In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds
Abstract
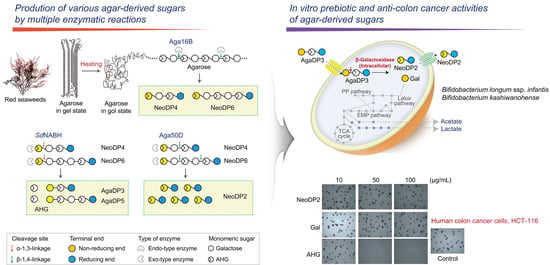
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Production of Agar-Derived Sugars with Various DPs
2.2. In Vitro Prebiotic Activity of Agar-Derived Sugars
2.3. Fermentation of AgaDP3 by Bifidobacteria
2.4. In Vitro Anti-Colon Cancer Activity of AHG
3. Materials and Methods
3.1. Preparation of Agar-Derived Sugars by Enzymatic Hydrolysis of Agarose
3.2. Purification of Agar-Derived Sugars by Size-Exclusion Chromatography
3.3. TLC Analysis
3.4. Screening of In Vitro Prebiotic Effects of Agar-Derived Sugars
3.5. Fermentation of AgaDP3 by Bifidobacteria
3.6. High-Performance Liquid Chromatography Analysis
3.7. High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection Analysis
3.8. Stability Test of AgaDP3 on Simulated Gastric Fluid
3.9. In Vitro Anti-Colon Cancer Activity Test of AHG Using Soft Agar Assay
3.10. In Vitro Anti-Colon Cancer Activity Test of AHG by Using Cell Viability Assay
3.11. In Vitro Anti-Colon Cancer Activity Test of AHG by 4′,6-Diamidino-2-Phenylindole Staining
3.12. In Vitro Anti-Colon Cancer Activity Test of AHG by Western Blot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Borba Gurpilhares, D.; Cinelli, L.P.; Simas, N.K.; Pessoa, A., Jr.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. β-Glucan, a dietary fiber in effective prevention of lifestyle diseases–An insight. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100187. [Google Scholar] [CrossRef]
- Lozupone, C.; Faust, K.; Raes, J.; Faith, J.J.; Frank, D.N.; Zaneveld, J.; Gordon, J.I.; Knight, R. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome Res. 2012, 22, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Kim, H.T.; Cho, K.M.; Yu, S.; Kim, S.; Choi, I.-G.; Kim, K.H. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour. Technol. 2016, 199, 311–318. [Google Scholar] [CrossRef]
- Knutsen, S.; Myslabodski, D.; Larsen, B.; Usov, A. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–170. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Zhang, N.; Mao, X.; Li, R.; Hou, E.; Wang, Y.; Xue, C.; Tang, Q.-J. Neoagarotetraose protects mice against intense exercise induced fatigue damage by modulating gut microbial composition and function. Mol. Nutr. Food Res. 2017, 61, 1600585. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Zhu, P.; Lin, J. Antioxidant activity and hepatoprotective potential of agaro-oligosaccharides in vitro and in vivo. Nutr. J. 2006, 5, 31. [Google Scholar] [CrossRef]
- Enoki, T.; Okuda, S.; Kudo, Y.; Takashima, F.; Sagawa, H.; Kato, I. Oligosaccharides from agar inhibit pro-inflammatory mediator release by inducing heme oxygenase 1. Biosci. Biotechnol. Biochem. 2010, 74, 766–770. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Associations among dietary seaweed intake, c-MYC rs6983267 polymorphism, and risk of colorectal cancer in a Korean population: A case–control study. Eur. J. Nutr. 2020, 59, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kanemura, S.; Oikawa, T.; Suzuki, S.; Hasegawa, Y.; Nishino, Y.; Fujiya, T.; Miura, K. Associations of Japanese food intake with survival of stomach and colorectal cancer: A prospective patient cohort study. Cancer Sci. 2020, 111, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Nam, S.J.; Kong, G.; Kim, M.K. A case-control study on seaweed consumption and the risk of breast cancer. Br. J. Nutr. 2010, 103, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK Pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Ha, S.; Lee, S.; Lee, J.; Kim, H.; Ko, H.-J.; Kim, K.; Choi, I.-G. Crystal structure of a key enzyme in the agarolytic pathway, α-neoagarobiose hydrolase from Saccharophagus degradans 2-40. Biochem. Biophys. Res. Commun. 2011, 412, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Lee, S.; Lee, D.; Kim, H.S.; Bang, W.G.; Kim, K.H.; Choi, I.-G. Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2-40: An exo-type β-agarase producing neoagarobiose. Appl. Microbiol. Biotechnol. 2010, 86, 227–234. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, E.J.; Seo, N.; Yu, S.; Kim, D.H.; Cho, K.M.; An, H.J.; Kim, J.H.; Choi, I.-G.; Kim, K.H. Enzymatic liquefaction of agarose above the sol-gel transition temperature using a thermostable endo-type β-agarase, Aga16B. Appl. Microbiol. Biotechnol. 2017, 101, 1111–1120. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Sakurama, H.; Kiyohara, M.; Nakajima, M.; Kitaoka, M.; Ashida, H.; Hirose, J.; Katayama, T.; Yamamoto, K.; Kumagai, H. Bifidobacterium longum subsp. infantis uses two different β-galactosidases for selectively degrading type-1 and type-2 human milk oligosaccharides. Glycobiology 2011, 22, 361–368. [Google Scholar] [CrossRef]
- Garrido, D.; Ruiz-Moyano, S.; Lemay, D.G.; Sela, D.A.; German, J.B.; Mills, D.A. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci. Rep. 2015, 5, 13517. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Bottacini, F.; Contreras, J.I.S.; Vigoureux, M.; Egan, M.; Motherway, M.O.; Holmes, E.; van Sinderen, D. Metabolism of the predominant human milk oligosaccharide fucosyllactose by an infant gut commensal. Sci. Rep. 2019, 9, 15427. [Google Scholar] [CrossRef]
- Kitaoka, M. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Adv. Nutr. 2012, 3, 422S–429S. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Liu, J.-J.; Lee, J.W.; Kwak, S.; Yu, S.; Kim, K.H.; Jin, Y.-S. Biosynthetic routes for producing various fucosyl-oligosaccharides. ACS Synth. Biol. 2019, 8, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Pluvinage, B.; Grondin, J.M.; Amundsen, C.; Klassen, L.; Moote, P.E.; Xiao, Y.; Thomas, D.; Pudlo, N.A.; Anele, A.; Martens, E.C.; et al. Molecular basis of an agarose metabolic pathway acquired by a human intestinal symbiont. Nat. Commun. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Yu, S.; Yun, E.J.; Kim, D.H.; Park, S.Y.; Kim, K.H. Dual agarolytic pathways in a marine bacterium, Vibrio sp. strain EJY3: Molecular and enzymatic verification. Appl. Environ. Microbiol. 2020, 86, e02724-19. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef]
- Wefers, D.; Dong, J.; Abdel-Hamid, A.M.; Paul, H.M.; Pereira, G.V.; Han, Y.; Dodd, D.; Baskaran, R.; Mayer, B.; Mackie, R.I.; et al. Enzymatic mechanism for arabinan degradation and transport in the thermophilic bacterium Caldanaerobius polysaccharolyticus. Appl. Environ. Microbiol. 2017, 83, e00794-17. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Jakob, C.A.; Aebi, M.; Tuor, U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl. Environ. Microbiol. 1999, 65, 3727–3729. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, E.J.; Yu, S.; Kim, Y.-A.; Liu, J.-J.; Kang, N.J.; Jin, Y.-S.; Kim, K.H. In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds. Mar. Drugs 2021, 19, 213. https://doi.org/10.3390/md19040213
Yun EJ, Yu S, Kim Y-A, Liu J-J, Kang NJ, Jin Y-S, Kim KH. In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds. Marine Drugs. 2021; 19(4):213. https://doi.org/10.3390/md19040213
Chicago/Turabian StyleYun, Eun Ju, Sora Yu, Young-Ah Kim, Jing-Jing Liu, Nam Joo Kang, Yong-Su Jin, and Kyoung Heon Kim. 2021. "In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds" Marine Drugs 19, no. 4: 213. https://doi.org/10.3390/md19040213
APA StyleYun, E. J., Yu, S., Kim, Y.-A., Liu, J.-J., Kang, N. J., Jin, Y.-S., & Kim, K. H. (2021). In Vitro Prebiotic and Anti-Colon Cancer Activities of Agar-Derived Sugars from Red Seaweeds. Marine Drugs, 19(4), 213. https://doi.org/10.3390/md19040213