, A.; Ben-Amotz, A.; Bitzur, R.; Rühl, R.; Shaish, A. Vitamin A-deficient diet accelerated atherogenesis in apolipoprotein E(−/−) mice and dietary β -carotene prevents this consequence. Biomed Res. Int. 2015, 2015, 758723. [Google Scholar] [CrossRef] [Green Version]
- Kaliappan, G.; Nagarajan, P.; Moorthy, R.; Kalai Gana Selvi, S.; Avinash Raj, T.; Mahesh Kumar, J. Ang II induce kidney damage by recruiting inflammatory cells and up regulates PPAR gamma and Renin 1 gene: Effect of β carotene on chronic renal damage. J. Thromb. Thrombolysis 2013, 36, 277–285. [Google Scholar] [CrossRef]
- Takahashi, N.; Kake, T.; Hasegawa, S.; Imai, M. Effects of post-administration of β-carotene on diet-induced atopic dermatitis in hairless mice. J. Oleo Sci. 2019, 68, 793–802. [Google Scholar] [CrossRef]
- Kake, T.; Imai, M.; Takahashi, N. Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef]
- Horváth, G.; Kemény, Á.; Barthó, L.; Molnár, P.; Deli, J.; Szente, L.; Bozó, T.; Pál, S.; Sándor, K.; Szőke, É.; et al. Effects of some natural carotenoids on TRPA1- and TRPV1-induced neurogenic inflammatory processes in vivo in the mouse skin. J. Mol. Neurosci. 2015, 56, 113–121. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, L.; Lin, S.; Chen, S.; Liu, Y.J.; Zhou, W.; Wang, X. Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 2018, 61, 92–99. [Google Scholar] [CrossRef]
- Zainal, Z.; Rahim, A.A.; Khaza’ai, H.; Chang, S.K. Effects of palm oil tocotrienol-rich fraction (TRF) and carotenes in ovalbumin (OVA)-challenged asthmatic brown Norway rats. Int. J. Mol. Sci. 2019, 20, 1764. [Google Scholar] [CrossRef] [Green Version]
- Fuke, N.; Aizawa, K.; Suganuma, H.; Takagi, T.; Naito, Y. Effect of combined consumption of Lactobacillus brevis KB290 and β-carotene on minor diarrhoea-predominant irritable bowel syndrome-like symptoms in healthy subjects: A randomised, double-blind, placebo-controlled, parallel-group trial. Int. J. Food Sci. Nutr. 2017, 68, 973–986. [Google Scholar] [CrossRef]
- Asemi, Z.; Alizadeh, S.A.; Ahmad, K.; Goli, M.; Esmaillzadeh, A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: A double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 2016, 35, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, D.H.; Won, C.H.; Kim, S.M.; Lee, S.; Lee, M.J.; Chung, J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology 2010, 221, 160–171. [Google Scholar] [CrossRef]
- Ribeiro, D.; Sousa, A.; Nicola, P.; Ferreira de Oliveira, J.M.P.; Rufino, A.T.; Silva, M.; Freitas, M.; Carvalho, F.; Fernandes, E. β-Carotene and its physiological metabolites: Effects on oxidative status regulation and genotoxicity in in vitro models. Food Chem. Toxicol. 2020, 141, 111392. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, C.; Zeng, F.; Zhu, H. Low levels of serum β-carotene and β-carotene/retinol ratio are associated with histological severity in nonalcoholic fatty liver disease patients. Ann. Nutr. Metab. 2019, 74, 156–164. [Google Scholar] [CrossRef]
- Chambaneau, A.; Filaire, M.; Jubert, L.; Bremond, M.; Filaire, E. Nutritional Intake, Physical Activity and Quality of Life in COPD Patients. Int. J. Sports Med. 2016, 37, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Brucker, N.; Durgante, J.; Bubols, G.; Bulcão, R.; Moro, A.; Charão, M.; Baierle, M.; Nascimento, S.; Gauer, B.; et al. Urinary 1-hydroxypyrene is associated with oxidative stress and inflammatory biomarkers in acute myocardial infarction. Int. J. Environ. Res. Public Health 2014, 11, 9024–9037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epplein, M.; Signorello, L.B.; Zheng, W.; Cai, Q.; Hargreaves, M.K.; Michel, A.; Pawlita, M.; Fowke, J.H.; Correa, P.; Blot, W.J. Helicobacter pylori prevalence and circulating micronutrient levels in a low-income United States population. Cancer Prev. Res. 2011, 4, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Muzáková, V.; Kand’ár, R.; Meloun, M.; Skalický, J.; Královec, K.; Záková, P.; Vojtísek, P. Inverse correlation between plasma Beta-carotene and interleukin-6 in patients with advanced coronary artery disease. Int. J. Vitam. Nutr. Res. 2010, 80, 369–377. [Google Scholar] [CrossRef]
- Munia, I.; Gafray, L.; Bringer, M.A.; Goldschmidt, P.; Proukhnitzky, L.; Jacquemot, N.; Cercy, C.; Otman, K.R.B.; Errera, M.H.; Ranchon-Cole, I. Cytoprotective effects of natural highly bio-available vegetable derivatives on human-derived retinal cells. Nutrients 2020, 12, 879. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhang, A.; Sun, R.; Xu, J.; Yin, T.; He, H.; Gou, J.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar] [CrossRef]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Y.; Fung, F.K.C.; Fu, Z.J.; Wong, D.; Chan, H.H.L.; Lo, A.C.Y. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: In vivo and in vitro studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5976–5984. [Google Scholar] [CrossRef] [Green Version]
- Fung, F.K.C.; Law, B.Y.K.; Lo, A.C.Y. Lutein attenuates both apoptosis and autophagy upon cobalt (II) chloride-induced hypoxia in rat Muller cells. PLoS ONE 2016, 11, e0167828. [Google Scholar] [CrossRef]
- Chao, S.C.; Nien, C.W.; Iacob, C.; Hu, D.N.; Huang, S.C.; Lin, H.Y. Effects of lutein on hyperosmoticity-induced upregulation of IL-6 in cultured corneal epithelial cells and its relevant signal pathways. J. Ophthalmol. 2016, 2016, 8341439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongcharoen, S.; Warnnissorn, P.; Leŗtkajornsin, O.; Limpeanchob, N.; Sutheerawattananonda, M. Protective effect of silk lutein on ultraviolet B-irradiated human keratinocytes. Biol. Res. 2013, 46, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.O.; Smith, A.; Liu, Y.; Du, P.; Blumberg, J.B.; Garlick, J. Photoprotection by pistachio bioactives in a 3-dimensional human skin equivalent tissue model. Int. J. Food Sci. Nutr. 2017, 68, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.H.; Park, J.G.; Yi, Y.; Park, K.W.; Rho, H.S.; Lee, M.; Yoo, J.W.; Kang, S.; Hong, Y.D.; et al. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediat. Inflamm. 2013, 2013, 787042. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef]
- Chung, R.W.S.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Effect of different anthocyanidin glucosides on lutein uptake by Caco-2 cells, and their combined activities on anti-oxidation and anti-inflammation in vitro and ex vivo. Molecules 2018, 23, 2035. [Google Scholar] [CrossRef] [Green Version]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226. [Google Scholar] [CrossRef]
- Wang, W.; Tam, K.C.; Ng, T.C.; Goit, R.K.; Chan, K.L.S.; Lo, A.C.Y. Long-term lutein administration attenuates retinal inflammation and functional deficits in early diabetic retinopathy using the Ins2 Akita/+ mice. BMJ Open Diabetes Res. Care 2020, 8, e001519. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef] [Green Version]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha, S.; Vallikannan, B. Fatty acids influence the efficacy of lutein in the modulation of α-crystallin chaperone function: Evidence from selenite induced cataract rat model. Biochem. Biophys. Res. Commun. 2020, 529, 425–431. [Google Scholar] [CrossRef]
- He, R.R.; Tsoi, B.; Lan, F.; Yao, N.; Yao, X.S.; Kurihara, H. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin. Med. 2011, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, S.C.; Vagaggini, T.; Nien, C.W.; Huang, S.C.; Lin, H.Y. Effects of lutein and zeaxanthin on LPS-induced secretion of IL-8 by uveal melanocytes and relevant signal pathways. J. Ophthalmol. 2015, 2015, 152854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muz, O.E.; Orhan, C.; Erten, F.; Tuzcu, M.; Ozercan, I.H.; Singh, P.; Morde, A.; Padigaru, M.; Rai, D.; Sahin, K. A novel integrated active herbal formulation ameliorates dry eye syndrome by inhibiting inflammation and oxidative stress and enhancing glycosylated phosphoproteins in rats. Pharmaceuticals 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cui, W.; Wang, L.; Xiong, Y.; Liu, L.; Sun, X.; Hao, L. Lutein prevents high fat diet-induced atherosclerosis in ApoE-deficient mice by inhibiting NADPH oxidase and increasing PPAR expression. Lipids 2015, 50, 261–273. [Google Scholar] [CrossRef]
- Kim, J.E.; Leite, J.O.; deOgburn, R.; Smyth, J.A.; Clark, R.M.; Fernandez, M.L. A Lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, Y.; Kobayashi, A.; Endo, S.; Takemura, J.; Takeda, M. Effect of lutein on the acute inflammation-induced c-Fos expression of rat trigeminal spinal nucleus caudalis and C1 dorsal horn neurons. Eur. J. Oral Sci. 2019, 127, 379–385. [Google Scholar] [CrossRef]
- Syoji, Y.; Kobayashi, R.; Miyamura, N.; Hirohara, T.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimazu, Y.; Takeda, M. Suppression of hyperexcitability of trigeminal nociceptive neurons associated with inflammatory hyperalgesia following systemic administration of lutein via inhibition of cyclooxygenase-2 cascade signaling. J. Inflamm. 2018, 15, 4–13. [Google Scholar] [CrossRef]
- AbuBakr, H.O.; Aljuaydi, S.H.; Abou-Zeid, S.M.; El-Bahrawy, A. Burn-induced multiple organ injury and protective effect of lutein in rats. Inflammation 2018, 41, 760–772. [Google Scholar] [CrossRef]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Du, S.Y.; Zhang, Y.L.; Bai, R.X.; Ai, Z.L.; Xie, B.S.; Yang, H.Y. Lutein prevents alcohol-induced liver disease in rats by modulating oxidative stress and inflammation. Int. J. Clin. Exp. Med. 2015, 8, 8785–8793. [Google Scholar] [PubMed]
- Cheng, F.; Zhang, Q.; Yan, F.F.; Wan, J.F.; Lin, C.S. Lutein protects against ischemia/reperfusion injury in rat skeletal muscle by modulating oxidative stress and inflammation. Immunopharmacol. Immunotoxicol. 2015, 37, 329–334. [Google Scholar] [CrossRef]
- Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Frederick, L.; Iii, F.; Elman, M.J.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary nutrient intake and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2020, 128, 425–442. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Bron, A.; Merle, B.M.J.; Savel, H.; Chêne, G.; Delcourt, C.; Creuzot-Garcher, C. Effect of dietary supplementation with lutein, zeaxanthin, and ω-3 on macular pigment: A randomized clinical trial. JAMA Ophthalmol. 2017, 135, 1259–1266. [Google Scholar] [CrossRef]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed. Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lu, X.R.; Lin, X.M. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: A randomised, double-blind, placebo-controlled trial. Br. J. Ophthalmol. 2015, 99, 371–375. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Recalde, S.; Alamán, A.S.; Robredo, P.F. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients 2013, 5, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Wolf-Schnurrbusch, U.E.K.; Zinkernagel, M.S.; Munk, M.R.; Ebneter, A.; Wolf, S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8069–8074. [Google Scholar] [CrossRef] [Green Version]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Morse, N.L.; Reid, A.J.; St-Onge, M. An open-label clinical trial assessing the efficacy and safety of Bend Skincare Anti-Aging Formula on minimal erythema dose in skin. Photodermatol. Photoimmunol. Photomed. 2018, 34, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Granger, C.; Aladren, S.; Delgado, J.; Garre, A.; Trullas, C.; Gilaberte, Y. Prospective evaluation of the efficacy of a food supplement in increasing photoprotection and improving selective markers related to skin photo-ageing. Dermatol. Ther. 2020, 10, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xiong, Y.; Xing, F.; Gao, H.; Wang, X.; He, L.; Ren, C.; Liu, L.; So, K.F.; Xiao, J. Precise regulation of mir-210 is critical for the cellular homeostasis maintenance and transplantation efficacy enhancement of mesenchymal stem cells in acute liver failure therapy. Cell Transpl. 2017, 26, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Gao, J.; Zheng, Y.; Wang, X.; Chen, C.; Cao, K.; Xu, J.; Li, Y.; Lu, W.; Liu, J.; et al. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014, 5, e1218. [Google Scholar] [CrossRef] [Green Version]
- Biswal, M.R.; Justis, B.D.; Han, P.; Li, H.; Gierhart, D.; Dorey, C.K.; Lewin, A.S. Daily zeaxanthin supplementation prevents atrophy of the retinal pigment epithelium (RPE) in a mouse model of mitochondrial oxidative stress. PLoS ONE 2018, 13, e0203816. [Google Scholar] [CrossRef]
- Sahin, K.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Ozercan, I.H.; Ali, S.; Yilmaz, I.; Juturu, V. (3R, 3’R)-zeaxanthin protects the retina from photo-oxidative damage via modulating the inflammation and visual health molecular markers. Cutan. Ocul. Toxicol. 2019, 38, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gunal, M.Y.; Sakul, A.A.; Caglayan, A.B.; Erten, F.; Kursun, O.E.D.; Kilic, E.; Sahin, K. Protective effect of lutein/zeaxanthin isomers in traumatic brain injury in mice. Neurotox. Res. 2021, 39, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef]
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015, 53, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Lv, Y.; Liu, Y.; Li, J.; Wang, X.; Zhou, Z.; Tipoe, G.L.; Ouyang, S.; Guo, Y.; Zhang, J.; et al. Wolfberry-derived zeaxanthin dipalmitate attenuates ethanol-induced hepatic damage. Mol. Nutr. Food Res. 2019, 63, e1801339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gan, T.; Fang, G.; Wang, S.; Mao, Y.; Ying, C. Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab. Brain Dis. 2018, 33, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Nagabhushanam, K. An open-label pilot study on Macumax supplementation for dry-type age-related macular degeneration. J. Med. Food. 2021, 24, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Azar, G.; Quaranta-El Maftouhi, M.; Masella, J.-J.; Mauget-Faysse, M. Macular pigment density variation after supplementation of lutein and zeaxanthin using the Visucam® 200 pigment module: Impact of age-related macular degeneration and lens status. J. Fr. Ophtalmol. 2017, 40, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Radkar, P.; Lakshmanan, P.S.; Mary, J.J.; Chaudhary, S.; Durairaj, S.K. A Novel multi-ingredient supplement reduces inflammation of the eye and improves production and quality of tears in humans. Ophthalmol. Ther. 2021, 10, 581–599. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef]
- Farruggia, C.; Kim, M.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Joo, M.; Park, Y.; Lee, J. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Xie, X.; Yao, D.; Ding, C.; Chen, M. Astaxanthin prevents against lipopolysaccharide-induced acute lung injury and sepsis via inhibiting activation of MAPK/NF-κB. Am. J. Transl. Res. 2019, 11, 1884–1894. [Google Scholar]
- Kang, H.; Lee, Y.; Bae, M.; Park, Y.-K.; Lee, J.-Y. Astaxanthin inhibits alcohol-induced inflammation and oxidative stress in macrophages in a Sirtuin 1-dependent manner. J. Nutr. Biochem. 2020, 85, 108477. [Google Scholar] [CrossRef]
- Binatti, E.; Zoccatelli, G.; Zanoni, F.; Donà, G.; Mainente, F.; Chignola, R. Phagocytosis of astaxanthin-loaded microparticles modulates TGFβ production and intracellular ROS levels in J774A.1 macrophages. Mar. Drugs 2021, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Hyang, Y.; Koh, H.; Kim, D. Down-regulation of IL-6 production by astaxanthin via ERK-, MSK-, and NF-κB-mediated signals in activated microglia. Int. Immunopharmacol. 2010, 10, 1560–1572. [Google Scholar] [CrossRef]
- Wen, X.; Xiao, L.; Zhong, Z.; Wang, L.; Li, Z.; Pan, X. Astaxanthin acts via LRP-1 to inhibit inflammation and reverse lipopolysaccharide-induced M1/M2 polarization of microglial cells. Oncotarget 2017, 8, 69370–69385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin suppresses PM2.5-induced neuroinflammation by regulating Akt phosphorylation in BV-2 microglial cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B. Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.J.; Lu, J.W.; Liu, F.C.; Lee, C.H.; Lee, H.S.; Ho, Y.J.; Hsieh, T.H.; Wu, C.C.; Wang, C.C. Astaxanthin attenuates joint inflammation induced by monosodium urate crystals. FASEB J. 2020, 34, 11215–11226. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E 2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion—Independent manner. Exp. Dermatol. 2012, 21, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef]
- Wan, F.C.; Zhang, C.; Jin, Q.; Wei, C.; Zhao, H.B.; Zhang, X.L.; You, W.; Liu, X.M.; Liu, G.F.; Liu, Y.F.; et al. Protective effects of astaxanthin on lipopolysaccharide-induced inflammation in bovine endometrial epithelial cells. Biol. Reprod. 2020, 102, 339–347. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, J.W.; Kim, H. Astaxanthin inhibits mitochondrial dysfunction and and interleukin-8 expression in Helicobacter pylori-infected gastric epithelial cells. Nutrients 2018, 10, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.; Kim, K.; Kim, S.; Mun, S.; Hong, S. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int. J. Mol. Sci. 2018, 19, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuvaneswari, S.; Baskaran, Y. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef]
- Han, J.H.; Ju, J.H.; Lee, Y.S.; Park, J.H.; Yeo, I.J.; Park, M.H.; Roh, Y.S. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci. Rep. 2018, 8, 14090. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin prevents alcoholic fatty liver disease by modulating mouse gut microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef]
- Shen, M.; Chen, K.; Lu, J.; Cheng, P.; Xu, L.; Dai, W.; Wang, F.; He, L.; Zhang, Y.; Chengfen, W.; et al. Protective effect of astaxanthin on liver fibrosis through modulation of TGF- β 1 expression and autophagy. Mediat. Inflamm. 2014, 2014, 954502. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Guo, C.; Jiang, H.; Han, B.; Wang, X.; Li, S.; Lv, Y.; Lv, Z.; Zhu, Y. Inflammation response after the cessation of chronic arsenic exposure and post-treatment of natural astaxanthin in liver: Potential role of cytokine-mediated cell-cell interactions. Food Funct. 2020, 11, 9252–9262. [Google Scholar] [CrossRef]
- Iskender, H.; Yenice, G.; Terim Kapakin, K.A.; Dokumacioglu, E.; Sevim, C.; Hayirli, A.; Altun, S. Effects of high fructose diet on lipid metabolism and the hepatic NF-κB/SIRT-1 pathway. Biotech. Histochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Chiu, C.; Chang, C.; Lin, S.; Chyau, C.; Peng, R. improved hepatoprotective effect of liposome-encapsulated astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Guo, L.; Fang, Q.; Yu, M.; Zhang, L.; You, C.; Wang, X.; Liu, Y.; Han, C. Astaxanthin protects against early acute kidney injury in severely burned rats by inactivating the TLR4/MyD88/NF-κB axis and upregulating heme oxygenase-1. Sci. Rep. 2021, 11, 6679. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J.; Hou, G.; Wang, L.; Wang, S.S.; Xiong, X.X. Astaxanthin suppresses lipopolysaccharide-induced myocardial injury by regulating MAPK and PI3K/AKT/mTOR/GSK3β signaling. Mol. Med. Rep. 2020, 22, 3338–3346. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J. 2021, 68, 451–459. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Guo, N.; Huang, P.; Mi, Y. Effects of astaxanthin on inflammation and insulin resistance in a mouse model of gestational diabetes mellitus. Dose Response 2020, 18, 1559325820926765. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Guo, Y.; Zhang, T.; Qiao, X.; Wang, J.; Xu, J.; Xue, C. Hydrophilic astaxanthin: PEGylated astaxanthin fights diabetes by enhancing the solubility and oral absorbability. J. Agric. Food Chem. 2020, 68, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Janani, R.; Anitha, R.E.; Perumal, M.K.; Divya, P.; Baskaran, V. Astaxanthin mediated regulation of VEGF through HIF1α and XBP1 signaling pathway: An insight from ARPE-19 cell and streptozotocin mediated diabetic rat model. Exp. Eye Res. 2021, 206, 108555. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, J.; Yin, W.; Ding, X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int. J. Clin. Exp. Pathol. 2015, 8, 6083–6094. [Google Scholar]
- Feng, Y.; Chu, A.; Luo, Q.; Wu, M.; Shi, X.; Chen, Y. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front. Pharmacol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Shen, L.; Chen, Z.; Xu, L.; Zhang, J.; Yu, X. Trans-astaxanthin attenuates lipopolysaccharide-induced neuroin fl ammation and depressive-like behavior in mice. Brain Res. 2016, 1649, 30–37. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, D.; Mulati, A.; Zhao, B.; Liu, F.; Liu, X. Development of astaxanthin-loaded layer-by-layer emulsions: Physicochemical properties and improvement of LPS-induced neuroinflammation in mice. Food Funct. 2021, 12, 5333–5350. [Google Scholar] [CrossRef]
- Wang, M.; Deng, X.; Xie, Y.; Chen, Y. Astaxanthin attenuates neuroinflammation in status epilepticus rats by regulating the ATP-P2X7R signal. Drug Des. Devel. Ther. 2020, 14, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Zhang, Q.; Wu, Q.; Li, W.; Jiang, T.; Hang, C. Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats. Brain Res. 2015, 1624, 113–124. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Wu, Q.; Dai, H.; Li, W.; Lv, S.; Zhou, X.; Zhang, X.; Hang, C.; Wang, J. Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 2019, 33, 722–737. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, Q.; Liu, F.; Jing, C.; Ding, L.; Zhang, L. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci. Lett. 2018, 662, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Dargahi, L.; Abbaszadeh, F.; Jorjani, M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res. Bull. 2018, 143, 217–224. [Google Scholar] [CrossRef]
- Fu, J.; Sun, H.; Wei, H.; Dong, M.; Zhang, Y.; Xu, W.; Fang, Y.; Zhao, J. Astaxanthin alleviates spinal cord ischemia- reperfusion injury via activation of PI3K/Akt/GSK-3β pathway in rats. J. Orthop. Surg. Res. 2020, 15, 275. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, T.J.; Chen, L.J.; Jiang, M.Y.; Wang, Y.J.; Tseng, G.F.; Chen, J.R. The effects of astaxanthin treatment on a rat model of Alzheimer’s disease. Brain Res. Bull. 2021, 172, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-B.; Zou, M.; Zhao, L.; Zhang, Y.-K. Astaxanthin attenuates acute cerebral infarction via Nrf-2/HO-1 pathway in rats. Curr. Res. Transl. Med. 2021, 69, 103271. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Lin, J.; Wang, G.; Guo, F. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhaliwal, N.; Dhaliwal, J.; Dharavath, R.N.; Chopra, K. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol. Rep. 2020, 72, 104–114. [Google Scholar] [CrossRef]
- Park, M.H.; Jung, J.C.; Hill, S.; Cartwright, E.; Dohnalek, M.H.; Yu, M.; Jun, H.J.; Han, S.B.; Hong, J.T.; Son, D.J. FlexPro MD®, a combination of krill oil, astaxanthin and hyaluronic acid, reduces pain behavior and inhibits inflammatory response in monosodium iodoacetate-induced osteoarthritis in rats. Nutrients 2020, 12, 956. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Lim, J.W.; Kim, H. Astaxanthin inhibits Helicobacter pylori-induced inflammatory and oncogenic responses in gastric mucosal tissues of mice. J. Cancer Prev. 2020, 25, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, S.; Jiao, D.; Yao, B.; Yang, S.; Li, P.; Long, M. Astaxanthin alleviates ochratoxin a-induced cecum injury and inflammation in mice by regulating the diversity of cecal microbiota and TLR4/MyD88/NF-κB signaling pathway. Oxid. Med. Cell. Longev. 2021, 2021, 8894491. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary astaxanthin inhibits colitis and colitis-associated colon carcinogenesis in mice via modulation of the inflammatory cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kochi, T.; Shimizu, M.; Sumi, T.; Kubota, M.; Shirakami, Y.; Tanaka, T. Inhibitory effects of astaxanthin on azoxymethane- induced colonic preneoplastic lesions in C57/BL/KsJ- db/db mice. BMC Gastroenterol. 2014, 14, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Yang, W.; Li, Y.; Hu, L.; Dai, Y.; Chen, J.; Xu, S. Astaxanthin ameliorates cerulein-induced acute pancreatitis in mice. Int. Immunopharmacol. 2018, 56, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Hong, S.; Mun, S.; Kim, S.-J.; Lee, S.; Kim, J.; Kang, K.; Yee, S. The protective effects of astaxanthin on the OVA-induced asthma mice model. Molecules 2017, 22, 2019. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Cui, R.; Li, Z.; Liu, C.; Zhang, J. Astaxanthin alleviated acute lung injury by inhibiting oxidative/nitrative stress and the inflammatory response in mice. Biomed. Pharmacother. 2017, 95, 974–982. [Google Scholar] [CrossRef]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S.; et al. Astaxanthin protects OTA-induced lung injury in mice through the Nrf2/NF-κB pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Jang, J.S.; Kim, K.C.; Park, M.H.; Lee, H.P.; Han, S.; Hong, J.T. Combination effect of titrated extract of Centella asiatica and astaxanthin in a mouse model of phthalic anhydride-induced atopic dermatitis. Allergy Asthma Immunol. Res. 2019, 11, 548–559. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved anti-inflammatory effects of liposomal astaxanthin on a phthalic anhydride-induced atopic dermatitis model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Andoh, T.; Matsunaga, K.; Ur Rehman, M.; Maoka, T.; Shimizu, T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS ONE 2016, 11, e0152288. [Google Scholar] [CrossRef]
- Harada, F.; Morikawa, T.; Lennikov, A.; Mukwaya, A.; Schaupper, M.; Uehara, O.; Takai, R.; Yoshida, K.; Sato, J.; Horie, Y.; et al. Protective effects of oral astaxanthin nanopowder against ultraviolet-induced photokeratitis in mice. Oxid. Med. Cell. Longev. 2017, 2017, 1956104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Seki, S.; Ueda, F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people—A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of composite supplement containing astaxanthin and sesamin on cognitive functions in people with mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J. Alzheimer’s Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Shokri-mashhadi, N.; Tahmasebi, M.; Mohammadiasl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef]
- Heo, S.; Yoon, W.; Kim, K.; Oh, C.; Choi, Y.; Yoon, K.; Kang, D.; Qian, Z.; Choi, I.; Jung, W. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef]
- Kim, K.; Heo, S.; Yoon, W.; Kang, S.; Ahn, G.; Yi, T.; Jeon, Y. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, X.; Wang, Y.; Hu, J.; Wu, H. Fucoxanthin alleviates palmitate-induced inflammation in RAW 264.7 cells through improving lipid metabolism and attenuating mitochondrial dysfunction. Food Funct. 2020, 11, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Vo, T.; Ngo, D.; Kim, S. Fucoxanthin ameliorates inflammation and oxidative reponses in microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef]
- Zhao, D.; Hwan, S.; Yoon, K.; Chun, S.; Yao, M. Anti-neuroinflammatory effects of fucoxanthin via inhibition of Akt/NF-κB and MAPKs/AP-1 pathways and activation of PKA/CREB pathway in lipopolysaccharide-activated BV-2 microglial cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Young, S.; Sun, W.; Lee, D.; Choi, G.; Yim, M.; Min, J.; Jung, W.; Gwang, S.; Seo, S.; Jae, S.; et al. Fucoxanthin inhibits pro fibrotic protein expression in vitro and attenuates bleomycin-induced lung fibrosis in vivo. Eur. J. Pharmacol. 2017, 811, 199–207. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [Green Version]
- Tavares, R.S.N.; Kawakami, C.M.; de Castro Pereira, K.; do Amaral, G.T.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for topical administration, a phototoxic vs. photoprotective potential in a tiered strategy assessed by in vitro methods. Antioxidants 2020, 9, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavares, R.S.N.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin irritation testing beyond tissue viability: Fucoxanthin effects on inflammation, homeostasis, and metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective effects of fucoxanthin on high glucose and 4-hydroxynonenal (4-HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Kanno, S.; Kodate, M.; Hosokawa, M.; Miyashita, K. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar. Drugs 2015, 13, 4799–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.; Seo, Y.; Pan, C.; Lee, O.; Kim, K.; Lee, B. Fucoxanthin suppresses lipid accumulation and ROS production during differentiation in 3T3-L1 adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Huang, W.; Liou, C. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun 2018, 495, 197–203. [Google Scholar] [CrossRef]
- Natsume, C.; Aoki, N.; Aoyama, T.; Senda, K.; Matsui, M.; Ikegami, A.; Tanaka, K.; Azuma, Y.T.; Fujita, T. Fucoxanthin ameliorates atopic dermatitis symptoms by regulating keratinocytes and regulatory innate lymphoid cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Kim, N.; Kim, S.; Lee, H.; Kim, S. Fucoxanthin inhibits the inflammation response in paw edema model through suppressing MAPKs, Akt, and NF-κB. J. Biochem. Mol. Toxicol. 2016, 30, 111–119. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, Q.; Zheng, S.; Zhou, M.; Zeng, Y.-M.; Zhou, T.-T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Chu, W.; Jiang, L.; Geng, C.; Li, J.; Ishikawa, N. Effect of fucoxanthin alone and in combination with d-glucosamine hydrochloride on carrageenan/kaolin-induced experimental arthritis in rats. Phytother. Res. 2014, 28, 1054–1063. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, G.; Lin, Q.; Tang, Z.; Yan, Q.; Yu, X. Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK-NF-κB pathway. Metab. Brain Dis. 2019, 34, 431–442. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Liu, K.; Li, M.; Luo, H.; Cui, L.; Huang, L.; Luo, L. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MyD88 signaling axis. Aging (Albany NY). 2020, 13, 2655–2667. [Google Scholar] [CrossRef]
- Yang, X.; Guo, G.; Dang, M.; Yan, L.; Kang, X.; Jia, K.; Ren, H. Assessment of the therapeutic effects of fucoxanthin by attenuating inflammation in ovalbumin-induced asthma in an experimental animal model. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 229–238. [Google Scholar] [CrossRef]
- Wu, S.-J.; Liou, C.-J.; Chen, Y.-L.; Cheng, S.-C.; Huang, W.-C. Fucoxanthin ameliorates oxidative stress and airway inflammation in tracheal epithelial cells and asthmatic mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Hou, Y. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, A.W.; Na, S.J.; Kim, W.K. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr. Res. Pr. 2013, 7, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Z.; Sudirman, S.; Hsu, Y.; Su, C.; Kuo, H. Fucoxanthin-rich brown algae extract improves male reproductive function on streptozotocin-nicotinamide-induced diabetic rat model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-ogura, Y. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [Green Version]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and fucoxanthin attenuate hepatic steatosis and inflammation of NAFLD through modulation of leptin/adiponectin axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef]
- Orhan, C.; Tuzcu, M.; Gencoglu, H.; Sahin, E.; Sahin, N.; Ozercan, I.H.; Namjoshi, T.; Srivastava, V.; Morde, A.; Rai, D.; et al. Different doses of β -cryptoxanthin may secure the retina from photooxidative injury resulted from common LED sources. Oxid. Med. Cell. Longev. 2021, 2021, 6672525. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef]
- Ni, Y.; Nagashimada, M.; Zhan, L.; Nagata, N.; Kobori, M.; Sugiura, M.; Ogawa, K.; Kaneko, S.; Ota, T. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology 2015, 156, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, D.; Wang, X.; Zhang, Y.; Duan, W.; Li, Y. β-cryptoxanthin alleviates myocardial ischaemia/reperfusion injury by inhibiting NF-κB-mediated inflammatory signalling in rats. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Horie, T.; Fukasawa, K.; Ozaki, K.; Onishi, Y.; Kanayama, T.; Iezaki, T.; Kaneda, K.; Sugiura, M.; Hinoi, E. Amelioration of the development of osteoarthritis by daily intake of β-cryptoxanthin. Biol. Pharm. Bull. 2017, 40, 1116–1120. [Google Scholar] [CrossRef]
- Liu, C.; Bronson, R.T.; Russell, R.M.; Wang, X.D. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev. Res. 2011, 4, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Haidari, F.; Hojhabrimanesh, A.; Helli, B.; Seyedian, S.S.; Ahmadi-Angali, K. An energy-restricted high-protein diet supplemented with β-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: A randomized controlled trial. Nutr. Res. 2020, 73, 15–26. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kim, Y.; Kim, Y.S.; Shin, J.H.; Rubin, L.P.; Kim, Y. β-Carotene exerts anti-colon cancer effects by regulating M2 macrophages and activated fibroblasts. J. Nutr. Biochem. 2020, 82, 108402. [Google Scholar] [CrossRef]
- Lu, M.S.; Fang, Y.J.; Chen, Y.M.; Luo, W.P.; Pan, Z.Z.; Zhong, X.; Zhang, C.X. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: A case–control study. Eur. J. Nutr. 2015, 54, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Liu, S.; Wang, Q.; Lin, X. Effect of β-carotene on immunity function and tumour growth in hepatocellular carcinoma rats. Molecules 2012, 17, 8595–8603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.M.; Luo, Y.; Yishake, D.; Liu, Z.Y.; He, T.T.; Luo, Y.; Zhang, Y.J.; Fang, A.P.; Zhu, H.L. Prediagnostic dietary intakes of vitamin A and β-carotene are associated with hepatocellular-carcinoma survival. Food Funct. 2020, 11, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, J.; Li, M.; Tang, L.; Wu, R.; Jin, L.; Liang, Z. β-carotene reverses tobacco smoke-induced gastric EMT via Notch pathway in vivo. Oncol. Rep. 2018, 39, 1867–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Kwon, O.; Kim, H.; Kim, J. Dietary carotenoids intake and the risk of gastric cancer: A case—control study in Korea. Nutrients 2018, 10, 1031. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, X.; Huang, T.; Chen, L.; Liu, Y.; Li, Q.; Song, J.; Ma, S.; Zhang, K.; Yang, B.; et al. β-Carotene synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal squamous cell carcinoma in vivo and in vitro. Toxicol. Lett. 2016, 261, 49–58. [Google Scholar] [CrossRef]
- Ge, X.X.; Xing, M.Y.; Yu, L.F.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1911–1918. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.M.; Yen, Y.T.; Huang, C.S.; Hu, M.L. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol. Nutr. Food Res. 2011, 55, 606–612. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, Y.S.; Kim, K.M.; Min, S.J.; Kim, Y. β-Carotene inhibits neuroblastoma tumorigenesis by regulating cell differentiation and cancer cell stemness. Biochem. Biophys. Res. Commun. 2014, 450, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Kushwah, V.; Garg, N.K.; Kesharwani, P.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: A preclinical study for breast cancer. Nanomedicine 2017, 12, 1851–1872. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Kushwah, V.; Ghoshal, G.; Jain, A.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Beta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: A preclinical study for breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and breast cancer survival: A systematic review and meta-analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.Y.; Weinstein, S.J.; Albanes, D.; Taylor, P.R.; Virtamo, J.; McGlynn, K.A.; Freedman, N.D. Association of serum α-tocopherol, β-carotene, and retinol with liver cancer incidence and chronic liver disease mortality. Br. J. Cancer 2014, 111, 2163–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Loarca-Pina, G.F.; Salgado, L.M.; Ramos-Gomez, M. Dietary supplementation of lutein reduces colon carcinogenesis in DMH-treated rats by modulating K-ras, PKB, and β-catenin proteins. Nutr. Cancer 2011, 63, 39–45. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Kwon, O.; Shin, A.; Kim, J. Dietary lutein plus zeaxanthin intake and DICER1 rs3742330 A > G polymorphism relative to colorectal cancer risk. Sci. Rep. 2019, 9, 3406. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Firdous, A.P.; Ramnath, V.; Kuttan, R. Effect of carotenoid lutein on N-nitrosodiethylamine-induced hepatocellular carcinoma and its mechanism of action. Eur. J. Cancer Prev. 2013, 22, 320–327. [Google Scholar] [CrossRef]
- Baraya, Y.S.A.; Yankuzo, H.M.; Wong, K.K.; Yaacob, N.S. Strobilanthes crispus bioactive subfraction inhibits tumor progression and improves hematological and morphological parameters in mouse mammary carcinoma model. J. Ethnopharmacol. 2021, 267, 113522. [Google Scholar] [CrossRef]
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid intake and circulating carotenoids are inversely associated with the risk of bladder cancer: A dose-response meta-analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Bock, C.H.; Ruterbusch, J.J.; Holowatyj, A.N.; Steck, S.E.; Van Dyke, A.L.; Ho, W.J.; Cote, M.L.; Hofmann, J.N.; Davis, F.; Graubard, B.I.; et al. Renal cell carcinoma risk associated with lower intake of micronutrients. Cancer Med. 2018, 7, 4087–4097. [Google Scholar] [CrossRef]
- Leoncini, E.; Edefonti, V.; Hashibe, M.; Parpinel, M.; Cadoni, G.; Ferraroni, M.; Serraino, D.; Matsuo, K.; Olshan, A.F.; Zevallos, J.P.; et al. Carotenoid intake and head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Eur. J. Epidemiol. 2016, 31, 369–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; William, R.; De Andrade, M.; Oberg, A.L.; Rabe, K.G.; Anderson, K.E.; Janet, E.; Sinha, R.; et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J. Gastrointest. Cancer 2013, 44, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Gorusupudi, A.; Li, B.; Blount, J.D.; Nwagbo, U.; Kim, H.J.; Sparrow, J.R.; Bernstein, P.S. Lutein and zeaxanthin reduce A2E and iso-A2E levels and improve visual performance in Abca4-/-/Bco2-/- double knockout mice. Exp. Eye Res. 2021, 209, 108680. [Google Scholar] [CrossRef]
- Xu, X.L.; Hu, D.N.; Iacob, C.; Jordan, A.; Gandhi, S.; Gierhart, D.L.; Rosen, R. Effects of zeaxanthin on growth and invasion of human uveal melanoma in nude mouse model. J. Ophthalmol. 2015, 2015, 392305. [Google Scholar] [CrossRef]
- Jeurnink, S.M.; Ros, M.M.; Leenders, M.; Van Duijnhoven, F.J.B.; Siersema, P.D.; Jansen, E.H.J.M.; Van Gils, C.H.; Bakker, M.F.; Overvad, K.; Roswall, N.; et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: A nested case-control study: Plasma micronutrients and pancreatic cancer risk. Int. J. Cancer 2015, 136, E665–E676. [Google Scholar] [CrossRef]
- Terlikowska, K.M.; Dobrzycka, B.; Kinalski, M.; Terlikowski, S.J. Serum concentrations of carotenoids and fat-soluble vitamins in relation to nutritional status of patients with ovarian cancer. Nutr. Cancer 2021, 73, 1480–1488. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, Y. Effects of β-carotene on expression of selected MicroRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J. Cancer Prev. 2019, 24, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Bu, S.; Ma, H.; Gao, L.; Cai, Y.; Zhang, Y.; Zhou, W. Preventive and therapeutic effects of astaxanthin on depressive-like behaviors in high-fat diet and streptozotocin-treated rats. Front. Pharmacol. 2020, 10, 1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.J.; Chen, W.P. Astaxanthin ameliorates cartilage damage in experimental osteoarthritis. Mod. Rheumatol. 2015, 25, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Akduman, H.; Tayman, C.; Çakir, U.; Çakir, E.; Dilli, D.; Türkmenoğlu, T.T.; Gönel, A. Astaxanthin prevents lung injury due to hyperoxia and inflammation. Comb. Chem. High Throughput Screen. 2020. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, Y.T.; Lee, G.S.; Cho, S.I.; Kim, J.M.; Lee, C.; Lim, B.K.; Ju, S.A.; An, W.G. Effects of astaxanthin on dinitrofluorobenzene-induced contact dermatitis in mice. Mol. Med. Rep. 2015, 12, 3632–3638. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Guo, C.; Wu, J. Astaxanthin in liver health and disease: A potential therapeutic agent. Drug Des. Devel. Ther. 2020, 14, 2275–2285. [Google Scholar] [CrossRef]
- Prabhu, P.N.; Ashokkumar, P.; Sudhandiran, G. Antioxidative and antiproliferative effects of astaxanthin during the initiation stages of 1,2-dimethyl hydrazine-induced experimental colon carcinogenesis. Fundam. Clin. Pharmacol. 2009, 23, 225–234. [Google Scholar] [CrossRef]
- Ohno, T.; Shimizu, M.; Shirakami, Y.; Miyazaki, T.; Ideta, T.; Kochi, T.; Kubota, M.; Sakai, H.; Tanaka, T.; Moriwaki, H. Preventive effects of astaxanthin on diethylnitrosamine-induced liver tumorigenesis in C57/BL/KsJ-db/db obese mice. Hepatol. Res. 2016, 46, E201–E209. [Google Scholar] [CrossRef]
- Nakao, R.; Nelson, O.L.; Park, J.S.; Mathison, B.D.; Thompson, P.A.; Chew, B.P. Effect of dietary astaxanthin at different stages of mammary tumor initiation in BALB/c mice. Anticancer Res. 2010, 30, 2171–2175. [Google Scholar] [PubMed]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NF-kB and COX-2. Invest. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef]
- Cui, L.; Xu, F.; Wang, M.; Li, L.; Qiao, T.; Cui, H.; Li, Z.; Sun, C. Dietary natural astaxanthin at an early stage inhibits N-nitrosomethylbenzylamine–induced esophageal cancer oxidative stress and inflammation via downregulation of NFκB and COX2 in F344 rats. Onco Targets Ther. 2019, 12, 5087–5096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavitha, K.; Thiyagarajan, P.; Rathna, J.; Mishra, R.; Nagini, S. Chemopreventive effects of diverse dietary phytochemicals against DMBA-induced hamster buccal pouch carcinogenesis via the induction of Nrf2-mediated cytoprotective antioxidant, detoxification, and DNA repair enzymes. Biochimie 2013, 95, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yu, H.; Wang, S.; Zhang, C.; Shen, S. Astaxanthin inhibits PC-3 xenograft prostate tumor growth in nude mice. Mar. Drugs 2017, 15, 66. [Google Scholar] [CrossRef] [Green Version]
- Haung, H.Y.; Wang, Y.C.; Cheng, Y.C.; Kang, W.; Hu, S.H.; Liu, D.; Xiao, C.; Wang, H.M.D.; Ali, D. A novel oral astaxanthin nanoemulsion from Haematococcus pluvialis induces apoptosis in lung metastatic melanoma. Oxid. Med. Cell. Longev. 2020, 2020, 2647670. [Google Scholar] [CrossRef]
- Terasaki, M.; Masaka, S.; Fukada, C.; Houzaki, M.; Endo, T.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Salivary glycine is a significant predictor for the attenuation of polyp and tumor microenvironment formation by fucoxanthin in AOM/DSS mice. In Vivo 2019, 33, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Kimura, R.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Continuity of tumor microenvironmental suppression in AOM/DSS mice by fucoxanthin may be able to track with salivary glycine. In Vivo 2020, 34, 3205–3215. [Google Scholar] [CrossRef]
- Terasaki, M.; Uehara, O.; Ogasa, S.; Sano, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Alteration of fecal microbiota by fucoxanthin results in prevention of colorectal cancer in AOM/DSS mice. Carcinogenesis 2021, 42, 210–219. [Google Scholar] [CrossRef]
- Terasaki, M.; Ikuta, M.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Dietary fucoxanthin induces anoikis in colorectal adenocarcinoma by suppressing integrin signaling in a murine colorectal cancer model. J. Clin. Med. 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Hamoya, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin prevents colorectal cancer development in dextran sodium sulfate-treated ApcMin/+ mice. Anticancer Res. 2021, 41, 1299–1305. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liu, Y. Anti-inflammatory and apoptotic signaling effect of fucoxanthin on benzo(A)pyrene-induced lung cancer in mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef]
- Mei, C.H.; Zhou, S.C.; Zhu, L.; Ming, J.X.; Zeng, F.D.; Xu, R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria japonica inhibits metastasis and enhances the sensitivity of lung cancer to gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine-induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Ye, G.; Lu, Q.; Zhao, W.; Du, D.; Jin, L.; Liu, Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biol. 2014, 35, 11261–11267. [Google Scholar] [CrossRef]
- Kim, K.N.; Ahn, G.; Heo, S.J.; Kang, S.M.; Kang, M.C.; Yang, H.M.; Kim, D.; Roh, S.W.; Kim, S.K.; Jeon, B.T.; et al. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Han, L.; Riaz, H.; Wang, S.; Cai, K.; Yang, L. The chemopreventive effect of β-cryptoxanthin from mandarin on human stomach cells (BGC-823). Food Chem. 2013, 136, 1122–1129. [Google Scholar] [CrossRef]
- Gao, M.; Dang, F.; Deng, C. β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur. J. Pharmacol. 2019, 859, 172528. [Google Scholar] [CrossRef]
- Lim, J.Y.; Liu, C.; Hu, K.Q.; Smith, D.E.; Wu, D.; Lamon-Fava, S.; Ausman, L.M.; Wang, X.D. Xanthophyll β-cryptoxanthin inhibits highly refined carbohydrate diet–promoted hepatocellular carcinoma progression in mice. Mol. Nutr. Food Res. 2020, 64, e1900949. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.Q.; Choi, S.W.; Ausman, L.M.; Wang, X.D. β-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.Q.; Liu, C.; Wang, X.D. β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic acetylcholine receptor A7 signaling. Cancer Prev. Res. 2016, 9, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.B.; Min, J.Y. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014, 105, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhu, X.; Zhu, L.; Hu, X.; Wen, S. Associations between serum carotenoid levels and the risk of non-Hodgkin lymphoma: A case-control study. Br. J. Nutr. 2020, 124, 1311–1319. [Google Scholar] [CrossRef]
- Huang, J.; Lu, M.S.; Fang, Y.J.; Xu, M.; Huang, W.Q.; Pan, Z.Z.; Chen, Y.M.; Zhang, C.X. Serum carotenoids and colorectal cancer risk: A case-control study in Guangdong, China. Mol. Nutr. Food Res. 2017, 61, 1700267. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Pan, M.X.; Mo, X.F.; Chen, Y.M.; Zhang, C.X. Specific carotenoid intake is inversely associated with the risk of breast cancer among Chinese women. Br. J. Nutr. 2014, 111, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, K.E.; Ke, L.; Gridley, G.; Chiu, B.C.H.; Ershow, A.G.; Lynch, C.F.; Graubard, B.I.; Cantor, K.P. Fruit, vegetables, fibre and micronutrients and risk of US renal cell carcinoma. Br. J. Nutr. 2012, 108, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Jinendiran, S.; Dahms, H.U.; Dileep Kumar, B.S.; Kumar Ponnusamy, V.; Sivakumar, N. Diapolycopenedioic-acid-diglucosyl ester and keto-myxocoxanthin glucoside ester: Novel carotenoids derived from Exiguobacterium acetylicum S01 and evaluation of their anticancer and anti-inflammatory activities. Bioorg. Chem. 2020, 103, 104149. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Sacanella, I.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive compounds of Mediterranean cooked tomato sauce (sofrito) modulate intestinal epithelial cancer cell growth through oxidative stress/arachidonic acid cascade regulation. ACS Omega 2020, 5, 17071–17077. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.; Lim, J.W.; Kim, H. β-Carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 2015, 10, 467. [Google Scholar] [CrossRef]
- Park, Y.; Lee, H.; Lim, J.W.; Kim, H. Inhibitory effect of β-carotene on Helicobacter pylori-induced TRAF expression and hyper-proliferation in gastric epithelial cells. Antioxidants 2019, 8, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lim, J.W.; Kim, H. β-carotene Inhibits Expression of c-Myc and Cyclin E in Helicobacter pylori-infected Gastric Epithelial Cells. J. Cancer Prev. 2019, 24, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhang, Y.; Li, Q.; Yang, L.; Zhang, N.; Ma, S.; Zhang, K.; Song, J.; Guan, F. β-Carotene induces apoptosis in human esophageal squamous cell carcinoma cell lines via the Cav-1/AKT/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2016, 30, 148–157. [Google Scholar] [CrossRef]
- Ngoc, N.B.; Lv, P.; Zhao, W.E. Suppressive effects of lycopene and β-carotene on the viability of the human esophageal squamous carcinoma cell line EC109. Oncol. Lett. 2018, 15, 6727–6732. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Surapaneni, B.K.; Bansal, A. Marked inhibition of cellular proliferation in the normal human esophageal epithelial cells and human esophageal squamous cancer cells in culture by carotenoids: Role for prevention and early treatment of esophageal cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Huei-Yan, C.; Chih-Min, Y.; Jen-Yin, C.; Te-Cheng, Y.; Miao-Lin, H. Multicarotenoids at physiological levels inhibit metastasis in human hepatocarcinoma SK-Hep-1 cells. Nutr. Cancer 2015, 67, 676–686. [Google Scholar] [CrossRef]
- Yurtcu, E.; Iseri, O.D.; Sahin, F.I. Effects of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cell line. Mol. Biol. Rep. 2011, 38, 4265–4272. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Sankaranarayanan, M.; Parasuraman, P.; Joshi, S.D.; Arunachalam, S.; Murugan, I. Design graph theoretical analysis and in silico modeling of Dunaliella bardawil biomass encapsulated N-succinyl chitosan nanoparticles for enhanced anticancer activity. Anticancer. Agents Med. Chem. 2018, 18, 1900–1918. [Google Scholar] [CrossRef] [PubMed]
- Naz, H.; Khan, P.; Tarique, M.; Rahman, S.; Meena, A.; Ahamad, S.; Luqman, S.; Islam, A.; Ahmad, F.; Hassan, M.I. Binding studies and biological evaluation of β-carotene as a potential inhibitor of human calcium/calmodulin-dependent protein kinase IV. Int. J. Biol. Macromol. 2017, 96, 161–170. [Google Scholar] [CrossRef]
- Haddad, N.F.; Teodoro, A.J.; Leite de Oliveira, F.; Soares, N.; de Mattos, R.M.; Hecht, F.; Dezonne, R.S.; Vairo, L.; dos Santos Goldenberg, R.C.; Gomes, F.C.A.; et al. Lycopene and Beta-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS ONE 2013, 8, e62773. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, W.E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Shiau, R.J.; Chen, K.Y.; Der Wen, Y.; Chuang, C.H.; Yeh, S.L. Genistein and β-carotene enhance the growth-inhibitory effect of trichostatin A in A549 cells. Eur. J. Nutr. 2010, 49, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sowmya Shree, G.; Yogendra Prasad, K.; Arpitha, H.S.; Deepika, U.R.; Nawneet Kumar, K.; Mondal, P.; Ganesan, P. β-carotene at physiologically attainable concentration induces apoptosis and down-regulates cell survival and antioxidant markers in human breast cancer (MCF-7) cells. Mol. Cell. Biochem. 2017, 436, 1–12. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Vijay, K.; Sowmya, P.R.R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS PharmSciTech. 2019, 20, 100. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, Y.-S.; Kim, Y. β-carotene regulates the murine liver microenvironment of a metastatic neuroblastoma. J. Cancer Prev. 2013, 18, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.Y.; Lee, B.J.; Chang, P.S.; Hsiao, H.Y.; Hsu, L.P.; Chang, C.H.; Lin, P.T. The risks of ubiquinone and β-carotene deficiency and metabolic disorders in patients with oral cancer. BMC Cancer 2020, 20, 310. [Google Scholar] [CrossRef] [Green Version]
- Rosa, C.; Franca, C.; Vieira, S.L.; Carvalho, A.; Penna, A.; Nogueira, C.; Lessa, S.; Ramalho, A. Reduction of serum concentrations and synergy between retinol, β-carotene, and zinc according to cancer staging and different treatment modalities prior to radiation therapy in women with breast cancer. Nutrients 2019, 11, 2953. [Google Scholar] [CrossRef] [Green Version]
- Nordström, T.; Van Blarigan, E.L.; Ngo, V.; Roy, R.; Weinberg, V.; Song, X.; Simko, J.; Carroll, P.R.; Chan, J.M.; Paris, P.L. Associations between circulating carotenoids, genomic instability and the risk of high-grade prostate cancer. Prostate 2016, 76, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Emri, S.; Kilickap, S.; Kadilar, C.; Halil, M.G.; Akay, H.; Besler, T. Serum levels of alpha-tocopherol, vitamin C, beta-carotene, and retinol in malignant pleural mesothelioma. Asian Pac. J. Cancer Prev. 2012, 13, 3025–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Weinstein, S.J.; Yu, K.; Männistö, S.; Albanes, D. Serum Beta-carotene and overall and cause-specific mortality: A prospective cohort study. Circ. Res. 2018, 123, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; De Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-De Jong, J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef]
- Yu, N.; Su, X.; Wang, Z.; Dai, B.; Kang, J. Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: A meta-analysis of 19 publications. Nutrients 2015, 7, 9309–9324. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Mahmoud, R.I.; Shareef, M.H.; Marei, L.S. Nutrient intake patterns and breast cancer risk among Jordanian women: A case-control study. Epidemiol. Health 2019, 41, e2019010. [Google Scholar] [CrossRef]
- Middha, P.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Mondul, A.M. β-Carotene supplementation and lung cancer incidence in the Alpha-tocopherol, Beta-carotene cancer prevention study: The role of tar and nicotine. Nicotine Tob. Res. 2019, 21, 1045–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein inhibits breast cancer cell growth by suppressing antioxidant and cell survival signals and induces apoptosis. J. Cell. Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef]
- Muhammad, S.N.H.; Yaacob, N.S.; Safuwan, N.A.M.; Fauzi, A.N. Antiglycolytic activities of Strobilanthes crispus active fraction and its bioactive components on triple-negative breast cancer cells in vitro. Anticancer Agents Med. Chem. 2021. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.R.; Swanson, H.M.; Rubin, L.P. Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ROS-mediated mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbahani, M. Evaluation of in vitro anticancer activity of Ocimum basilicum, Alhagi maurorum, Calendula officinalis and their parasite Cuscuta campestris. PLoS ONE 2014, 9, e116049. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.Y.; Gong, W.; Li, X.; Huang, H.B.; Zhu, X.M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.L.; Wang, P.C.; Yan, M.X.; Chen, J. Effect of lutein and doxorubicin combinatorial therapy on S180 cell proliferation and tumor growth. Eur. Rev. Med. Pharmacol. Sci. 2021, 22, 1514–1520. [Google Scholar] [CrossRef]
- Grudzinski, W.; Piet, M.; Luchowski, R.; Reszczynska, E.; Welc, R.; Paduch, R.; Gruszecki, W.I. Different molecular organization of two carotenoids, lutein and zeaxanthin, in human colon epithelial cells and colon adenocarcinoma cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Kanakasabai, S.; Gokarn, S.V.; Krueger, E.G.; Bright, J.J. Dietary lutein modulates growth and survival genes in prostate cancer cells. J. Med. Food 2015, 18, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhao, Y.N.; Shi, Z.Z.; Cong, D.; Bai, Y.S. Lutein inhibits cell growth and activates apoptosis via the PI3K/AKT/mTOR signaling pathway in A549 human non-small-cell lung cancer cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350. [Google Scholar] [CrossRef]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, Beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef]
- Sheng, Y.N.; Luo, Y.H.; Liu, S.B.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Wang, C.Y.; et al. Zeaxanthin induces apoptosis via ROS-regulated MAPK and Akt signaling pathway in human gastric cancer cells. Onco Targets. Ther. 2020, 13, 10995–11006. [Google Scholar] [CrossRef]
- Baudelet, P.H.; Gagez, A.L.; Bérard, J.B.; Juin, C.; Bridiau, N.; Kaas, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. Antiproliferative activity of Cyanophora paradoxa pigments in melanoma, breast and lung cancer cells. Mar. Drugs 2013, 11, 4390–4406. [Google Scholar] [CrossRef] [Green Version]
- Bi, M.C.; Rosen, R.; Zha, R.Y.; McCormick, S.A.; Song, E.; Hu, D.N. Zeaxanthin induces apoptosis in human uveal melanoma cells through Bcl-2 family proteins and intrinsic apoptosis pathway. Evid. Based Complement. Altern. Med. 2013, 2013, 205082. [Google Scholar] [CrossRef]
- Wu, N.L.; Chiang, Y.C.; Huang, C.C.; Fang, J.Y.; Chen, D.F.; Hung, C.F. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp. Dermatol. 2010, 19, 173–181. [Google Scholar] [CrossRef]
- Ahn, Y.T.; Kim, M.S.; Kim, Y.S.; An, W.G. Astaxanthin reduces stemness markers in BT20 and T47D breast cancer stem cells by inhibiting expression of pontin and mutant p53. Mar. Drugs 2020, 18, 577. [Google Scholar] [CrossRef]
- Badak, B.; Aykanat, N.E.B.; Kacar, S.; Sahinturk, V.; Arik, D.; Canaz, F. Effects of astaxanthin on metastasis suppressors in ductal carcinoma. A preliminary study. Ann. Ital. Chir. 2021, 10, S0003469X21035648. [Google Scholar]
- Su, X.-Z.; Chen, R.; Wang, C.-B.; Ouyang, X.-L.; Jiang, Y.; Zhu, M.-Y. Astaxanthin combine with human serum albumin to abrogate cell proliferation, migration, and drug-resistant in human ovarian carcinoma SKOV3 cells. Anticancer Agents Med. Chem. 2019, 19, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gu, H.; Zhang, H.; Li, J.H.; Zhao, W.E. PPARγ-dependent pathway in the growth-inhibitory effects of K562 cells by carotenoids in combination with rosiglitazone. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Nakamura, S.; Maoka, T.; Yamada, T.; Imai, T.; Ohba, T.; Yako, T.; Hayashi, M.; Endo, K.; Saio, M.; et al. Antitumour effects of astaxanthin and adonixanthin on glioblastoma. Mar. Drugs 2020, 18, 474. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ni, Y.; Yang, J.; Lin, X.; Li, J.; Zhang, L. Astaxanthin inhibits proliferation and induces apoptosis and cell cycle arrest of mice H22 hepatoma cells. Med. Sci. Monit. 2016, 22, 2152. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.T. Astaxanthin inhibits proliferation of human gastric cancer cell lines by interrupting cell cycle progression. Gut Liver 2016, 10, 369. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhang, J.; Wang, M.; Liu, W.; Gu, X.; Lv, C.-J. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lim, J.W.; Kim, H. Effect of astaxanthin on activation of autophagy and inhibition of apoptosis in Helicobacter pylori- infected gastric epithelial cell line AGS. Nutrients 2020, 12, 1750. [Google Scholar] [CrossRef]
- Hormozi, M.; Ghoreishi, S.; Baharvand, P. Astaxanthin induces apoptosis and increases activity of antioxidant enzymes in LS-180 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 891–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Sowmya, P.R.R.; Arathi, B.P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef]
- Liu, X.; Song, M.; Gao, Z.; Cai, X.; Dixon, W.; Chen, X.; Cao, Y.; Xiao, H. Stereoisomers of astaxanthin inhibit human colon cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. J. Agric. Food Chem. 2016, 64, 7750–7759. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a beam of light: Photoprotective activities of the marine carotenoids astaxanthin and fucoxanthin in suppression of inflammation and cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, 5993. [Google Scholar] [CrossRef] [Green Version]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr. Cancer 2018, 70, 350. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chaudhary, O.; Rodríguez-Morales, P.; Sun, X.; Chen, D.; Zappasodi, R.; Xu, Z.; Pinto, A.F.M.; Williams, A.; Schulze, I.; et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 2021, 54, 1561–1577.e7. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Yu, R.X.; Hu, X.M.; Xu, S.Q.; Jiang, Z.J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef]
- Yu, R.X.; Yu, R.T.; Liu, Z. Inhibition of two gastric cancer cell lines induced by fucoxanthin involves downregulation of Mcl-1 and STAT3. Hum. Cell 2018, 31, 50–63. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, J.; Min, Z.; Yin, T.; Zhang, R.; Zhang, W.; Hu, L.; Cui, Z.; Gao, C.; Xu, S.; et al. Effects of fucoxanthin on autophagy and apoptosis in SGC-7901cells and the mechanism. J. Cell Biochem. 2018, 119, 7274–7284. [Google Scholar] [CrossRef]
- Long, Y.; Cao, X.; Zhao, R.; Gong, S.; Jin, L.; Feng, C. Fucoxanthin treatment inhibits nasopharyngeal carcinoma cell proliferation through induction of autophagy mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Heal. A 2017, 80, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, S.M. Application of microalgal fucoxanthin for the reduction of colon cancer risk: Inhibitory activity of fucoxanthin against beta-glucuronidase and DLD-1 cancer cells. Nat. Prod. Commun. 2014, 9, 921–924. [Google Scholar]
- Tamura, S.; Narita, T.; Fujii, G.; Miyamoto, S.; Hamoya, T.; Kurokawa, Y.; Takahashi, M.; Miki, K.; Matsuzawa, Y.; Komiya, M.; et al. Inhibition of NF-kappaB transcriptional activity enhances fucoxanthinol-induced apoptosis in colorectal cancer cells. Genes Environ. 2019, 41, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Gu, Y.; Lu, Y.; Yu, C.; Zheng, J.; Qi, H. Fucoxanthin@polyvinylpyrrolidone nanoparticles promoted oxidative stress-induced cell death in Caco-2 human colon cancer cells. Mar. Drugs 2021, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.; Kurrey, N.; Manabe, Y.; Sugawara, T.; Baskaran, V. Polymeric chitosan-glycolipid nanocarriers for an effective delivery of marine carotenoid fucoxanthin for induction of apoptosis in human colon cancer cells (Caco-2 cells). Mater. Sci. Eng. C 2018, 91, 785–795. [Google Scholar] [CrossRef]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NF-κB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.H.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2018, 21, e00296. [Google Scholar] [CrossRef]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell Biol. Toxicol. 2014, 30, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Hou, L.; Gao, C.; Chen, L.; Hu, G.; Xie, S. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Ye, G.; Wang, L.; Yang, K.; Wang, C. Fucoxanthin may inhibit cervical cancer cell proliferation via downregulation of HIST1H3D. J. Int. Med. Res. 2020, 48, 300060520964011. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt/NF-κB signaling pathway. Med. Sci. Monit. 2018, 24, 11–18. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.W.; Choi, H.J.; Lee, J.Y.; Jeong, H.S.; Kim, C.H.; Joo, M.; Choi, J.Y.; Han, C.W.; Kim, S.Y.; Choi, J.S.; et al. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Reses. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.; Wu, R.; Fang, M.; Kong, A.; Drugs, B.; District, C.L.; City, T. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.G.; Oliveira, K.A.; Lopes, R.G.; Poluceno, G.G.; Simioni, C.; Pescador, G.D.S.; Bauer, C.M.; Maraschin, M.; Derner, R.B.; Garcez, R.C.; et al. Anti-cancer effects of fucoxanthin on human glioblastoma cell line. Anticancer Res. 2020, 40, 6799–6815. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Kopanitsa, L.; Módos, D.; Kletnieks, E.; Samarova, E.; Bender, A.; Gomez, L.D.; Bailey, D.S. Transcriptomics predicts compound synergy in drug and natural product treated glioblastoma cells. PLoS ONE 2020, 15, e0239551. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Fu, X.Y.; Cao, W.Q.; Xiang, W.Z.; Hou, Y.J.; Ma, J.K.; Wang, Y.; Fan, C.D. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J. Agric. Food Chem. 2019, 67, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Tafuku, S.; Ishikawa, C.; Yasumoto, T.; Mori, N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012, 28, 1512–1518. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. Vitr. 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Almeida, T.P.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A.A. Cytotoxic activity of fucoxanthin, alone and in combination with the cancer drugs imatinib and doxorubicin, in CML cell lines. Environmen. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Millán, C.S.; Soldevilla, B.; Martín, P.; Gil-Calderón, B.; Compte, M.; Pérez-Sacristán, B.; Donoso, E.; Peña, C.; Romero, J.; Granado-Lorencio, F.; et al. β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through ΔNP73 negative regulation in colon cancer. Clin. Cancer Res. 2015, 21, 4398–4409. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. https://doi.org/10.3390/md19100531
Ávila-Román J, García-Gil S, Rodríguez-Luna A, Motilva V, Talero E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Marine Drugs. 2021; 19(10):531. https://doi.org/10.3390/md19100531
Chicago/Turabian StyleÁvila-Román, Javier, Sara García-Gil, Azahara Rodríguez-Luna, Virginia Motilva, and Elena Talero. 2021. "Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids" Marine Drugs 19, no. 10: 531. https://doi.org/10.3390/md19100531
APA StyleÁvila-Román, J., García-Gil, S., Rodríguez-Luna, A., Motilva, V., & Talero, E. (2021). Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Marine Drugs, 19(10), 531. https://doi.org/10.3390/md19100531