Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina
Abstract
:1. Introduction
2. Results
2.1. Phytoene Production with Combined Treatment of Different Inhibitors and Red Light
2.1.1. Phytoene Desaturase Inhibitors
2.1.2. Pigment Inhibitors Other Than PDS Inhibitors
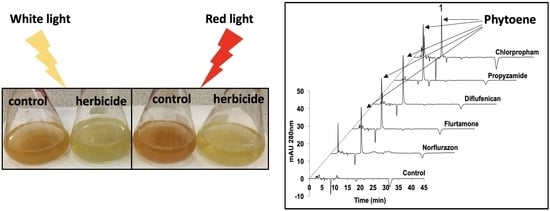
2.1.3. Cell Division Inhibitors
2.1.4. Growth Regulators
2.1.5. Amino Acid Synthesis Inhibitors
2.1.6. Other Inhibitors
2.2. Cellular Composition of Carotenoids in Cultures Maintained under White or Red Light with Selected Heribicides
3. Discussion
4. Materials and Methods
4.1. Herbicides
4.2. Algal Strain and Cultivation
4.3. Pigment Analysis
4.4. Data Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stinco, C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018, 67, 91–103. [Google Scholar]
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Soudant, E.; Ben-Amotz, A. A carotenoid algal preparation containing phytoene and phytofluene inhibited LDL oxidation in vitro. Plant Foods Hum. Nutr. 2008, 63, 83–86. [Google Scholar]
- Benítez-González, A.; Stinco, C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef]
- Welsch, R.; Wust, F.; Bar, C.; Al-Babili, S.; Beyer, P. A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 2008, 147, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Eonseon, J.; Lee, C.-G.; Polle, J.E.W. Secondary carotenoid accumulation in Haematococcus (Chlorophyceae): Biosynthesis, regulation, and biotechnology. Appl. Microbiol. Biotechnol. 2006, 16, 821–831. [Google Scholar]
- Giuliano, G.; Giliberto, L.; Rosati, C. Carotenoid isomerase: A tale of light and isomers. Trends Plant Sci. 2002, 7, 427–429. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Koschmieder, J.; Fehling-Kaschek, M.; Schaub, P.; Brausemann, A.; Timmer, J. Plant-type phytoene desaturase: Functional evaluation of structural implications. PLoS ONE 2017, 12, e0187628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Harvey, P.J. Carotenoid production by Dunaliella salina under red light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitenbach, J.; Zhu, C.; Sandmann, G. Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with the cofactors. J. Agric. Food Chem. 2001, 49, 5270–5272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Harvey, P. Phytoene and phytofluene overproduction by Dunaliella salina using the mitosis inhibitor chlorpropham. Algal Res. 2020, 52, 102126. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Laje, K.; Seger, M.; Dungan, B.; Cooke, P.; Polle, J.; Holguin, F.O. Phytoene accumulation in the novel microalga Chlorococcum sp. using the pigment synthesis inhibitor fluridone. Mar. Drugs 2019, 17, 187. [Google Scholar] [CrossRef] [Green Version]
- León, R.; Vila, M.; Hernánz, D.; Vilchez, C. Production of phytoene by herbicide-treated microalgae Dunaliella bardawil in two-phase systems. Biotechnol. Bioeng. 2005, 92, 695–701. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Gressel, J.; Avron, M. Massive accumulation of phytoene induced by norflurazon in Dunaliella bardawil (chlorophyceae) prevents recovery from photoinhibition. J. Phycol. 1987, 23, 176–181. [Google Scholar] [CrossRef]
- Harker, M.; Young, A.J. Inhibition of astaxanthin synthesis in the green alga, Haematococcus pluvialis. Eur. J. Phycol. 1995, 30, 179–187. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O. Herbicides, Carotenoid Biosynthesis Inhibitors; American Cancer Society: Hoboken, NJ, USA, 2003. [Google Scholar]
- Morillo, E.; Undabeytia, T.; Cabrera, A.; Villaverde, J.; Maqueda, C. Effect of soil type on adsorption-desorption, mobility, and activity of the herbicide norflurazon. J. Agric. Food Chem. 2004, 52, 884–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathishkumar, P.; Mangalaraja, R.V.; Rozas, O.; Vergara, C.; Mansilla, H.D.; Gracia-Pinilla, M.A.; Anandan, S. Sonophotocatalytic mineralization of Norflurazon in aqueous environment. Chemosphere 2016, 146, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, S.; Gothandam, K.M. Accumulation of phytoene, a colorless carotenoid by inhibition of phytoene desaturase (PDS) gene in Dunaliella salina V-101. Bioresour. Technol. 2017, 242, 311–318. [Google Scholar]
- Jeong, S.-W.; Kang, C.K.; Choi, Y.J. Metabolic Engineering of Deinococcus radiodurans for the production of phytoene. Appl. Microbiol. Biotechnol. 2018, 28, 1691–1699. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; Rodríguez, H.; Vargas, M.Á. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsenani, F.; Wass, T.J.; Ma, R.; Eltanahy, E.; Netzel, M.E.; Schenk, P.M. Transcriptome-wide analysis of Chlorella reveals auxin-induced carotenogenesis pathway in green microalgae. Algal Res. 2019, 37, 320–335. [Google Scholar] [CrossRef]
- Cruz, A.B.; Bianchetti, R.E.; Alves, F.R.R.; Purgatto, E.; Peres, L.E.P.; Rossi, M.; Freschi, L. Light, ethylene and auxin signaling interaction regulates carotenoid biosynthesis during tomato fruit ripening. Front. Plant Sci. 2018, 9, 1370. [Google Scholar] [CrossRef]
- Doddaiah, K.M.; Narayan, A.; Aswathanarayana, R.G.; Ravi, S. Effect of metabolic inhibitors on growth and carotenoid production in Dunaliella bardawil. J. Food Sci. Technol. 2011, 50, 1130–1136. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, K.C.; Lehnen, L.P. Mitotic disrupter herbicides. Weed Sci. 1991, 39, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Brausemann, A.; Gemmecker, S.; Koschmieder, J.; Einsle, O. Structure of phytoene desaturase provides insights into herbicide binding and reaction mechanisms involved in carotene desaturation. Structure 2017, 25, 1222–1232.e3. [Google Scholar] [CrossRef]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; vom Lintig, J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Huq, E. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, P.J.; Xu, Y. Production of Dunaliella. WO/2019/097219, 23 May 2019. [Google Scholar]





| Herbicide | Herbicide Active Ingredient (IUPAC Name; CAS Number) | Site of Action | WSSA Group |
|---|---|---|---|
| (i) Phytoene desaturase inhibitors | |||
| Norflurazon | 4-chloro-5-(methylamino)-2-[3-(trifluoromethyl)phenyl]pyridazin-3-one; 27314-13-2 | Phytoene desaturase (PDS) inhibitor | 12 |
| Diflufenican | N-(2,4-difluorophenyl)-2-[3-(trifluoromethyl)phenoxy]pyridine-3-carboxamide; 83164-33-4 | 12 | |
| Flurtamone | 5-(methylamino)-2-phenyl-4-[3-(trifluoromethyl)phenyl]furan-3-one; 96525-23-4 | 12 | |
| (ii) Other pigment inhibitors | |||
| Amitrole | 1H-1,2,4-triazol-5-amine; 61-82-5 | Lycopene cyclase inhibitor, ζ-carotene desaturase inhibitor | 11 |
| Clomazone | 2-(2-chlorobenzyl)-4,4-dimethyl-1,2-oxazolidin-3-one; 81777-89-1 | DOXP (1-deoxy-d-xyulose 5-phosphate synthase) inhibitor | 13 |
| Cinmethylin | 1-methyl-2-[(2-methylphenyl)methoxy]-4-propan-2-yl-7-oxabicyclo[2,2.1]heptane; 87818-31-3 | HPPD (4-hydroxyphenyl-pyruvate-dioxygenase) inhibitor (blockage of plastoquinone synthesis) | 27 |
| (iii) Cell division inhibitors | |||
| Propyzamide | 3,5-dichloro-N-(1,1-dimethylprop-2-ynyl) benzamide; 23950-58-5 | Microtubule polymerization inhibitor | 3 |
| Dimethenamid | 2-Chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl) acetamide; 87674-68-8 | Long-chain fatty acid inhibitor | 15 |
| Chlorpropham | propan-2-yl N-(3-chlorophenyl) carbamate; 101-21-3 | Microtubule organisation inhibitor | 23 |
| (iv) Growth regulators | |||
| Aminopyralid | 4-amino-3,6-dichloropyridine-2-carboxylic acid; 150114-71-9 | Synthetic auxins | 4 |
| Diflufenzopyr-sodium | sodium;2-[(E)-N-[(3,5-difluorophenyl)carbamoylamino]-C-methylcarbonimidoyl]pyridine-3-carboxylate; 109293-98-3 | Auxin transport inhibitor | 19 |
| (v) Amino acid synthesis inhibitors | |||
| Chlorsulfuron | 1-(2-chlorophenyl)sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)urea; 64902-72-3 | ALS (acetolactate synthase) inhibitors | 2 |
| Glyphosate | 2-(phosphonomethylamino)acetic acid; 1071-83-6 sulfosate (glyphosate-trimesium) 2-(phosphonomethylamino)acetate;trimethylsulfanium; 81591-81-3 | EPSP (5-enolpyruvyl-shikimate3-phosphate) synthase inhibitor | 9 |
| (vi) Other inhibitors | |||
| Aminoethyl Sulfate | 2-Aminoethyl hydrogen sulfate; 926-39-6 | GABA transaminase inhibitor (increased GABA levels) | - |
| cis-1,2,3,6-Tetrahydrophthalimide | (3aR,7aS)-3a,4,7,7a-tetrahydroisoindole-1,3-dione, 1469-48-3 | Germination inhibitor | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Harvey, P.J. Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina. Mar. Drugs 2021, 19, 595. https://doi.org/10.3390/md19110595
Xu Y, Harvey PJ. Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina. Marine Drugs. 2021; 19(11):595. https://doi.org/10.3390/md19110595
Chicago/Turabian StyleXu, Yanan, and Patricia J. Harvey. 2021. "Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina" Marine Drugs 19, no. 11: 595. https://doi.org/10.3390/md19110595
APA StyleXu, Y., & Harvey, P. J. (2021). Mitosis Inhibitors Induce Massive Accumulation of Phytoene in the Microalga Dunaliella salina. Marine Drugs, 19(11), 595. https://doi.org/10.3390/md19110595