The Expanded Role of Chitosan in Localized Antimicrobial Therapy
Abstract
:1. Introduction
1.1. Localized Therapy
- Achieving a high drug concentration at the infection site,
- Limiting systemic drug exposure often responsible for faster development of AMR,
- Consequently, reducing the systemic side effects,
- Improving the safety drug profile in, for example, pregnant patients [10].
1.2. Structure of Review
2. Polymers’ Role in Antimicrobial Therapy
2.1. Polymers Used in Localized Antimicrobial Therapy
2.2. Chitosan’s Antimicrobial Properties and Its Role in Localized Antimicrobial Therapy
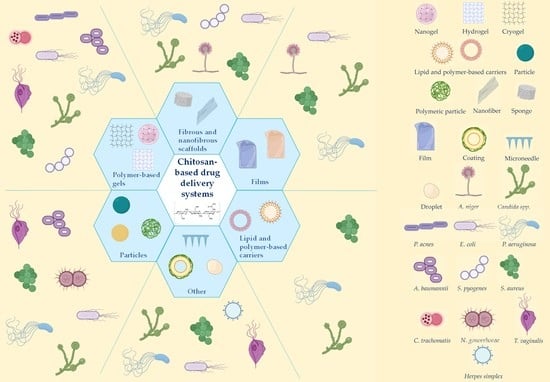
3. Chitosan-Based Drug Delivery Systems for Localized Antimicrobial Therapy
3.1. Particles and Carriers
3.2. Coating Material and Excipients
3.3. Polymer-Based Gels
3.4. Scaffolds
4. Role of Chitosan in Localized Therapy of Skin Infections
4.1. Common Skin Infections and Microorganisms
4.2. Challenges of Antimicrobial Treatment and Delivery to the Skin
4.3. Tackling the Challenges of Infected Skin—The Delivery Strategies, Systems, and Scaffolds
4.3.1. Particles and Carriers
4.3.2. Coating Material and Excipients
4.3.3. Polymer-Based Gels
4.3.4. Scaffolds
5. Chitosan and Vaginal Infections
5.1. Common Vaginal Infections
5.2. Challenges of Localized Therapy of Vaginal Infections
5.3. Antimicrobial Chitosan-Based Systems for Vaginal Application
5.3.1. Particles and Carriers
5.3.2. Coating Material and Excipients
5.3.3. Polymer-Based Vaginal Gels
5.3.4. Vaginal Films
6. Other Localized Antimicrobial Therapies
6.1. Ocular Infections
6.2. Buccal Treatment
6.3. Nasal Route
7. General Considerations
7.1. Toxicity and Irritation
7.2. Limitations
8. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.J.; Villapún, V.M.; Addison, O.; Webber, M.A.; Lowther, M.; Louth, S.E.T.; Mountcastle, S.E.; Brunet, M.Y.; Cox, S.C. A call for action to the biomaterial community to tackle antimicrobial resistance. Biomater. Sci. 2020, 8, 4951–4974. [Google Scholar] [CrossRef]
- Wan, M.-C.; Qin, W.; Lei, C.; Li, Q.-H.; Meng, M.; Fang, M.; Song, W.; Chen, J.-H.; Tay, F.; Niu, L.-N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Nanomedicines for the topical treatment of vulvovaginal infections: Addressing the challenges of antimicrobial resistance. Adv. Drug Deliv. Rev. 2021, 178, 113855. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [Green Version]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial Activities of Chitosan Derivatives. Pharmaceutics 2021, 13, 1639. [Google Scholar] [CrossRef]
- Felipe, V.; Breser, M.L.; Bohl, L.P.; Rodrigues da Silva, E.; Morgante, C.A.; Correa, S.G.; Porporatto, C. Chitosan disrupts biofilm formation and promotes biofilm eradication in Staphylococcus species isolated from bovine mastitis. Int. J. Biol. Macromol. 2019, 126, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of Chitosan Gels to Disrupt Bacterial Biofilms and Their Applications in the Treatment of Bacterial Vaginosis. J. Pharm. Sci. 2013, 102, 2096–2101. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Gomes Neto, R.J.; Genevro, G.M.; de Almeida Paulo, A.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Characterization and in vitro evaluation of chitosan/konjac glucomannan bilayer film as a wound dressing. Carbohydr. Polym. 2019, 212, 59–66. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, Y.-Y.; Wu, G.-J.; Huang, C.-H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M.; et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Minagawa, T.; Okamura, Y.; Shigemasa, Y.; Minami, S.; Okamoto, Y. Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing. Carbohydr. Polym. 2007, 67, 640–644. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Leal, E.C.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Chitosan-based dressings loaded with neurotensin—An efficient strategy to improve early diabetic wound healing. Acta Biomater. 2014, 10, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Argenziano, M.; Bressan, B.; Luganini, A.; Finesso, N.; Genova, T.; Troia, A.; Giribaldi, G.; Banche, G.; Mandras, N.; Cuffini, A.M.; et al. Comparative Evaluation of Different Chitosan Species and Derivatives as Candidate Biomaterials for Oxygen-Loaded Nanodroplet Formulations to Treat Chronic Wounds. Mar. Drugs 2021, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Julin, K.; Ahsan, L.; Basnet, P.; Johannessen, M.; Škalko-Basnet, N. Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar. Drugs 2021, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. A 2003, 64A, 551–559. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Hurler, J.; Škalko-Basnet, N. Potentials of chitosan-based delivery systems in wound therapy: Bioadhesion study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Jøraholmen, M.W.; Vanić, Ž.; Tho, I.; Škalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Cintia Alejandra Briones, N.; Mercedes, V.; Alicia Graciela, C.; Analía Irma, R.; José María, B. Chitosan Applications on Pharmaceutical Sciences: A Review. Drug Deliv. Lett. 2019, 9, 167–181. [Google Scholar] [CrossRef]
- Dias, C.; Rauter, A.P. Membrane-targeting antibiotics: Recent developments outside the peptide space. Future Med. Chem. 2019, 11, 211–228. [Google Scholar] [CrossRef]
- Machul, A.; Mikołajczyk, D.; Regiel-Futyra, A.; Heczko, P.B.; Strus, M.; Arruebo, M.; Stochel, G.; Kyzioł, A. Study on inhibitory activity of chitosan-based materials against biofilm producing Pseudomonas aeruginosa strains. J. Biomater. Appl. 2015, 30, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Qian, L.-H.; Xie, J. Effect of chitosan on membrane permeability and cell morphology of Pseudomonas aeruginosa and Staphyloccocus aureus. Carbohydr. Polym. 2011, 86, 969–974. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Schollmeyer, E. Anticandidal action of fungal chitosan against Candida albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef]
- Park, S.-C.; Nah, J.-W.; Park, Y. pH-dependent mode of antibacterial actions of low molecular weight water-soluble chitosan (LMWSC) against various pathogens. Macromol. Res. 2011, 19, 853–860. [Google Scholar] [CrossRef]
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chitosan Polymer as Bioactive Coating and Film against Aspergillus niger Contamination. J. Food Sci. 2005, 70, M100–M104. [Google Scholar] [CrossRef]
- Champer, J.; Patel, J.; Fernando, N.; Salehi, E.; Wong, V.; Kim, J. Chitosan against cutaneous pathogens. AMB Express 2013, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuero, R.G.; Osuji, G.; Washington, A. N-carboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett. 1991, 13, 441–444. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Zhu, Y.-H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Blume-Peytavi, U.; Vogt, A. Utilization of biodegradable polymeric materials as delivery agents in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ghaznavi-Rad, E.; Abtahi, H.; Sarlak, N. Antimicrobial Activity, Stability and Wound Healing Performances of Chitosan Nanoparticles Loaded Recombinant LL37 Antimicrobial Peptide. Int. J. Pept. Res. Ther. 2021, 27, 2505–2515. [Google Scholar] [CrossRef]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, characterisation and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Niamlang, P.; Tongrain, T.; Ekabutr, P.; Chuysinuan, P.; Supaphol, P. Preparation, characterization and biocompatibility of poly (vinyl alcohol) films containing tetracycline hydrochloride-loaded quaternized chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; Chaves, M.P.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Vaz, J.M.; Taketa, T.B.; Hernandez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. Sci. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, Y.; Cong, H.; Hu, H.; Xu, F.-J. An overview of chitosan and its application in infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Hydrogels as intrinsic antimicrobials. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 309–328. [Google Scholar]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Lotfipour, F.; Jelvehgari, M. A brief overview on nano-sized materials used in the topical treatment of skin and soft tissue bacterial infections. Expert Opin. Drug Deliv. 2019, 16, 1313–1331. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Lei, V.; Petty, A.J.; Atwater, A.R.; Wolfe, S.A.; MacLeod, A.S. Skin Viral Infections: Host Antiviral Innate Immunity and Viral Immune Evasion. Front. Immunol. 2020, 11, 593901. [Google Scholar] [CrossRef]
- O’Dell, M.L. Skin and Wound Infections: An Overview. Am. Fam. Physician 1998, 57, 2424–2432. [Google Scholar] [PubMed]
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016, 29, 109–115. [Google Scholar] [CrossRef]
- Leong, H.N.; Kurup, A.; Tan, M.Y.; Kwa, A.L.H.; Liau, K.H.; Wilcox, M.H. Management of complicated skin and soft tissue infections with a special focus on the role of newer antibiotics. Infect. Drug Resist. 2018, 11, 1959–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The Insights of Microbes’ Roles in Wound Healing: A Comprehensive Review. Pharmaceutics 2021, 13, 981. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M. Epidemiology of superficial fungal infections. Clin. Dermatol. 2010, 28, 197–201. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Dowd, S.E.; Delton Hanson, J.; Rees, E.; Wolcott, R.D.; Zischau, A.M.; Sun, Y.; White, J.; Smith, D.M.; Kennedy, J.; Jones, C.E. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J. Wound Care 2011, 20, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. The increasing problem of treatment-resistant fungal infections: A call for antifungal stewardship programs. Int. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Thandi, C.S.; Whittam, L. Diagnosis and management of common viral skin infections. Prescriber 2021, 32, 10–14. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Park, K.C.; Han, W.S. Viral Skin Infections. Drugs 2002, 62, 479–490. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Gajula, B.; Munnamgi, S.; Basu, S. How bacterial biofilms affect chronic wound healing: A narrative review. Int. J. Surg. Glob. Health 2020, 3, e16. [Google Scholar] [CrossRef]
- Tipton, C.D.; Wolcott, R.D.; Sanford, N.E.; Miller, C.; Pathak, G.; Silzer, T.K.; Sun, J.; Fleming, D.; Rumbaugh, K.P.; Little, T.D.; et al. Patient genetics is linked to chronic wound microbiome composition and healing. PLoS Pathog. 2020, 16, e1008511. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Kaiser, P.; Wächter, J.; Windbergs, M. Therapy of infected wounds: Overcoming clinical challenges by advanced drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Gariazzo, L.; Cioni, M.; Trave, I.; Parodi, A. The microbiome and its relevance in complex wounds. Eur. J. Dermatol. 2019, 29, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-K.; Cheng, N.-C.; Cheng, C.-M. Biofilms in Chronic Wounds: Pathogenesis and Diagnosis. Trends Biotechnol. 2019, 37, 505–517. [Google Scholar] [CrossRef]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Wang, M.; Khan, I.M.; Xu, J.; Peng, C.; Wang, Z. Preparation, characterization, and antibiofilm activity of cinnamic acid conjugated hydroxypropyl chitosan derivatives. Int. J. Biol. Macromol. 2021, 189, 657–667. [Google Scholar] [CrossRef]
- Raff, A.B.; Kroshinsky, D. Cellulitis: A Review. JAMA 2016, 316, 325–337. [Google Scholar] [CrossRef]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef] [Green Version]
- Lam, P.L.; Lee, K.K.H.; Wong, R.S.M.; Cheng, G.Y.M.; Bian, Z.X.; Chui, C.H.; Gambari, R. Recent advances on topical antimicrobials for skin and soft tissue infections and their safety concerns. Crit. Rev. Microbiol. 2018, 44, 40–78. [Google Scholar] [CrossRef]
- Martinez, N. Skin and Soft-Tissue Infections: It’s More Than Just Skin Deep. Adv. Emerg. Nurs. J. 2020, 42, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wiraja, C.; Chew, S.W.T.; Xu, C. Nanodelivery Systems for Topical Management of Skin Disorders. Mol. Pharm. 2021, 18, 491–505. [Google Scholar] [CrossRef]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the Skin Permeation Barrier: Challenges and Opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Badilli, U.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Chapter 9—Lipid-based nanoparticles for dermal drug delivery. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 369–413. [Google Scholar]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Maciel Tabosa, M.A.; Hoppel, M.; Bunge, A.L.; Guy, R.H.; Delgado-Charro, M.B. Predicting topical drug clearance from the skin. Drug Deliv. Transl. Res. 2021, 11, 729–740. [Google Scholar] [CrossRef]
- Alnasif, N.; Zoschke, C.; Fleige, E.; Brodwolf, R.; Boreham, A.; Rühl, E.; Eckl, K.-M.; Merk, H.-F.; Hennies, H.C.; Alexiev, U.; et al. Penetration of normal, damaged and diseased skin—An in vitro study on dendritic core–multishell nanotransporters. J. Control. Release 2014, 185, 45–50. [Google Scholar] [CrossRef]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical Antimicrobial Therapy for Treating Chronic Wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Basnet, P.; Škalko-Basnet, N. Nanodelivery systems for improved topical antimicrobial therapy. Curr. Pharm. Des. 2013, 19, 7237–7243. [Google Scholar] [CrossRef]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef] [Green Version]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Alberti, T.; Coelho, D.S.; Voytena, A.; Pitz, H.; de Pra, M.; Mazzarino, L.; Kuhnen, S.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Nanotechnology: A Promising Tool Towards Wound Healing. Curr. Pharm. Des. 2017, 23, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Veiga, M.; Tavaria, F.K.; Pintado, M.M. Exploring chitosan nanoparticles as effective inhibitors of antibiotic resistant skin microorganisms—From in vitro to ex vitro testing. Carbohydr. Polym. 2018, 201, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Ktari, N.; Ben Salah, R.; Boufi, S.; Debeaufort, F.; Nasri, M. Development of Nanocomposite Films Based on Chitosan and Gelatin Loaded with Chitosan-Tripolyphosphate Nanoparticles: Antioxidant Potentials and Applications in Wound Healing. J. Polym. Environ. 2021, 1–22. [Google Scholar] [CrossRef]
- Vila-Sanjurjo, C.; Hembach, L.; Netzer, J.; Remuñán-López, C.; Vila-Sanjurjo, A.; Goycoolea, F.M. Covalently and ionically, dually crosslinked chitosan nanoparticles block quorum sensing and affect bacterial cell growth on a cell-density dependent manner. J. Colloid Interface Sci. 2020, 578, 171–183. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanić, Ž.; Škalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.M.; Mohd Amin, M.C.; Ng, S.-F.; Katas, H.; Shah, S.U.; Khan, N.R. Formulation and Evaluation of Microwave-Modified Chitosan-Curcumin Nanoparticles—A Promising Nanomaterials Platform for Skin Tissue Regeneration Applications Following Burn Wounds. Polymers 2020, 12, 2608. [Google Scholar] [CrossRef] [PubMed]
- Fras Zemljič, L.; Maver, U.; Kraševac Glaser, T.; Bren, U.; Knez Hrnčič, M.; Petek, G.; Peršin, Z. Electrospun Composite Nanofibrous Materials Based on (Poly)-Phenol-Polysaccharide Formulations for Potential Wound Treatment. Materials 2020, 13, 2631. [Google Scholar] [CrossRef]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yuen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE 2017, 12, e0174888. [Google Scholar] [CrossRef] [Green Version]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Rozman, N.A.S.; Tong, W.Y.; Leong, C.R.; Anuar, M.R.; Karim, S.; Ong, S.K.; Yusof, F.A.M.; Tan, W.-N.; Sulaiman, B.; Ooi, M.L.; et al. Homalomena pineodora essential oil nanoparticle inhibits diabetic wound pathogens. Sci. Rep. 2020, 10, 3307. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Ghanem, R.A.; Al-Saggaf, M.S.; Elebeedy, D.; Abd El Maksoud, A.I. Application of Fish Collagen-Nanochitosan-Henna Extract Composites for the Control of Skin Pathogens and Accelerating Wound Healing. Int. J. Polym. Sci. 2021, 2021, 1907914. [Google Scholar] [CrossRef]
- Aramwit, P.; Yamdech, R.; Ampawong, S. Controlled Release of Chitosan and Sericin from the Microspheres-Embedded Wound Dressing for the Prolonged Anti-microbial and Wound Healing Efficacy. AAPS J. 2016, 18, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Zhan, B.; Zhang, W.; Qin, D.; Xia, G.; Zhang, H.; Peng, M.; Li, S.-A.; Zhang, Y.; Gao, Y.; et al. Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing. Int. J. Nanomed. 2018, 13, 5771–5786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Pourhajibagher, M.; Hosseini, N.; Boluki, E.; Chiniforush, N.; Bahador, A. Photoelimination Potential of Chitosan Nanoparticles-Indocyanine Green Complex Against the Biological Activities of Acinetobacter baumannii Strains: A Preliminary In Vitro Study in Burn Wound Infections. J. Lasers Med. Sci. 2020, 11, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.M.; Silva, S.; Vicente, S.; Neto, C.; Castro, P.M.; Veiga, M.; Madureira, R.; Tavaria, F.; Pintado, M.M. Chitosan nanoparticles as alternative anti-staphylococci agents: Bactericidal, antibiofilm and antiadhesive effects. Mater. Sci. Eng. C 2017, 79, 221–226. [Google Scholar] [CrossRef]
- Marangon, C.A.; Martins, V.C.A.; Ling, M.H.; Melo, C.C.; Plepis, A.M.G.; Meyer, R.L.; Nitschke, M. Combination of Rhamnolipid and Chitosan in Nanoparticles Boosts Their Antimicrobial Efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Mocktar, C.; Govender, T. Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2020, 147, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, C.; Yu, X.; Su, W.; Yuan, Z. Chitosan/zinc nitrate microneedles for bacterial biofilm eradication. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 911–920. [Google Scholar] [CrossRef]
- Yi, X.; Wang, C.; Yu, X.; Yuan, Z. A novel bacterial biofilms eradication strategy based on the microneedles with antibacterial properties. Procedia CIRP 2020, 89, 159–163. [Google Scholar] [CrossRef]
- Frade, M.L.; De Annunzio, S.R.; Calixto, G.M.; Victorelli, F.D.; Chorilli, M.; Fontana, C.R. Assessment of Chitosan-Based Hydrogel and Photodynamic Inactivation against Propionibacterium acnes. Molecules 2018, 23, 473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artunduaga Bonilla, J.J.; Honorato, L.; Cordeiro de Oliveira, D.F.; Araújo Gonçalves, R.; Guimarães, A.; Miranda, K.; Nimrichter, L. Silver chitosan nanocomposites as a potential treatment for superficial candidiasis. Med. Mycol. 2021, 59, 993–1005. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2020, 147, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P.; Hsieh, C.-M.; Tsai, T.; Yang, J.-C.; Chen, C.-T. Optimization and Evaluation of a Chitosan/Hydroxypropyl Methylcellulose Hydrogel Containing Toluidine Blue O for Antimicrobial Photodynamic Inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [Green Version]
- Neff, J.A.; Bayramov, D.F.; Patel, E.A.; Miao, J. Novel Antimicrobial Peptides Formulated in Chitosan Matrices are Effective Against Biofilms of Multidrug-Resistant Wound Pathogens. Mil. Med. 2020, 185, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Pati, B.A.; Kurata, W.E.; Horseman, T.S.; Pierce, L.M. Antibiofilm activity of chitosan/epsilon-poly-L-lysine hydrogels in a porcine ex vivo skin wound polymicrobial biofilm model. Wound Repair Regen. 2021, 29, 316–326. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C 2016, 60, 317–323. [Google Scholar] [CrossRef]
- Huber, D.; Tegl, G.; Mensah, A.; Beer, B.; Baumann, M.; Borth, N.; Sygmund, C.; Ludwig, R.; Guebitz, G.M. A Dual-Enzyme Hydrogen Peroxide Generation Machinery in Hydrogels Supports Antimicrobial Wound Treatment. ACS Appl. Mater. Interfaces 2017, 9, 15307–15316. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical Properties and Anti-Biofilm Activity of Chitosan-Immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Harrison, Z.L.; Bumgardner, J.D.; Fujiwara, T.; Baker, D.L.; Jennings, J.A. In vitro evaluation of loaded chitosan membranes for pain relief and infection prevention. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym. 2017, 158, 11–19. [Google Scholar] [CrossRef]
- Gil, J.; Natesan, S.; Li, J.; Valdes, J.; Harding, A.; Solis, M.; Davis, S.C.; Christy, R.J. A PEGylated fibrin hydrogel-based antimicrobial wound dressing controls infection without impeding wound healing. Int. Wound J. 2017, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Natesan, S.; Stowers, R.S.; Mullens, C.; Baer, D.G.; Suggs, L.J.; Christy, R.J. A PEGylated fibrin-based wound dressing with antimicrobial and angiogenic activity. Acta Biomater. 2011, 7, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Cerchiara, T.; Abruzzo, A.; Ñahui Palomino, R.A.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F.; et al. Spanish Broom (Spartium junceum L.) fibers impregnated with vancomycin-loaded chitosan nanoparticles as new antibacterial wound dressing: Preparation, characterization and antibacterial activity. Eur. J. Pharm. Sci. 2017, 99, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Doostan, M.; Maleki, H.; Doostan, M.; Khoshnevisan, K.; Faridi-Majidi, R.; Arkan, E. Effective antibacterial electrospun cellulose acetate nanofibrous patches containing chitosan/erythromycin nanoparticles. Int. J. Biol. Macromol. 2021, 168, 464–473. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmed, N.; Qindeel, M.; Asad, M.I.; Khan, G.M.; ur.Rehman, A. Development of novel biopolymer-based nanoparticles loaded cream for potential treatment of topical fungal infections. Drug Dev. Ind. Pharm. 2021, 1–10. [Google Scholar] [CrossRef]
- Costa-Fernandez, S.; Matos, J.K.R.; Scheunemann, G.S.; Salata, G.C.; Chorilli, M.; Watanabe, I.-S.; de Araujo, G.L.B.; Santos, M.F.; Ishida, K.; Lopes, L.B. Nanostructured lipid carriers containing chitosan or sodium alginate for co-encapsulation of antioxidants and an antimicrobial agent for potential application in wound healing. Int. J. Biol. Macromol. 2021, 183, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Prato, M.; Magnetto, C.; Allizond, V.; Giribaldi, G.; Argenziano, M.; Khadjavi, A.; Gulino, G.R.; Finesso, N.; Mandras, N.; et al. Antimicrobial chitosan nanodroplets: New insights for ultrasound-mediated adjuvant treatment of skin infection. Future Microbiol. 2015, 10, 929–939. [Google Scholar] [CrossRef]
- Chhabra, P.; Chauhan, G.; Kumar, A. Augmented healing of full thickness chronic excision wound by rosmarinic acid loaded chitosan encapsulated graphene nanopockets. Drug Dev. Ind. Pharm. 2020, 46, 878–888. [Google Scholar] [CrossRef]
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly(vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241. [Google Scholar] [CrossRef]
- Zan, P.; Than, A.; Duong, P.K.; Song, J.; Xu, C.; Chen, P. Antimicrobial Microneedle Patch for Treating Deep Cutaneous Fungal Infection. Adv. Ther. 2019, 2, 1900064. [Google Scholar] [CrossRef]
- Permana, A.D.; Anjani, Q.K.; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; Donnelly, R.F. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C 2021, 120, 111786. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wei, D.; Du, M.; Ming, S.; Ding, Q.; Tan, R. Targeting Antibacterial Effect and Promoting of Skin Wound Healing After Infected with Methicillin-Resistant Staphylococcus aureus for the Novel Polyvinyl Alcohol Nanoparticles. Int. J. Nanomed. 2021, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Daghian, S.G.; Farahpour, M.R.; Jafarirad, S. Biological fabrication and electrostatic attractions of new layered silver/talc nanocomposite using Lawsonia inermis L. and its chitosan-capped inorganic/organic hybrid: Investigation on acceleration of Staphylococcus aureus and Pseudomonas aeruginosa infected wound healing. Mater. Sci. Eng. C 2021, 128, 112294. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound healing applications of sericin/chitosan-capped silver nanoparticles incorporated hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; Amalraj, J. Chitosan capped copper oxide/copper nanoparticles encapsulated microbial resistant nanocomposite films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.; AbouSamra, M.M.; Awad, G.A.; Mansy, S.S. A potential antibacterial wound dressing of cefadroxil chitosan nanoparticles in situ gel: Fabrication, in vitro optimization and in vivo evaluation. Int. J. Pharm. 2018, 544, 129–140. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surf. B 2017, 158, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Gao, X.; Shi, Y.; Cheng, C.; Shi, Z.; Jiao, M.; Cao, F.; Xu, Z.; Li, X.; Zhang, J. Augmented Graphene Quantum Dot-Light Irradiation Therapy for Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2020, 12, 40153–40162. [Google Scholar] [CrossRef]
- AbdelSamie, S.M.; Kamel, A.O.; Sammour, O.A.; Ibrahim, S.M. Terbinafine hydrochloride nanovesicular gel: In vitro characterization, ex vivo permeation and clinical investigation. Eur. J. Pharm. Sci. 2016, 88, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, L.; Yu, Q.; Chen, Y.; Liu, Y. Polysaccharide-Based Supramolecular Hydrogel for Efficiently Treating Bacterial Infection and Enhancing Wound Healing. Biomacromolecules 2021, 22, 534–539. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, 2001591. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; Sun, T.; et al. Evaluation of genipin-crosslinked chitosan hydrogels as a potential carrier for silver sulfadiazine nanocrystals. Colloids Surf. B 2016, 148, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Thattaruparambil Raveendran, N.; Mohandas, A.; Ramachandran Menon, R.; Somasekharan Menon, A.; Biswas, R.; Jayakumar, R. Ciprofloxacin- and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Appl. Bio Mater. 2019, 2, 243–254. [Google Scholar] [CrossRef]
- Fasiku, V.O.; Omolo, C.A.; Devnarain, N.; Ibrahim, U.H.; Rambharose, S.; Faya, M.; Mocktar, C.; Singh, S.D.; Govender, T. Chitosan-Based Hydrogel for the Dual Delivery of Antimicrobial Agents against Bacterial Methicillin-Resistant Staphylococcus aureus Biofilm-Infected Wounds. ACS Omega 2021, 6, 21994–22010. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.-W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Kim, E.; Lei, M.; Dai, K.; Tan, X.; Xu, M.; Li, J.; Liu, Y.; Shi, X.; et al. Bio-inspired redox-cycling antimicrobial film for sustained generation of reactive oxygen species. Biomaterials 2018, 162, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, H.; Zhang, C.; Lei, Y.; Lei, M.; Xu, M.; Jin, D.; Li, P.; Yin, M.; Payne, G.F.; et al. Electrofabrication of functional materials: Chloramine-based antimicrobial film for infectious wound treatment. Acta Biomater. 2018, 73, 190–203. [Google Scholar] [CrossRef]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Schoung, J.-Y.; Huang, Y.-B.; Tsai, Y.-H.; Hao, J.-Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, S.; Pang, L.; Ma, J.; Liu, Y.; Du, L.; Wu, Y.; Jin, Y. ICG-loaded photodynamic chitosan/polyvinyl alcohol composite nanofibers: Anti-resistant bacterial effect and improved healing of infected wounds. Int. J. Pharm. 2020, 588, 119797. [Google Scholar] [CrossRef]
- Shi, L.; Lin, F.; Zhou, M.; Li, Y.; Li, W.; Shan, G.; Xu, Y.; Xu, J.; Yang, J. Preparation of biocompatible wound dressings with dual release of antibiotic and platelet-rich plasma for enhancing infected wound healing. J. Biomater. Appl. 2021, 36, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, Z.; Li, M.; Liu, L.; Luo, H.; Wang, P.; Zhang, D.; Yang, X.; Zhou, K.; Lei, S. Graphene Oxide/Copper Nanoderivatives-Modified Chitosan/Hyaluronic Acid Dressings for Facilitating Wound Healing in Infected Full-Thickness Skin Defects. Int. J. Nanomed. 2020, 15, 8231–8247. [Google Scholar] [CrossRef]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr. Polym. 2020, 227, 115296. [Google Scholar] [CrossRef] [PubMed]
- Alshamsan, A.; Aleanizy, F.S.; Badran, M.; Alqahtani, F.Y.; Alfassam, H.; Almalik, A.; Alosaimy, S. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J. Microbiol. Methods 2019, 156, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Kesavan, K. Effect of chitosan coating on microemulsion for effective dermal clotrimazole delivery. Pharm. Dev. Technol. 2017, 22, 617–626. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Aguzzi, C.; Mori, M.; Grisoli, P.; Cerezo, P.; Tenci, M.; Viseras, C.; et al. Montmorillonite–chitosan–silver sulfadiazine nanocomposites for topical treatment of chronic skin lesions: In vitro biocompatibility, antibacterial efficacy and gap closure cell motility properties. Carbohydr. Polym. 2014, 102, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xi, Z.; Wu, F.; Song, S.; Huang, X.; Chu, X.; Wang, Z.; Wang, Y.; Zhang, Q.; Meng, N.; et al. Quaternized Chitosan-Coated Montmorillonite Interior Antimicrobial Metal–Antibiotic In Situ Coordination Complexation for Mixed Infections of Wounds. Langmuir 2019, 35, 15275–15286. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.F.; Ribeiro, C.A.; Leyva, M.E.; Marques, P.S.; Soares, C.R.J.; Alencar de Queiroz, A.A. Biophysical properties of electrospun chitosan-grafted poly(lactic acid) nanofibrous scaffolds loaded with chondroitin sulfate and silver nanoparticles. J. Biomater. Appl. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Wu, G.; Yi, Y.; Huang, M.; Liu, R.; Shi, X.; Deng, H. Layer-by-layer immobilization of amphoteric carboxymethyl chitosan onto biocompatible silk fibroin nanofibrous mats. Carbohydr. Polym. 2019, 210, 9–16. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef]
- Grimling, B.; Karolewicz, B.; Nawrot, U.; Włodarczyk, K.; Górniak, A. Physicochemical and Antifungal Properties of Clotrimazole in Combination with High-Molecular Weight Chitosan as a Multifunctional Excipient. Mar. Drugs 2020, 18, 591. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bölgen, N.; Demir, D.; Yalçın, M.S.; Özdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Liu, Y.; Feng, F.; Zhou, J.; Feng, X.; Fan, Y. Polysaccharide-Peptide Cryogels for Multidrug-Resistant-Bacteria Infected Wound Healing and Hemostasis. Adv. Healthc. Mater. 2020, 9, 1901041. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; García-González, C.A. Jet Cutting Technique for the Production of Chitosan Aerogel Microparticles Loaded with Vancomycin. Polymers 2020, 12, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Ouyang, Q.-Q.; Hu, Z.; Lin, Z.-P.; Quan, W.-Y.; Deng, Y.-F.; Li, S.-D.; Li, P.-W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [Green Version]
- Özcan, İ.; Abacı, Ö.; Uztan, A.H.; Aksu, B.; Boyacıoğlu, H.; Güneri, T.; Özer, Ö. Enhanced Topical Delivery of Terbinafine Hydrochloride with Chitosan Hydrogels. AAPS PharmSciTech 2009, 10, 1024. [Google Scholar] [CrossRef] [Green Version]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Padgett, M.L.; Llamas, C.B.; Barnett, H.H.; Bunnell, B.A.; Caldorera-Moore, M.E. Development of Responsive Chitosan–Genipin Hydrogels for the Treatment of Wounds. ACS Appl. Bio Mater. 2019, 2, 2879–2888. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial peptides-loaded smart chitosan hydrogel: Release behavior and antibacterial potential against antibiotic resistant clinical isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, L.M.; Giordani, B.; Pettersen, A.K.; Vitali, B.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydr. Polym. 2021, 262, 117939. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, S.; Haeri, A.; Mahboubi, A.; Mortazavi, A.; Dadashzadeh, S. Chitosan gel-embedded moxifloxacin niosomes: An efficient antimicrobial hybrid system for burn infection. Int. J. Biol. Macromol. 2016, 85, 625–633. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Pereira dos Santos, E.; Nicácio, P.H.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/Essential Oils Formulations for Potential Use as Wound Dressing: Physical and Antimicrobial Properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [Green Version]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [Green Version]
- Akyuz, L.; Kaya, M.; Mujtaba, M.; Ilk, S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Cakmak, Y.S.; Islek, C. Supplementing capsaicin with chitosan-based films enhanced the anti-quorum sensing, antimicrobial, antioxidant, transparency, elasticity and hydrophobicity. Int. J. Biol. Macromol. 2018, 115, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, V.; Rajarajeswari, G.R. In vivo wound healing efficiency of curcumin-incorporated pectin-chitosan biodegradable films. New J. Chem. 2021, 45, 17918–17929. [Google Scholar] [CrossRef]
- Kausar, R.; Khan, A.-U.; Jamil, B.; Shahzad, Y.; Ul-Haq, I. Development and pharmacological evaluation of vancomycin loaded chitosan films. Carbohydr. Polym. 2021, 256, 117565. [Google Scholar] [CrossRef]
- Bavarsad, N.; Kouchak, M.; Mohamadipour, P.; Sadeghi-Nejad, B. Preparation and physicochemical characterization of topical chitosan-based film containing griseofulvin-loaded liposomes. J. Adv. Pharm. Technol. Res. 2016, 7, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Ambrogi, V.; Pietrella, D.; Nocchetti, M.; Casagrande, S.; Moretti, V.; De Marco, S.; Ricci, M. Montmorillonite–chitosan–chlorhexidine composite films with antibiofilm activity and improved cytotoxicity for wound dressing. J. Colloid Interface Sci. 2017, 491, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hissae Yassue-Cordeiro, P.; Henrique Zandonai, C.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Luisa Garcia, M.; Regina Camargo Fernandes-Machado, N.; Severino, P.; Souto, E.B.; Ferreira da Silva, C. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.-S.; Huang, T.-B. Nanocomposites of Genipin-Crosslinked Chitosan/Silver Nanoparticles—Structural Reinforcement and Antimicrobial Properties. Macromol. Biosci. 2008, 8, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. Fabrication, Characterization of Chitosan/Nanosilver Film and Its Potential Antibacterial Application. J. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2214. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindra, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; MohanaRaju, K. Fabrication of porous chitosan films impregnated with silver nanoparticles: A facile approach for superior antibacterial application. Colloids Surf. B 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Pansara, C.; Mishra, R.; Mehta, T.; Parikh, A.; Garg, S. Formulation of Chitosan Stabilized Silver Nanoparticle-Containing Wound Healing Film: In Vitro and In Vivo Characterization. J. Pharm. Sci. 2020, 109, 2196–2205. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Pan, S.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Qi, Z. Evaluation of New Film Based on Chitosan/Gold Nanocomposites on Antibacterial Property and Wound-Healing Efficacy. Adv. Mater. Sci. Eng. 2020, 2020, 6212540. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Desoky, W.M.; Hussein, E.M.; El-Shaer, N.H.; Gomaa, M.; Gamal, A.A.; Esawy, M.A.; Guirguis, O.W. Biological applications study of bio-nanocomposites based on chitosan/TiO2 nanoparticles polymeric films modified by oleic acid. J. Biomed. Mater. Res. A 2021, 109, 232–247. [Google Scholar] [CrossRef]
- Foster, L.J.R.; Butt, J. Chitosan films are NOT antimicrobial. Biotechnol. Lett. 2011, 33, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gómez Chabala, L.F.; Cuartas, C.E.; López, M.E. Release Behavior and Antibacterial Activity of Chitosan/Alginate Blends with Aloe vera and Silver Nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [Green Version]
- Bueno, C.Z.; Moraes, Â.M. Influence of the incorporation of the antimicrobial agent polyhexamethylene biguanide on the properties of dense and porous chitosan-alginate membranes. Mater. Sci. Eng. C 2018, 93, 671–678. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, X.; Li, M.; Lu, Y.; Ai, C.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antifungal activity of silver nanoparticles synthesized by iturin against Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 2021, 105, 3759–3770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, X.; Li, M.; Yan, L.; Lu, Y.; Jiang, C.; Liu, Y.; Pan, Z.; Shi, J. Antibacterial and wound healing–promoting effect of sponge-like chitosan-loaded silver nanoparticles biosynthesized by iturin. Int. J. Biol. Macromol. 2021, 181, 1183–1195. [Google Scholar] [CrossRef]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Profire, L.; Nita, L.E.; Dragostin, O.M.; Ghetu, N.; Pieptu, D.; Vasile, C. Sulfadiazine—Chitosan Conjugates and Their Polyelectrolyte Complexes with Hyaluronate Destined to the Management of Burn Wounds. Materials 2015, 8, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Burkatovskaya, M.; Castano, A.P.; Demidova-Rice, T.N.; Tegos, G.P.; Hamblin, M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008, 16, 425–431. [Google Scholar] [CrossRef]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Tegos, G.P.; Burkatovskaya, M.; Castano, A.P.; Hamblin, M.R. Chitosan Acetate Bandage as a Topical Antimicrobial Dressing for Infected Burns. Antimicrob. Agents Chemother. 2009, 53, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Kalalinia, F.; Taherzadeh, Z.; Jirofti, N.; Amiri, N.; Foroghinia, N.; Beheshti, M.; Bazzaz, B.S.F.; Hashemi, M.; Shahroodi, A.; Pishavar, E.; et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int. J. Biol. Macromol. 2021, 177, 100–110. [Google Scholar] [CrossRef]
- Faccendini, A.; Ruggeri, M.; Miele, D.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Vigani, B.; Sandri, G.; et al. Norfloxacin-Loaded Electrospun Scaffolds: Montmorillonite Nanocomposite vs. Free Drug. Pharmaceutics 2020, 12, 325. [Google Scholar] [CrossRef] [Green Version]
- Khosravimelal, S.; Chizari, M.; Farhadihosseinabadi, B.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. J. Mater. Sci. Mater. Med. 2021, 32, 114. [Google Scholar] [CrossRef]
- Bösiger, P.; Tegl, G.; Richard, I.M.T.; Le Gat, L.; Huber, L.; Stagl, V.; Mensah, A.; Guebitz, G.M.; Rossi, R.M.; Fortunato, G. Enzyme functionalized electrospun chitosan mats for antimicrobial treatment. Carbohydr. Polym. 2018, 181, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial activity of chitosan nanofiber meshes with liposomes immobilized releasing gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Gosecka, M.; Gosecki, M. Antimicrobial Polymer-Based Hydrogels for the Intravaginal Therapies—Engineering Considerations. Pharmaceutics 2021, 13, 1393. [Google Scholar] [CrossRef] [PubMed]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef] [Green Version]
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int. J. Pharm. 2021, 598, 120375. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic vaginitis: No longer a stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef] [PubMed]
- Malli, S.; Bories, C.; Bourge, M.; Loiseau, P.M.; Bouchemal, K. Surface-dependent endocytosis of poly(isobutylcyanoacrylate) nanoparticles by Trichomonas vaginalis. Int. J. Pharm. 2018, 548, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Pradines, B.; Bories, C.; Vauthier, C.; Ponchel, G.; Loiseau, P.M.; Bouchemal, K. Drug-Free Chitosan Coated Poly(isobutylcyanoacrylate) Nanoparticles Are Active against Trichomonas vaginalis and Non-Toxic towards Pig Vaginal Mucosa. Pharm. Res. 2015, 32, 1229–1236. [Google Scholar] [CrossRef]
- Unemo, M.; Seifert, H.S.; Hook, E.W.; Hawkes, S.; Ndowa, F.; Dillon, J.-A.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef]
- Cole, M.J.; Tan, W.; Fifer, H.; Brittain, C.; Duley, L.; Hepburn, T.; Lawrence, T.; Montgomery, A.A.; Sprange, K.; Thandi, S.; et al. Gentamicin, azithromycin and ceftriaxone in the treatment of gonorrhoea: The relationship between antibiotic MIC and clinical outcome. J. Antimicrob. Chemother. 2019, 75, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Durukan, D.; Read, T.R.H.; Murray, G.; Doyle, M.; Chow, E.P.F.; Vodstrcil, L.A.; Fairley, C.K.; Aguirre, I.; Mokany, E.; Tan, L.Y.; et al. Resistance-Guided Antimicrobial Therapy Using Doxycycline–Moxifloxacin and Doxycycline–2.5 g Azithromycin for the Treatment of Mycoplasma genitalium Infection: Efficacy and Tolerability. Clin. Infect. Dis. 2019, 71, 1461–1468. [Google Scholar] [CrossRef]
- Unemo, M.; Jensen, J.S. Antimicrobial-resistant sexually transmitted infections: Gonorrhoea and Mycoplasma genitalium. Nat. Rev. Urol. 2017, 14, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, K.M.; Rockey, D.D. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef] [Green Version]
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gallarate, M. Recent Advances in Nanosystems and Strategies for Vaginal Delivery of Antimicrobials. Nanomaterials 2021, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Broad, C.E.; Furegato, M.; Harrison, M.A.; Pond, M.J.; Tan, N.; Okala, S.; Fuller, S.S.; Harding-Esch, E.M.; Sadiq, S.T. High prevalence of coinfection of azithromycin-resistant Mycoplasma genitalium with other STIs: A prospective observational study of London-based symptomatic and STI-contact clinic attendees. Sex. Transm. Infect. 2021, 97, 63–68. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J.; et al. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2021, 13, 26. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinatto, W.M.; Galante, J.; Mesquita, L.; Silva, D.S.; Martins dos Santos, D.; Moraes, T.B.; Campana-Filho, S.P.; Colnago, L.A.; Sarmento, B.; das Neves, J. Clotrimazole-loaded N-(2-hydroxy)-propyl-3-trimethylammonium, O-palmitoyl chitosan nanoparticles for topical treatment of vulvovaginal candidiasis. Acta Biomater. 2021, 125, 312–321. [Google Scholar] [CrossRef]
- Arumugam, G.; Rajendran, R. Callophycin A loaded chitosan and spicules based nanocomposites as an alternative strategy to overcome vaginal candidiasis. Int. J. Biol. Macromol. 2020, 161, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.E.; de Oliveira, A.A.; Cabral, M.d.S.; Costa, A.F.; Silva, B.C.; do Carmo Silva, L.; de Menezes, L.B.; de Almeida Soares, C.M.; Amaral, A.C.; Pereira, M. Investigation of thiosemicarbazide free or within chitosan nanoparticles in a murine model of vulvovaginal candidiasis. Braz. J. Microbiol. 2020, 51, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.C.; Saavedra, P.H.V.; Oliveira Souza, A.C.; de Melo, M.T.; Tedesco, A.C.; Morais, P.C.; Soares Felipe, M.S.; Bocca, A.L. Miconazole loaded chitosan-based nanoparticles for local treatment of vulvovaginal candidiasis fungal infections. Colloids Surf. B 2019, 174, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Costa, A.; Evangelista Araujo, D.; Santos Cabral, M.; Teles Brito, I.; Borges de Menezes Leite, L.; Pereira, M.; Correa Amaral, A. Development, characterization, and in vitro–in vivo evaluation of polymeric nanoparticles containing miconazole and farnesol for treatment of vulvovaginal candidiasis. Med. Mycol. 2018, 57, 52–62. [Google Scholar] [CrossRef]
- Moreno, M.A.; Gómez-Mascaraque, L.G.; Arias, M.; Zampini, I.C.; Sayago, J.E.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; López-Rubio, A.; Isla, M.I. Electrosprayed chitosan microcapsules as delivery vehicles for vaginal phytoformulations. Carbohydr. Polym. 2018, 201, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Cover, N.F.; Lai-Yuen, S.; Parsons, A.K.; Kumar, A. Synergetic effects of doxycycline-loaded chitosan nanoparticles for improving drug delivery and efficacy. Int. J. Nanomed. 2012, 7, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Maestrelli, F.; Jug, M.; Cirri, M.; Kosalec, I.; Mura, P. Characterization and microbiological evaluation of chitosan-alginate microspheres for cefixime vaginal administration. Carbohydr. Polym. 2018, 192, 176–183. [Google Scholar] [CrossRef]
- Elmi, T.; Rahimi Esboei, B.; Sadeghi, F.; Zamani, Z.; Didehdar, M.; Fakhar, M.; Chabra, A.; Hajialiani, F.; Namazi, M.J.; Tabatabaie, F. In Vitro Antiprotozoal Effects of Nano-chitosan on Plasmodium falciparum, Giardia lamblia and Trichomonas vaginalis. Acta Parasitol. 2021, 66, 39–52. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Sreekumar, S.; Svetaz, L.A.; Lamas, M.C.; Moerschbacher, B.M.; Leonardi, D. Design and Characterization of Chitosan Nanoformulations for the Delivery of Antifungal Agents. Int. J. Mol. Sci. 2019, 20, 3686. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Pérez, B.; Quintanar-Guerrero, D.; Tapia-Tapia, M.; Cisneros-Tamayo, R.; Zambrano-Zaragoza, M.L.; Alcalá-Alcalá, S.; Mendoza-Muñoz, N.; Piñón-Segundo, E. Controlled-release biodegradable nanoparticles: From preparation to vaginal applications. Eur. J. Pharm. Sci. 2018, 115, 185–195. [Google Scholar] [CrossRef]
- Oliveira, D.A.J.; Amaral, J.G.; Garcia, L.B.; dos Santos, M.S.; Silva, L.A.O.; Almeida, M.P.; Gomes, A.F.; Barros, D.R.P.; Lopes, N.P.; Pereira, G.R.; et al. Associating chitosan and microemulsion as a topical vehicle for the administration of herbal medicines. Carbohydr. Polym. 2021, 255, 117482. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Grisin, T.; Bories, C.; Bombardi, M.; Loiseau, P.M.; Rouffiac, V.; Solgadi, A.; Mallet, J.-M.; Ponchel, G.; Bouchemal, K. Supramolecular Chitosan Micro-Platelets Synergistically Enhance Anti-Candida albicans Activity of Amphotericin B Using an Immunocompetent Murine Model. Pharm. Res. 2017, 34, 1067–1082. [Google Scholar] [CrossRef]
- Darwesh, B.; Aldawsari, H.M.; Badr-Eldin, S.M. Optimized Chitosan/Anion Polyelectrolyte Complex Based Inserts for Vaginal Delivery of Fluconazole: In Vitro/In Vivo Evaluation. Pharmaceutics 2018, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar] [CrossRef] [Green Version]
- Rodero, C.F.; Fioramonti Calixto, G.M.; Cristina dos Santos, K.; Sato, M.R.; Aparecido dos Santos Ramos, M.; Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Bauab, T.M.; Sotomayor, C.E.; et al. Curcumin-Loaded Liquid Crystalline Systems for Controlled Drug Release and Improved Treatment of Vulvovaginal Candidiasis. Mol. Pharm. 2018, 15, 4491–4504. [Google Scholar] [CrossRef]
- Fitaihi, R.A.; Aleanizy, F.S.; Elsamaligy, S.; Mahmoud, H.A.; Bayomi, M.A. Role of chitosan on controlling the characteristics and antifungal activity of bioadhesive fluconazole vaginal tablets. Saudi Pharm. J. 2018, 26, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Chanaj-Kaczmarek, J.; Romaniuk-Drapała, A.; Rubiś, B.; Szymanowska, D.; Kobus-Cisowska, J.; Szymańska, E.; Winnicka, K.; Cielecka-Piontek, J. Mucoadhesive Chitosan Delivery System with Chelidonii Herba Lyophilized Extract as a Promising Strategy for Vaginitis Treatment. J. Clin. Med. 2020, 9, 1208. [Google Scholar] [CrossRef] [Green Version]
- Abd Ellah, N.H.; Abdel-Aleem, J.A.; Abdo, M.N.; Abou-Ghadir, O.F.; Zahran, K.M.; Hetta, H.F. Efficacy of ketoconazole gel-flakes in treatment of vaginal candidiasis: Formulation, in vitro and clinical evaluation. Int. J. Pharm. 2019, 567, 118472. [Google Scholar] [CrossRef]
- Permana, A.D.; Utomo, E.; Pratama, M.R.; Amir, M.N.; Anjani, Q.K.; Mardikasari, S.A.; Sumarheni, S.; Himawan, A.; Arjuna, A.; Usmanengsi, U.; et al. Bioadhesive-Thermosensitive In Situ Vaginal Gel of the Gel Flake-Solid Dispersion of Itraconazole for Enhanced Antifungal Activity in the Treatment of Vaginal Candidiasis. ACS Appl. Mater. Interfaces 2021, 13, 18128–18141. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Saladini, B.; Gallucci, M.C.; Cruciani, F.; Vitali, B.; Luppi, B. Chitosan/alginate complexes for vaginal delivery of chlorhexidine digluconate. Carbohydr. Polym. 2013, 91, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Bigucci, F.; Abruzzo, A.; Vitali, B.; Saladini, B.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: Preparation, characterization and antimicrobial activity. Int. J. Pharm. 2015, 478, 456–463. [Google Scholar] [CrossRef]
- Perioli, L.; Ambrogi, V.; Pagano, C.; Scuota, S.; Rossi, C. FG90 chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int. J. Pharm. 2009, 377, 120–127. [Google Scholar] [CrossRef]
- Lupo, N.; Fodor, B.; Muhammad, I.; Yaqoob, M.; Matuszczak, B.; Bernkop-Schnürch, A. Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets. Acta Biomater. 2017, 64, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Malli, S.; Bories, C.; Pradines, B.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. In situ forming pluronic® F127/chitosan hydrogel limits metronidazole transmucosal absorption. Eur. J. Pharm. Biopharm. 2017, 112, 143–147. [Google Scholar] [CrossRef]
- Malli, S.; Loiseau, P.M.; Bouchemal, K. Trichomonas vaginalis Motility Is Blocked by Drug-Free Thermosensitive Hydrogel. ACS Infect. Dis. 2020, 6, 114–123. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Ribeiro, M.; Palmeira-de-Oliveira, R.; Gaspar, C.; Costa-de-Oliveira, S.; Correia, I.; Vaz, C.P.; Martinez-de-Oliveira, J.; Queiroz, J.; Rodrigues, A. Anti-Candida activity of a chitosan hydrogel: Mechanism of action and cytotoxicity profile. Gynecol. Obstet. Investig. 2010, 70, 322–327. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Palmeira-de-Oliveira, R.; Gaspar, C.; Salgueiro, L.; Cavaleiro, C.; Martinez-de-Oliveira, J.; Queiroz, J.; Rodrigues, A. Association of Thymbra capitata essential oil and chitosan (TCCH hydrogel): A putative therapeutic tool for the treatment of vulvovaginal candidosis. Flavour Fragr. J. 2013, 28, 354–359. [Google Scholar] [CrossRef]
- Campos, L.M.; de Oliveira Lemos, A.S.; da Cruz, L.F.; de Freitas Araújo, M.G.; de Mello Botti, G.C.R.; Júnior, J.L.R.; Rocha, V.N.; Denadai, Â.M.L.; da Silva, T.P.; Tavares, G.D.; et al. Development and in vivo evaluation of chitosan-gel containing Mitracarpus frigidus methanolic extract for vulvovaginal candidiasis treatment. Biomed. Pharmacother. 2020, 130, 110609. [Google Scholar] [CrossRef]
- Şenyiğit, Z.A.; Karavana, S.Y.; Eraç, B.; Gürsel, Ö.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, M.K.; Kreutz, T.; Danielli, L.J.; De Marchi, J.G.B.; Pippi, B.; Koester, L.S.; Fuentefria, A.M.; Limberger, R.P. A chitosan hydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 56, 101527. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Palmeira-de-Oliveira, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Queiroz, J.A.; Pina-Vaz, C.; Rodrigues, A.G. Anti-biofilm activity of low-molecular weight chitosan hydrogel against Candida species. Med. Microbiol. Immunol. 2014, 203, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Arany, P.; Papp, I.; Zichar, M.; Regdon, G.; Béres, M.; Szalóki, M.; Kovács, R.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; et al. Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics 2021, 13, 1714. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyamoto, Y.; Ihara, S.; Yang, J.Z.; Zuill, D.E.; Angsantikul, P.; Zhang, Q.; Gao, W.; Zhang, L.; Eckmann, L. Composite Thermoresponsive Hydrogel with Auranofin-Loaded Nanoparticles for Topical Treatment of Vaginal Trichomonad Infection. Adv. Ther. 2019, 2, 1900157. [Google Scholar] [CrossRef]
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross-linked chitosan and pectin. J. Therm. Anal. Calorim. 2017, 130, 1683–1695. [Google Scholar] [CrossRef]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef]
- Parodi, B.; Russo, E.; Caviglioli, G.; Baldassari, S.; Gaglianone, N.; Schito, A.M.; Cafaggi, S. A chitosan lactate/poloxamer 407-based matrix containing Eudragit RS microparticles for vaginal delivery of econazole: Design and in vitro evaluation. Drug Dev. Ind. Pharm. 2013, 39, 1911–1920. [Google Scholar] [CrossRef]
- Tentor, F.; Siccardi, G.; Sacco, P.; Demarchi, D.; Marsich, E.; Almdal, K.; Bose Goswami, S.; Boisen, A. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery. J. Mater. Sci. Mater. Med. 2020, 31, 25. [Google Scholar] [CrossRef] [PubMed]
- Abilova, G.K.; Kaldybekov, D.B.; Irmukhametova, G.S.; Kazybayeva, D.S.; Iskakbayeva, Z.A.; Kudaibergenov, S.E.; Khutoryanskiy, V.V. Chitosan/Poly(2-ethyl-2-oxazoline) Films with Ciprofloxacin for Application in Vaginal Drug Delivery. Materials 2020, 13, 1709. [Google Scholar] [CrossRef] [Green Version]
- Hopper, M.; Yun, J.-F.; Zhou, B.; Le, C.; Kehoe, K.; Le, R.; Hill, R.; Jongeward, G.; Debnath, A.; Zhang, L.; et al. Auranofin inactivates Trichomonas vaginalis thioredoxin reductase and is effective against trichomonads in vitro and in vivo. Int. J. Antimicrob. Agents 2016, 48, 690–694. [Google Scholar] [CrossRef] [Green Version]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.K.; Nirmal, J.; Garg, P.; Venuganti, V.V.K. Corneal delivery of moxifloxacin and dexamethasone combination using drug-eluting mucoadhesive contact lens to treat ocular infections. Int. J. Pharm. 2020, 591, 120023. [Google Scholar] [CrossRef] [PubMed]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central composite optimization of ocular mucoadhesive cubosomes for enhanced bioavailability and controlled delivery of voriconazole. J. Drug Deliv. Sci. Technol. 2021, 61, 102075. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Hassan, N.; Mirza, M.A.; Aslam, M.; Mahmood, S.; Iqbal, Z. Doe guided chitosan based nano-ophthalmic preparation against fungal keratitis. Mater. Today Proc. 2021, 41, 19–29. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Gilani, S.J.; Jahangir, M.A.; Zafar, A.; Alshehri, S.; Yasir, M.; Kala, C.; Taleuzzaman, M.; Imam, S.S. Clarithromycin-Loaded Ocular Chitosan Nanoparticle: Formulation, Optimization, Characterization, Ocular Irritation, and Antimicrobial Activity. Int. J. Nanomed. 2020, 15, 7861–7875. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Taghe, S.; Asare-Addo, K.; Nokhodchi, A. Polyvinyl Alcohol/Chitosan Single-Layered and Polyvinyl Alcohol/Chitosan/Eudragit RL100 Multi-layered Electrospun Nanofibers as an Ocular Matrix for the Controlled Release of Ofloxacin: An In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Jain, N.; Pandey, J.; Nagaich, U. Investigating the Retention Potential of Chitosan Nanoparticulate Gel: Design, Development, In Vitro & Ex Vivo Characterization. Recent Pat. Anti-Infect. Drug Dis. 2020, 15, 41–67. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Tiwari, A.; Saraf, S.; Panda, P.K.; Jain, S.K. Promising Antifungal Potential of Engineered Non-ionic Surfactant-Based Vesicles: In Vitro and In Vivo Studies. AAPS PharmSciTech 2021, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.B.; Robinson, J.R. Oral cavity as a site for bioadhesive drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 43–74. [Google Scholar] [CrossRef]
- Abouhussein, D.; El Nabarawi, M.A.; Shalaby, S.H.; El-Bary, A.A. Cetylpyridinium chloride chitosan blended mucoadhesive buccal films for treatment of pediatric oral diseases. J. Drug Deliv. Sci. Technol. 2020, 57, 101676. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Wróblewska, M.; Kurowska, I.; Maciejczyk, M.; Basa, A.; Wolska, E.; Wilczewska, A.Z.; Winnicka, K. Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole. Pharmaceutics 2021, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Timur, S.S.; Yüksel, S.; Akca, G.; Şenel, S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int. J. Pharm. 2019, 559, 102–112. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef]
- Armenta-Rojas, E.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; López-Maldonado, E.A.; Olivas-Sarabia, A.; Castillo-Martínez, N.A.; Villarreal-Gómez, L.J.; Hurtado-Ayala, L.A. Nystatin-loaded Polyelectrolyte Complex Films as a Mucoadhesive Drug Delivery System for Potential Buccal Application. Biointerface Res. Appl. Chem. 2022, 12, 4384–4398. [Google Scholar] [CrossRef]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C 2017, 79, 140–150. [Google Scholar] [CrossRef]
- Ossama, M.; Lamie, C.; Tarek, M.; Wagdy, H.A.; Attia, D.A.; Elmazar, M.M. Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: In-vitro and in-vivo evaluation. Drug Deliv. 2021, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.E.; Mosgaard, M.D.; Kjeldsen, R.B.; Boisen, A.; Meyer, R.L.; Nielsen, L.H. Management of oral biofilms by nisin delivery in adhesive microdevices. Eur. J. Pharm. Biopharm. 2021, 167, 83–88. [Google Scholar] [CrossRef]
- Caricato, D.; Primavilla, S.; Scuota, S.; Ricci, M.; Perioli, L.; Marinozzi, M.; Giovagnoli, S. Rojo Duro Red Onion Extract Loaded Spray Thermogel as a Sustainable Platform for the Treatment of Oral Mucosa Lesions. J. Pharm. Sci. 2021, 110, 2974–2985. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.Y.; Steinbach-Rankins, J.M.; Demuth, D.R. Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J. Control. Release 2019, 297, 3–13. [Google Scholar] [CrossRef]
- Xia, M.-Y.; Xie, Y.; Yu, C.-H.; Chen, G.-Y.; Li, Y.-H.; Zhang, T.; Peng, Q. Graphene-based nanomaterials: The promising active agents for antibiotics-independent antibacterial applications. J. Control. Release 2019, 307, 16–31. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Covarrubias, C.; Trepiana, D.; Corral, C. Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mater. J. 2018, 37, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic Effect of Newly Introduced Root Canal Medicaments; Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef]
- Namangkalakul, W.; Benjavongkulchai, S.; Pochana, T.; Promchai, A.; Satitviboon, W.; Howattanapanich, S.; Phuprasong, R.; Ungvijanpunya, N.; Supakanjanakanti, D.; Chaitrakoonthong, T.; et al. Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 2020, 123, 181.e1–181.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of Chitosan on the Infectivity of Murine Norovirus, Feline Calicivirus, and Bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Gao, G.F.; Cheng, S. Carbohydrate-Functionalized Chitosan Fiber for Influenza Virus Capture. Biomacromolecules 2011, 12, 3962–3969. [Google Scholar] [CrossRef]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 28729. [Google Scholar] [CrossRef] [Green Version]
- Dornish, M.; Kaplan, D.S.; Arepalli, S.R. Regulatory Status of Chitosan and Derivatives. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; Sarmento, B., das Neves, J., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2012; pp. 463–481. [Google Scholar]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Wiegand, C.; Winter, D.; Hipler, U.C. Molecular-Weight-Dependent Toxic Effects of Chitosans on the Human Keratinocyte Cell Line HaCaT. Skin Pharmacol. Physiol. 2010, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]



| Delivery System/Scaffold | System | Microorganisms | Findings | Ref. |
|---|---|---|---|---|
| MW | ||||
| DDA | ||||
| Particles and carriers | CNPs: Chitosan 107 kDa DDA: 75–85% | S. aureus MRSA MRSE | In vitro antibiofilm activity: The CNPs were able to inhibit biofilm formation in all strains, however, only by around 30% for MRSE, while S. aureus and MRSA had inhibitions of more than 90% and approximately 75%, respectively. | [151] |
| CNPs: Chitosan 1.6 kDa DDA: 92% Rhamnolipid | S. aureus S. epidermidis Klebsiella oxytoca | In vitro antibiofilm activity: CNPs with the surface-active compound rhamnolipid demonstrated superior antibiofilm activity against S. aureus and S. epidermidis; however, the antibiofilm action in the Gram-negative bacteria K. oxytoca was absent. | [152] | |
| Excipient and coatings | Chitosan-coated NPs or CNPs incorporated into microneedles: Chitosan 50–190 kDa DDA: n.a. Doxycycline | S. aureus P. aeruginosa | In vitro antibiofilm activity: The bacterial eradication of doxycycline-loaded NPs with chitosan was superior to the free drug in all strains. More than 99% of the bacterial biofilm was eradicated at 4× MIC. Ex vivo porcine antibiofilm activity: the chitosan-coated NPs incorporated in microneedles eradicated upwards of 97% of the bacteria in all strains. | [153] |
| Lipid-polymer hybrid nanovesicles: Chitosan ≈250 kDa DDA: 75–85% Vancomycin | MRSA | In vitro antibiofilm activity: The lipid-polymer hybrid nanovesicles with vancomycin demonstrated superior eradication of MRSA biofilm. | [154] | |
| Microneedles: Chitosan MW: n.a. DDA: n.a. Zinc | E. coli S. aureus | In vitro antibiofilm activity: The zinc-loaded microneedles displayed superior antibiofilm activity in a concentration-dependent manner. In the highest zinc concentrations, almost all bacteria were killed. The unloaded microneedles killed more bacteria than the control but were less effective than the zinc-loaded microneedles. | [155,156] | |
| Hydrogel: Chitosan LMW DDA: n.a. Methylene blue | Propionibacterium acnes | In vitro antibiofilm activity: The chitosan/poloxamer hydrogel displayed moderate but significant antibiofilm activity; however, no additional antibiofilm effects from methylene blue were observed. | [157] | |
| Composite matrix: Chitosan 50–190 kDa DDA: ≥85% Silver NPs | C. albicans | In vitro antibiofilm activity: Compared to the control, the chitosan matrix with silver NPs and silver NPs alone reduced the number of viable cells in both C. albicans strains. | [158] | |
| Film: Chitosan 120 kDa DDA: n.a. Ciprofloxacin | S. aureus P. aeruginosa | In vitro antibiofilm activity: The ciprofloxacin-loaded films comprising chitosan and bacterial cellulose eradicated bacteria within 1 h of treatment. Inhibition towards both strains; however, stronger in P. aeruginosa | [159] | |
| Polymer-based gels | Hydrogel: Chitosan 25–35 kDa DDA: >90% Toluidine blue O | S. aureus P. aeruginosa | In vitro antibiofilm activity: The hydrogels comprising chitosan and HPMC with toluidine blue O displayed good anti-biofilm activity in biofilms produced by S. aureus or P. aeruginosa with 1- to 3-log bacterial killing and proper biofilm penetration. | [160] |
| Hydrogel: Chitosan 320 kDa DDA: n.a. Antimicrobial peptides: (ASP)-1 ASP-2 | P. aeruginosa A. baumannii MRSA | In vitro antibiofilm activity: The peptide-loaded hydrogels had strong anti-biofilm activity in P. aeruginosa and MRSA, especially in P. aeruginosa, where the formulation was superior to a commercialized silver product. Ex vivo porcine antibiofilm activity: The peptide-loaded hydrogels surpassed the commercial product in all three strains and exhibited a strong eradication of the biofilms. | [161] | |
| Hydrogel: Chitosan LMW DDA: 95.6% ε-poly-L-lysine | MDR- P. aeruginosa MRSA C. albicans | Ex vivo antibiofilm activity: The ε-poly-l-lysine loaded hydrogels reduced the thickness of the polymicrobial biofilm and reduced the bacterial load of P. aeruginosa; however, the bacterial burden of the other organisms was not reduced. | [162] | |
| Hydrogel: Chitosan 190–375 kDa DDA: n.a. Silver NPs | P. aeruginosa MRSA | Polymicrobial biofilm activity: The silver NP-loaded chitosan hydrogel significantly reduced the bacterial load of MRSA in all concentrations of the nanoparticles. The bacterial load of P. aeruginosa was also reduced; however, the reduction was lower than for MRSA, and higher NP concentrations were required. | [163] | |
| Hydrogel: Succinyl chitosan 200 kDa DDA: 87% Cellobiose dehydrogenase Cellulase from Trichoderma longibrachiatum | S. aureus E. coli | In vitro antibiofilm activity: The unloaded hydrogel demonstrated anti-biofilm activity against both S. aureus and E. coli. The enzyme-loaded hydrogel had approximately the same level of inhibition. | [164] | |
| Scaffolds | Matrix: Chitosan 200 kDa or 350 kDa DDA: n.a. Papain | S. aureus S. epidermidis | In vitro antibiofilm activity: Slightly improved activity from MMW chitosan with papain compared to HMW chitosan. | [165] |
| Membrane: Chitosan 311.5 kDa DDA: 71% Cis-2-decenoic acid Bupivacaine | MRSA | In vitro antibiofilm activity: Almost all membranes displayed significant antibiofilm effects both upon evaluating the growth on the dressings and in wells. | [166] |
| Delivery System/Scaffold | System | Microorganisms | Findings | Ref. |
|---|---|---|---|---|
| MW | ||||
| DDA | ||||
| Particles and carriers | CNPs: Chitosan 50–190 KDa DDA: 75–85% Antimicrobial peptide LL-37 | MRSA | MRSA-infected wound model in mice: No growth of MRSA was observed in the group treated with LL-37 loaded CNPs after 7 days. This antibacterial effect was superior to all other treatment conditions. | [64] |
| CNPs: Chitosan 50–190 kDa DDA: n.a. Cefadroxil | S. aureus | S. aureus-infected wound model in rats: CNPs loaded with cefadroxil embedded in in situ poloxamer 407 hydrogel showed a significant reduction in the bacterial burden in the wounds and complete healing after 5 days. | [184] | |
| CNPs: Chitosan LMW DDA: 75–85% Vancomycin hydrochloride | MRSA | MRSA-infected wound model in mice: the rats treated with pH-responsive CNPs comprising gemini surfactants loaded with vancomycin displayed a significantly reduced bacterial burden compared with both drug-free CNPs and free vancomycin. | [185] | |
| Excipient and coatings | Beads: Chitosan MW: n.a. DDA: 90% Zinc oxide NPs | Noninfected wound model in mice: The bacterial growth in the wound without induced infections treated with any of the chitosan/PVA/zinc beads was lower than the control. Almost no growth was observed in mice treated with chitosan, chitosan/PVA, or the loaded beads. | [176] | |
| Chitosan-functionalized graphene quantum dots: Chitosan oligosaccharide MW: n.a. DDA: n.a. Graphene quantum dots | S. aureus | S. aureus-infected wound model in rats: the rats treated with the chitosan-functionalized quantum dots composite together with illumination exhibited improved wound healing compared to all the other groups. | [186] | |
| Polymer-based gels | Hydrogel: Chitosan LMW DDA: >85% Terbinafine hydrochloride | C. albicans | C. albicans-infected wound in rats: chitosan hydrogel loaded with vesicles comprising penetration enhancers produced a significant reduction of C. albicans in the wound bed. | [187] |
| Hydrogel: Cyclodextrin-modified chitosan 15–22 kDa DDA: 75−80% Diclofenac Silver ions | P. aeruginosa | P. aeruginosa-infected wound in a murine model: β-cyclodextrin modified chitosan supramolecular hydrogel loaded with diclofenac and silver ions displayed improved wound healing and reduced the bacterial load in the wound bed. | [188] | |
| Hydrogel: Chitosan MW: n.a. DDA: ≥95% Silver nitrate Calcium sulfate dehydrate Zinc nitrate hexahydrate Copper nitrate trihydrate | S. aureus | S. aureus-infected wound model in mice: The chitosan/ion hydrogel in gauzes surpassed chitosan alone and the control group in wound healing. Additionally, the group treated with the chitosan/ion hydrogel in gauzes significantly reduced bacterial load in the wound bed compared with chitosan alone. | [189] | |
| Hydrogel: Chitosan 300–450 kDa DDA: 85–95% Silver sulfadiazine | S. aureus | S. aureus-infected burn and wound model in mice: The healing rate of the wounds treated with the silver sulfadiazine nanocrystal in the hydrogel was faster, and the overall healing was superior to a cream formulation in both the burn and wound model. | [190] | |
| Hydrogel: Chitosan MW: 100−150 kDa DDA: 85% Ciprofloxacin Fluconazole | S. aureus E. coli C. albicans | Polymicrobial wound model in rats: The ciprofloxacin and fluconazole-loaded fibrin NPs loaded in chitosan hydrogel bandage displayed a significant reduction in microbial load in the infected wound compared to the unloaded- fibrin NPs loaded in chitosan. However, there was still some microbial growth after 14 days. | [191] | |
| Hydrogel: Chitosan (maleic anhydride grafted chitosan) MW: n.a. DDA: n.a. Antimicrobial peptide Hydrogen peroxide | MRSA | MRSA biofilm-infected wound model in mice: The hydrogel loaded with antimicrobial peptide and hydrogen peroxide displayed a significant reduction in bacterial viability compared to all other treatments; however, not complete eradication. Chitosan alone reduced bacterial viability. Wound closure also improved in the groups treated with the coloaded hydrogel. | [192] | |
| Scaffolds | Film: Chitosan 50–190 kDa DDA: 75–85% S-nitrosoglutathione | MRSA | MRSA biofilm-infected wound model in mice: Both loaded and unloaded chitosan films reduced the bacterial burden in the wound and improved the healing rate compared to the control group. However, the NO-releasing film displayed a significantly improved healing and dispersal of the biofilm. | [193] |
| Film: Chitosan 200 kDa DDA: 85% Catechol | MRSA | MRSA-infected wound model in rats: The bacterial load in the group treated with the catechol-chitosan film at a reduced state was significantly reduced compared with the other groups. Additionally, the tissue in this group appeared normal. | [194] | |
| Film: Chlorinated chitosan MMW DDA: 75–85% Chloramine | MRSA | MRSA-infected wound model in mice: Chlorinated chitosan film produced with electrofabrication induced faster wound healing and reduced the wound’s bacterial burden, compared to the control and plain chitosan. | [195] | |
| Membrane: Chitosan MW: n.a. DDA: 87% Silver sulfadiazine | P. aeruginosa, S. aureus | S. aureus and P. aeruginosa infected-wound model in rats: The membranes significantly reduced the bacterial load in the wounds compared to the control group with a rapid initial eradication. | [196] | |
| Dressing: Chitosan 190–310 kDa DDA: 75–85% Silver NPs | P. aeruginosa | P. aeruginosa-infected wound model in mice: The mice treated with the polyelectrolyte complex had a reduced bacterial load in the tissue after 14 days of treatment and higher survival than mice treated with gauze. | [197] | |
| Nanofibers: Chitosan ≈250 kDa DDA: n.a. Indocyanine green | MRSA | MRSA-infected wound model in rats: The indocyanine green-loaded chitosan/PVA nanofibers and illumination demonstrated improved wound healing and reduced bacterial burden in the wound bed compared to all other treatment groups. | [198] | |
| Dressing loaded with microspheres: Chitosan MMW DDA: ≥85% Gentamycin sulfate | S. aureus E. coli | S. aureus and E. coli-infected wound model in rats: The group treated with gelatin microspheres loaded with gentamycin and platelet-rich plasma on chitosan dressing displayed reduced bacterial load and a faster wound healing rate than the group treated with gauze. | [199] | |
| Dressing: Chitosan MMW DDA: 97% Graphene oxide Copper NPs | S. aureus | S. aureus-infected wound model in mice: The group treated with the graphene oxide–copper composite in chitosan/hyaluronic acid hydrogel improved wound healing compared with all other groups. | [200] | |
| Sponge: Chitosan 10–30 kDa DDA: ≥95% Silver NPs | S. aureus | S. aureus-infected wound model in rabbits: the group treated with the silver NP-sponge healed faster than the control group, and although not statistically significant, faster than the marketed silver dressing. | [201] | |
| Sponge: Chitosan MW: 500 kDa DDA: 90% Quaternary ammonium CNPs | S. aureus | S. aureus-infected wound model in mice: the chitosan sponges loaded with quaternary ammonium CNPs exhibited superior antimicrobial activity compared to sponges without CNPs and untreated mice on days 7 and 10. | [202] |
| Formulation/Role of Chitosan | Targeted Vaginal Infection | Active Ingredient | Main Finding(s) | Ref. |
|---|---|---|---|---|
| Particles and carriers | Candidiasis | Clotrimazole | Decreased antifungal activity in vitro but improved safety profile for CNP-associated clotrimazole | [285] |
| Callophycin A | Synergetic and improved antifungal effect both in vitro and in vivo by Callophycin A in CNPs | [286] | ||
| Thiosemicarbazide | CNP-associated thiosemicarbazide obtained a prominent reduction of fungal burden in vivo | [287] | ||
| Miconazole | CNPs containing a seven-fold lower miconazole concentration than conventional miconazole cream obtained equal therapeutic effect | [288] | ||
| Miconazole and farnesol | The combination of miconazole and farnesol in CNPs expressed a greater antifungal effect in vivo | [289] | ||
| Argentinean medicinal plants | Chitosan microcapsules containing active substances exhibited strong antifungal capacity in vitro | [290] | ||
| Bacterial vaginosis | Doxycycline | CNP-associated doxycycline expressed a significant reduction in E. coli viability in vitro | [291] | |
| Metronidazole | Superior in vitro inhibition of B. fragilis growth by chitosan-alginate microspheres containing metronidazole | [265] | ||
| Aerobic vaginitis | Cefixime | Cefixime microspheres reduced E. coli viability in vitro | [292] | |
| Trichomoniasis | - | CNPs expressed concentrations and time-dependent antimicrobial activity against T. vaginalis in vitro | [293] | |
| Gonorrhoea | - | CNPs expressed antigonoccocal activity against all tested strains, including high-level resistant N. gonorrhoeae | [294] | |
| Coating material and excipient | Candidiasis | Tioconazole and econazole | Chitosan-coated nanocapsules maintained the antifungal activity for both drugs in vitro | [295] |
| Clotrimazole | Chitosan-coated PLGA NPs increased the antifungal activity of clotrimazole in vitro | [296] | ||
| Metronidazole | Fungal inhibition was equal for the chitosan-based formulation containing metronidazole and the drug-free formulation | [27] | ||
| Herbal medicine | All constituents of the microemulsion expressed antifungal activity in vitro, including chitosan | [297] | ||
| Metronidazole | In vitro antifungal activity was increased in the presence of chitosan and independent of metronidazole | [298] | ||
| Amphotericin B | The hydrogel containing amphotericin B-loaded chitosan microplatelets obtained a complete cure of infection in vivo | [299] | ||
| Fluconazole | Chitosan-based vaginal inserts containing fluconazole showed improved antifungal activity both in vitro and in vivo compared to free drug | [300] | ||
| Curcumin | Curcumin liquid crystal system containing chitosan increased the antifungal potency of curcumin in vitro | [301] | ||
| Curcumin liquid crystal system containing chitosan significantly decreased fungal burden in vivo and efficiently reduced the growth of biofilm in vitro | [302] | |||
| Fluconazole | Chitosan-based vaginal tablets increased the antifungal activity of fluconazole | [303] | ||
| Chelidonii herba extract | Chitosan-based vaginal tablets detained the in vitro antimicrobial activity of the extract | [304] | ||
| Ketoconazole | Ketoconazole-containing chitosan and gellan gum gel flakes in thermosensitive gel expressed antifungal effect in vivo | [305] | ||
| Itraconazole | Thermosensitive gel with a chitosan gel-flake system significantly improved the antifungal effect of itraconazole in vivo | [306] | ||
| Chlorhexidine | Chitosan-based vaginal inserts increased the antifungal activity of chlorhexidine in vitro | [307] | ||
| Chitosan-based vaginal inserts increased the antifungal activity of chlorhexidine in vitro | [308] | |||
| Bacterial vaginosis | - | Chitosan-coated liposomes expressed in vitro antibacterial effect against S. epidermidis and S. aureus | [8] | |
| Metronidazole | Vaginal tablets containing chitosan and metronidazole inhibited B. fragilis growth in vitro | [309] | ||
| Aerobic vaginitis | Chlorhexidine | Chitosan-based vaginal inserts increased the antimicrobial activity against E. coli of chlorhexidine in vitro | [307] | |
| Chitosan-based vaginal inserts increased the antimicrobial activity against E. coli of chlorhexidine in vitro | [308] | |||
| Metronidazole | Metronidazole vaginal tablets containing chitosan exhibited in vitro antimicrobial effect against E. coli | [310] | ||
| Trichomoniasis | - | In vitro antimicrobial effect was related to the PIBCA NPs and dependent on chitosan coating of NPs | [271] | |
| Metronidazole | Increased in vitro antimicrobial activity of chitosan-coated NPs compared to noncoated | [270] | ||
| Antimicrobial effect of metronidazole was maintained when in chitosan delivery system | [311] | |||
| - | The hydrogel containing chitosan proved to reduce T. vaginalis motility in biological fluids | [312] | ||
| Vaginal gel | Candidiasis | - | Chitosan hydrogel was confirmed to have intrinsic antifungal properties in vitro | [313] |
| Thymbra capitata essential oil | Chitosan hydrogel with essential oil showed increased in vitro antifungal activity and the ability to disrupt biofilm in a dose-dependent manner | [314] | ||
| Mitracarpus frigidus extract | Chitosan hydrogel with the extract obtained antifungal effect in vivo comparable to the marketed product | [315] | ||
| Miconazole or econazole | Superior in vitro antifungal activity by LMW chitosan hydrogel containing miconazole | [316] | ||
| Pelargonium graveolens essential oil | Chitosan hydrogel-thickened nanoemulsion containing essential oil expressed antifungal activity in vitro | [317] | ||
| - | Chitosan hydrogel significantly reduced biofilm formations both in vitro and in an in vivo model | [318] | ||
| Iminoboronate | Fungicidal activity in biomimetic conditions and inhibition of biofilm formation in vitro was obtained | [319] | ||
| Bacterial vaginosis | - | Low concentration chitosan hydrogel efficiently eradicated Pseudomonas aeruginosa biofilms in vitro | [24] | |
| Superior in vitro activity against S. aureus and S. epidermidis by chitosan formulated as hydrogel | [8] | |||
| Metronidazole | 3D printed vaginal ring containing chitosan and metronidazole obtained a bactericidal effect against E. coli and confirmed synergistic antibacterial effect by chitosan and metronidazole | [320] | ||
| Trichomoniasis | Auranofin | The chitosan-based hydrogel containing auranofin NPs managed to completely inhibit parasite growth in vitro in a dose-dependent manner | [321] | |
| Chlamydia | Resveratrol | Superior antichlamydial activity in vitro was by resveratrol liposomes-in-hydrogel in the lower concentrations | [276] | |
| Vaginal film | Candidiasis | Fluconazole | The chitosan-based vaginal film obtained in vitro fungal growth inhibition comparable to the marketed product | [322] |
| Tioconazole | Drug-free chitosan vaginal film expressed in vitro fungicidal activity; however, superior activity when loaded with tioconazole | [323] | ||
| Econazole | Chitosan-based matrices containing econazole microparticles expressed antifungal activity in vitro | [324] | ||
| Bacterial vaginosis | Metronidazole | Chitosan-based membrane did not restrain the effect of metronidazole against S. aureus and G. vaginalis in vitro | [325] | |
| Aerobic vaginitis | Ciprofloxacin | Vaginal films enhanced the activity of ciprofloxacin against E. coli and S. aureus in vitro | [326] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmingsen, L.M.; Škalko-Basnet, N.; Jøraholmen, M.W. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Mar. Drugs 2021, 19, 697. https://doi.org/10.3390/md19120697
Hemmingsen LM, Škalko-Basnet N, Jøraholmen MW. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Marine Drugs. 2021; 19(12):697. https://doi.org/10.3390/md19120697
Chicago/Turabian StyleHemmingsen, Lisa Myrseth, Nataša Škalko-Basnet, and May Wenche Jøraholmen. 2021. "The Expanded Role of Chitosan in Localized Antimicrobial Therapy" Marine Drugs 19, no. 12: 697. https://doi.org/10.3390/md19120697
APA StyleHemmingsen, L. M., Škalko-Basnet, N., & Jøraholmen, M. W. (2021). The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Marine Drugs, 19(12), 697. https://doi.org/10.3390/md19120697