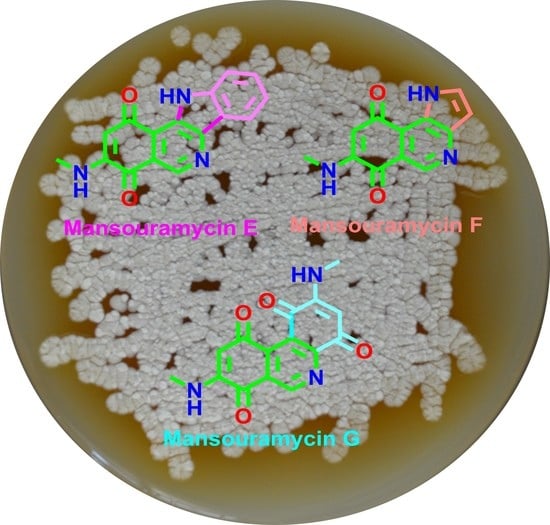
Mansouramycins E–G, Cytotoxic Isoquinolinequinones from Marine Streptomycetes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Biological Activities
3. Materials and Methods
3.1. General Procedures
3.2. Isolation and Taxonomy of the Producing Strain
3.3. Fermentation and Working Up
3.4. Isolation and Purification
3.5. Cytotoxicity Assays
3.6. DFT-Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukumi, H.; Kurihara, H.; Hata, T.; Tamura, C.; Mishima, H.; Kubo, A.; Arai, T. Mimosamycin, a novel antibiotic produced by Streptomyces lavendulae No. 314: Structure and synthesis. Tetrahedron Lett. 1977, 18, 3825–3828. [Google Scholar] [CrossRef]
- Kubo, A.; Kitahara, Y.; Nakahara, S.; Iwata, R.; Numata, R. Synthesis of mimocin, an isoquinolinequinone antibiotic from Streptomyces lavendulae, and its congeners. Chem. Pharm. Bull. 1988, 36, 4355–4363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettit, G.R.; Collins, J.C.; Herald, D.L.; Doubek, D.L.; Boyd, M.R.; Schmidt, J.M.; Hooper, J.N.A.; Tackett, L.P. Isolation and structure of cribrostatins 1 and 2 from the blue marine sponge Cribrochalina sp. Can. J. Chem. 1992, 70, 1170–1175. [Google Scholar] [CrossRef]
- Pettit, G.R.; Knight, J.C.; Collins, J.C.; Herald, D.L.; Pettit, R.K.; Boyd, M.R.; Young, V.G. Antineoplastic agents 430. Isolation and structure of cribrostatins 3, 4, and 5 from the republic of maldives Cribrochalina species. J. Nat. Prod. 2000, 63, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Frincke, J.M.; Faulkner, D.J.J. Antimicrobial metabolites of the sponge Reniera sp. J. Am. Chem. Soc. 1982, 104, 265–269. [Google Scholar] [CrossRef]
- Milanowski, D.J.; Gustafson, K.R.; Kelley, J.A.; McMahon, J.B. Caulibugulones A–F, novel cytotoxic isoquinoline quinones and iminoquinones from the marine bryozoan Caulibugula intermis. J. Nat. Prod. 2004, 67, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nakahara, S.; Iwata, R.; Takahashi, K.; Arai, T. Mimocin, a new isoquinolinequinone antibiotic. Tetrahedron Lett. 1980, 21, 3207–3208. [Google Scholar] [CrossRef]
- Mckee, T.C.; Ireland, C.M. Cytotoxic and antimicrobial alkaloids from the Fijian sponge Xestospongia caycedoi. J. Nat. Prod. 1987, 50, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Speitling, M. Vergleich der Metabolischen Kapazität Mariner und Terrestrischer Mikroorganismen—Isolierung und Strukturaufklärung von Branimycin, Brom-alterochromid A/B und weiteren Stoffwechselprodukten. Ph.D. Thesis, Georg-August University, Göttingen, Germany, 1998. [Google Scholar]
- Hawas, U.W.; Shaaban, M.; Shaaban, K.A.; Speitling, M.; Maier, A.; Kelter, G.; Fiebig, H.H.; Meiners, M.; Helmke, E.; Laatsch, H. Mansouramycins A-D, Cytotoxic Isoquinolinequinones from a marine Streptomycete. J. Nat. Prod. 2009, 72, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, C.; Zhang, W.; Zhu, Y.; Ma, L.; Fang, Z.; Zhang, C. Albumycin, a new isoindolequinone from Streptomyces albus J1074 harboring the fluostatin biosynthetic gene cluster. J. Antibiot. 2019, 72, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Schröder, D.; Shaaban, K.A.; Helmke, E.; Wagner-Döbler, I.; Laatsch, H. Flazin, Perlolyrin, and other new β-Carbolines from marine-derived Bacteria. Rev. Latinoam. Quim. 2007, 35, 58–67. [Google Scholar]
- Shaaban, K.A.; Shaaban, M.; Nair, V.; Schuhmann, I.; Win, H.Y.; Lei, L.; Dittrich, B.; Helmke, E.; Schüffler, A.; Laatsch, H. Structure Elucidation and Synthesis of Hydroxylated Isatins from Streptomycetes. Z. Naturforsch. B 2016, 71, 1191–1198. [Google Scholar] [CrossRef]
- Shaaban, M. Bioactive Secondary Metabolites from Marine and Terrestrial Bacteria: Isoquinolinequinones, Bacterial Compounds with a Novel Pharmacophor. Ph.D. Thesis, Georg-August University, Göttingen, Germany, 2004. [Google Scholar]
- Lindel, T.; Junker, J.; Koeck, M. 2D-NMR-guided constitutional analysis of organic compounds employing the computer program COCON. Eur. J. Org. Chem. 1999, 1999, 573–577. [Google Scholar] [CrossRef]
- SPARTAN’20; Wavefunction, Inc.: Irvine, CA, USA, 2020.
- Shaaban, M.; Abou-El-Wafa, G.S.E.; Golz, C.; Laatsch, H. New haloterpenes from the marine red alga Laurencia papillosa: Structure elucidation and biological activity. Mar. Drugs 2021, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Arzel, E.; Rocca, P.; Marsais, F.; Godard, A.; Quéguiner, G. First total synthesis of cryptomisrine. Tetrahedron 1999, 55, 12149–12156. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin-ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Structure of mescengricin, a novel neuronal cell protecting substance produced by Streptomyces griseoflavus. Tetrahedron Lett. 1997, 38, 3431–3434. [Google Scholar] [CrossRef]
- Dengler, W.A.; Schulte, J.; Berger, D.P.; Mertelsmann, R.; Fiebig, H.H. Development of a propidium iodide fluorescence assay for proliferation and cytotoxicity assays. Anticancer Drugs 1995, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Roemer, E.; Lange, C.; Huang, X.; Maier, A.; Kelter, G.; Jiang, Y.; Xu, L.; Menzel, K.-D.; Grabley, S.; et al. Structure, derivatisation, and antitumor activity of new griseusins from Nocardiopsis sp. J. Med. Chem. 2007, 50, 5168–5175. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, H.H.; Maier, A.; Burger, A.M. Clonogenic assay with established human tumour xenografts: Correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur. J. Cancer 2004, 40, 802–820. [Google Scholar] [CrossRef] [PubMed]


| Analytical Methods | Mansouramycin E (1a) | Mansouramycin F (2a) | Mansouramycin G (3a) |
|---|---|---|---|
| Appearance | Red powder | Dark red solid | Red solid |
| Rf a | 0.76 (CH2Cl2/7% MeOH) | 0.50 (CH2Cl2/7% MeOH) | 0.23 (CH2Cl2/7% MeOH). |
| Anisaldehyde/H2SO4 reagent | yellow | yellow | yellow |
| Staining with NaOH | no color change | no color change | no color change |
| Molecular Formula | C16H11N3O2 | C12H9N3O2 | C15H11N3O4 |
| UV/vis λmax (log ε) | (MeOH): 244 (4.17), 264 (4.20), 287 sh (4.17), 314 sh (3.71), 377 (3.94), 448 sh (3.28), 509 sh (3.17); (MeOH + 1n NaOH): 243 (4.16), 263 (4.21), 287 sh (4.17), 313 sh (3.77) 378 (3.94), 449 sh (3.47), 508 sh (3.17); (MeOH + 1n HCl) 245 sh (4.04), 267 (4.14), 284 sh (4.02) 314 sh (3.31) 387 (3.91), 510 (3.17) nm | (MeOH): 234 (3.66), 288 (3.26), 373 (3.22), 481 sh (2.38); (MeOH+ 1n HCl): 237 (3.53), 313 (3.38), 378 (3.12), 485 sh (2.38) nm; (MeOH+1n NaOH): 233 (3.64), 289 (3.28), 375 (3.19), 485 sh (2.38) nm | (MeOH): 244 (4.13), 299 sh (3.65), 382 (3.42), 435 nm (3.47); (MeOH + 1n HCl): 243 (4.05), 303 (3.65), 377 (3.46), 436 (3.47); (MeOH + 1n NaOH): 245 (4.07), 302 sh (3.57), 384 (3.35), 437 (3.35) nm |
| IR (KBr) νmax (KBr) | 3434, 2925, 2855, 1672, 1625, 1598, 1510, 1491, 1412, 1384, 1354, 1311, 1268, 1208, 1050 cm−1 | 3419, 2926, 2856, 1669, 1595, 1543, 1515, 1489, 1420, 1384, 1336, 1264, 1097, 1028, 764, cm−1 | 3426, 2925, 2855, 1616, 1559, 1544, 1458, 1412, 1384, 1325, 1261, 1028 cm−1 |
| CI-MS: m/z (%) | 245.0 ([M+NH4]+, 5), 228.0 ([M+H]+, 100) | ||
| (+)-ESI-MS: m/z (%) | 278 ([M+H]+) | 320.2 ([M+Na]+, 31), 617.0 ([2M+Na]+, 100) | |
| EI-MS: m/z (%) | 277 [M]+ (84), 256 (8), 249 (12), 236 (15), 220 (11), 195 (8), 192 (13), 179 (9), 166 (24), 138 (13), 102 (8), 97 (15), 82 (28), 73 (36), 69 (42), 57 (72), 43 (76), 44 (100) | 227 ([M]+., 100), 199 ([M-CO]+., 8), 186 (16), 145 (9), 116 (8), 59 (12), 43 (8) | |
| (+)-ESI-HRMS: m/z | 228.07663 [M+H]+ | 298.08203 [M+H]+ | |
| Calcd. | 277.0846 for C16H11N3O2 | 228.07667 for C12H10N3O2 [M+H]+ | 298.08223 for C15H12N3O4 [M+H]+ |
| EI HRMS: m/z | 277.0848 |
| Position | Mansouramycin E (1a) | Mansouramycin F (2a) | Mansouramycin G (3a) | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (Mult, J in [Hz]) (a) | δC, Type | δH (Mult, J in [Hz]) (b) | δC, type | δH (Mult, J in [Hz]) (a) | |
| 1 | 139.0, CH | 9.01 (s) | 140.7, CH | 8.90 (s) | 150.4, CH | 9.31 (s) |
| 3 | 149.9, C | 153.5, C | 153.4, C | |||
| 4 | 127.6, C | 122.4, C | 127.0, C | |||
| 4a | 120.3, C | 121.9, C | 141.5, C | |||
| 5 | 182.8, C | 182.8, C | 178.2, C | |||
| 6 | 99.6, CH | 5.71 (s) | 99.0, CH | 5.62 (s) | 100.8, CH | 5.75 (s) |
| 7 | 149.9 (c), C | 149.9, C | 148.9, C | |||
| 8 | 181.4, C | 181.2, C | 179.8, C | |||
| 8a | 120.4, C | 117.3, C | 126.7, C | |||
| 9 | 7.83 (brs) | 7.81 (brq, 5.2) | 7.84 (brq, 5.1) | |||
| 10 | 29.0, CH3 | 2.85 (d, 4.9) | 28.9, CH3 | 2.83 (d, 5.2) | 28.9, CH3 | 2.82 (d, 5.1) |
| 1′ | 11.98 (brs) | 11.88 (brs) | 177.6, C | |||
| 1′a | 144.7 (c), C | |||||
| 2′ | 113.3, CH | 7.79 (d, 7.9) | 137.6, CH | 7.93 (t, 3.05) | 99.6, CH | 5.77 (s) |
| 3′ | 129.9, CH | 7.61 (td, 7.9, 1.3) | 102.7, CH | 6.70 (dd, 3.05, 1.83) | 152.2, C | |
| 4′ | 120.6, CH | 7.31 (td, 8.1, 1.4) | 180.9, C | |||
| 5′ | 120.8, CH | 8.23 (d, 8.1) | ||||
| 5′a | 120.1, C | |||||
| 5′ | 8.04 (brq, 4.9) | |||||
| 6′ | 29.0, CH3 | 2.83 (brd, 4.9) | ||||
| Compound | Potency | Tumor Selectivity | ||||
|---|---|---|---|---|---|---|
| Mean IC50 μM (μgmL−1) | Mean IC70 μM (μgmL−1) | Selectivity */Total | % Selectivity | Rating ** | Internal Code | |
| Mansouramycin A (5) | 13.44 (2.902) | 26.26 (5.671) | 4/36 | 11% | ++ | MNSG078 |
| Mansouramycin C (4b) | 0.089 (0.022) | 0.167 (0.041) | 10/36 | 28% | +++ | MNSG091 |
| Mansouramycin E (1a) | 23.10 (6.398) | 33.95 (9.405) | 0/18 | 0% | - | MNSG089 |
| Mansouramycin F (2a) | 7.92 (1.797) | 15.19 ((3.449) | 7/36 | 19% | ++ | MNSG090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaaban, M.; Shaaban, K.A.; Kelter, G.; Fiebig, H.H.; Laatsch, H. Mansouramycins E–G, Cytotoxic Isoquinolinequinones from Marine Streptomycetes. Mar. Drugs 2021, 19, 715. https://doi.org/10.3390/md19120715
Shaaban M, Shaaban KA, Kelter G, Fiebig HH, Laatsch H. Mansouramycins E–G, Cytotoxic Isoquinolinequinones from Marine Streptomycetes. Marine Drugs. 2021; 19(12):715. https://doi.org/10.3390/md19120715
Chicago/Turabian StyleShaaban, Mohamed, Khaled A. Shaaban, Gerhard Kelter, Heinz Herbert Fiebig, and Hartmut Laatsch. 2021. "Mansouramycins E–G, Cytotoxic Isoquinolinequinones from Marine Streptomycetes" Marine Drugs 19, no. 12: 715. https://doi.org/10.3390/md19120715
APA StyleShaaban, M., Shaaban, K. A., Kelter, G., Fiebig, H. H., & Laatsch, H. (2021). Mansouramycins E–G, Cytotoxic Isoquinolinequinones from Marine Streptomycetes. Marine Drugs, 19(12), 715. https://doi.org/10.3390/md19120715