Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size Distribution
2.2. Drug Content Estimation
2.3. Production Yield
2.4. In Vitro Dissolution Study
2.5. FTIR Spectroscopy
2.6. Differential Scanning Calorimetry
2.7. X-ray Diffractometry
2.8. Scanning Electron Microscopy (SEM)
2.9. In Vitro Antitumor Activity
2.10. Antimicrobial Activity
2.11. Antioxidant Activity
2.12. Stability Study
3. Materials and Methods
3.1. Materials
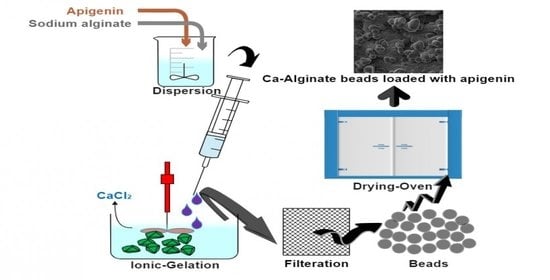
3.2. Development of Apigenin-Loaded Calcium-Alginate Beads
3.3. Particle Size Distribution
3.4. Drug Content Estimation
3.5. Production Yield
3.6. In Vitro Dissolution Study
3.7. FTIR Spectroscopy
3.8. Differential Scanning Calorimetry
3.9. X-ray Diffractometry
3.10. Scanning Electron Microscopy (SEM)
3.11. In Vitro Antitumor Activity
3.11.1. Cell Culture Condition and Treatment
3.11.2. Cell Survival Assay
3.12. Antimicrobial Activity
3.12.1. In Vitro Antibacterial Activity
3.12.2. In Vitro Antifungal Activity
3.13. Antioxidant Activity
3.14. Stability Study
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, H.-K.; Seo, C.H.; Park, Y. The Effects of Marine Carbohydrates and Glycosylated Compounds on Human Health. Int. J. Mol. Sci. 2015, 16, 6018–6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekumar, K.; Bindhu, B. A comprehensiapve review on alginates. Int. J. Recent Technol. Eng. 2019, 8, 1095–1097. [Google Scholar]
- Hay, I.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Prus-Walendziak, W.; Kozlowska, J. Lyophilized Emulsions in the Form of 3D Porous Matrices as a Novel Material for Topical Application. Materials 2021, 14, 950. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Sangeetha, S.; Venkatesh, D.N.; Adhiyaman, R.; Santhi, K.; Suresh, B. Formulation of Sodium Alginate Nanospheres Containing Amphotericin B for the Treatment of Systemic Candidiasis. Trop. J. Pharm. Res. 2007, 6, 653–659. [Google Scholar] [CrossRef]
- Rasel, M.A.T.; Hasan, M. Formulation and Evaluation of Floating Alginate Beads of Diclofenac Sodium. Dhaka Univ. J. Pharm. Sci. 2012, 11, 29–35. [Google Scholar] [CrossRef]
- Sharma, V.; Dash, S.K.; Manhas, A.; Radhakrishnan, J.; Jagavelu, K.; Verma, R.S. Injectable hydrogel for co-delivery of 5-azacytidine in zein protein nanoparticles with stem cells for cardiac function restoration. Int. J. Pharm. 2021, 603, 120673. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef]
- Ahirrao, S.; Gide, P.; Shrivastav, B.; Sharma, P. Extended Release of Theophylline Through Sodium Alginate Hydrogel Beads: Effect of Glycerol on Entrapment Efficiency, Drug Release. Part. Sci. Technol. 2014, 32, 105–111. [Google Scholar] [CrossRef]
- Arıca, B.; Çalış, S.; Kaş, H.; Sargon, M.; Hıncal, A. 5-Fluorouracil encapsulated alginate beads for the treatment of breast cancer. Int. J. Pharm. 2002, 242, 267–269. [Google Scholar] [CrossRef]
- Almurisi, S.H.; Doolaanea, A.A.; Akkawi, M.E.; Chatterjee, B.; Sarker, Z.I. Taste masking of paracetamol encapsulated in chitosan-coated alginate beads. J. Drug Deliv. Sci. Technol. 2020, 56, 101520. [Google Scholar] [CrossRef]
- Abdelwahed, N.A.M.; El-Naggar, N.E.A. Repeated batch production of vancomycin using synthetic cotton fibers. Afr. J. Biotechnol. 2011, 10, 12244–12251. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, J.; Jeong, N.Y.; Chung, H.-J. The natural plant flavonoid apigenin is a strong antioxidant that effectively delays peripheral neurodegenerative processes. Anat. Sci. Int. 2019, 94, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; AlShehri, S.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.A.; Mohsin, K.; Alanazi, F.K.; Alsarra, I. Solubility and thermodynamic parameters of apigenin in different neat solvents at different temperatures. J. Mol. Liq. 2017, 234, 73–80. [Google Scholar] [CrossRef]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.; Kumar, S.S.; Krishnamoorthy, B.; Basu, S.K. Development and evaluation of calcium alginate beads prepared by sequential and simultaneous methods. Braz. J. Pharm. Sci. 2010, 46, 785–793. [Google Scholar] [CrossRef]
- Al-Tahami, K. Preparation of Alginate Microspheres for the Delivery of Risperidone. Yemeni J. Med. Sci. 2014, 8. Available online: https://ust.edu/ojs/index.php/yjmp/article/view/779 (accessed on 11 June 2021).
- Alshehri, S.M.; Shakeel, F.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.; Mohsin, K.; Almeanazel, O.T.; Alkholief, M.; Alshetaili, A.; Alsulays, B.; et al. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharm. J. 2018, 27, 264–273. [Google Scholar] [CrossRef]
- Lupo, B.; Maestro, A.; Gutierrez, J.M.; Gonzalez, C. Characterization of alginate beads with encapsulated cocoa extract to prepare functional food: Comparison of two gelation mechanisms. Food Hydrocoll. 2015, 49, 25–34. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altaf, M.; Casagrande, N.; Mariotto, E.; Baig, N.; Kawde, A.-N.; Corona, G.; Larcher, R.; Borghese, C.; Pavan, C.; Seliman, A.A.; et al. Potent In Vitro and In Vivo Anticancer Activity of New Bipyridine and Bipyrimidine Gold (III) Dithiocarbamate Derivatives. Cancers 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telange, D.R.; Patil, A.T.; Pethe, A.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Gim-Krumm, M.; Ruby-Figueroa, R.; Troncoso, E.; Estay, H. Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis. Minerals 2019, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Ahmed, M.M.; Alshetaili, A.; Almutairy, B.K.; Alalaiwe, A.; Fatima, F.; Ansari, M.N.; Iqbal, M. Preparation of spray dried amorphous solid dispersion of diosmin in soluplus with improved hepato-renoprotective activity: In vitro anti-oxidant and in-vivo safety studies. J. Drug Deliv. Sci. Technol. 2020, 60, 102101. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ansari, M.J.; Das, S.S.; Alshahrani, S.M. Development and characterization of ethyl cellulose nanosponges for sustained release of brigatinib for the treatment of non-small cell lung cancer. J. Polym. Eng. 2020, 40, 823–832. [Google Scholar] [CrossRef]
- Almutairy, B.K.; Alshetaili, A.; Alali, A.S.; Ahmed, M.M.; Anwer, M.K.; Aboudzadeh, M.A. Design of Olmesartan Medoxomil-Loaded Nanosponges for Hypertension and Lung Cancer Treatments. Polymers 2021, 13, 2272. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Aldawsari, M.F.; Alsaidan, Y.S.M.; Alfaiz, S.A.; Haque, A.; Az, A.; Alhazzani, K. Development and characterization of Brigatinib loaded solid lipid nanoparticles: In-vitro cytotoxicity against human carcinoma A549 lung cell lines. Chem. Phys. Lipids 2020, 233, 105003. [Google Scholar] [CrossRef]
- Mohammed, M.; Alnafisah, M.S.; Anwer, M.K.; Fatima, F.; Almutairy, B.K.; Alshahrani, S.; Alshetaili, A.S.; Alalaiwe, A.; Fayed, M.H.; Alanazi, A.Z.; et al. Chitosan surface modified PLGA nanoparticles loaded with brigatinib for the treatment of non-small cell lung cancer. J. Polym. Eng. 2019, 39, 909–916. [Google Scholar] [CrossRef]
- Septisetyani, E.P.; Ningrum, R.A.; Romadhani, Y.; Wisnuwardhani, P.H.; Santoso, A. Optimization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with wst-1 assay in mcf-7 cells. Indones. J. Pharm. 2014, 25, 245. [Google Scholar] [CrossRef]
- Moglad, E.H.; Fatima, F.; A, M.M.; Devanathad, V.; Anw, M.K.; Aldawsa, M.F. Development of Topical Antibacterial Gel Loaded with Cefadroxil Solid Lipid Nanoparticles: In vivo Wound Healing Activity and Epithelialization Study. Int. J. Pharmacol. 2020, 16, 298–309. [Google Scholar] [CrossRef]
- Chandrasekar, D.; Madhusudhana, K.; Ramakrishna, S.; Diwan, P.V. Determination of DPPH free radical scavenging activity by reversed-phase HPLC: A sensitive screening method for polyherbal formulations. J. Pharm. Biomed. Anal. 2006, 40, 460–464. [Google Scholar] [CrossRef] [PubMed]










| Formulation Code | Composition | Characterization | ||||
|---|---|---|---|---|---|---|
| AGN | SAG | CaCl2 | PS | DL | Yield | |
| mg | mg | % | µm | % | % | |
| BD1 | 100 | 200 | 10 | 1413 ± 0.02 | 49.87 ± 1.8 | 84.98 ± 0.5 |
| BD2 | 100 | 225 | 10 | 1376 ± 0.03 | 55.76 ± 2.1 | 88.76 ± 0.6 |
| BD3 | 100 | 250 | 10 | 1265 ± 0.01 | 59.87 ± 1.8 | 99.91 ± 0.8 |
| BD4 | 100 | 275 | 10 | 1181 ± 0.07 | 66.67 ± 2.3 | 90.98 ± 0.3 |
| BD5 | 100 | 300 | 10 | 1102 ± 0.05 | 70.34 ± 1.5 | 94.89 ± 0.1 |
| BD6 | 100 | 325 | 10 | 1043 ± 0.02 | 76.65 ± 2.0 | 96.78 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldawsari, M.F.; Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Katakam, P.; Khan, A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar. Drugs 2021, 19, 467. https://doi.org/10.3390/md19080467
Aldawsari MF, Ahmed MM, Fatima F, Anwer MK, Katakam P, Khan A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Marine Drugs. 2021; 19(8):467. https://doi.org/10.3390/md19080467
Chicago/Turabian StyleAldawsari, Mohammed F., Mohammed Muqtader Ahmed, Farhat Fatima, Md. Khalid Anwer, Prakash Katakam, and Abdullah Khan. 2021. "Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities" Marine Drugs 19, no. 8: 467. https://doi.org/10.3390/md19080467
APA StyleAldawsari, M. F., Ahmed, M. M., Fatima, F., Anwer, M. K., Katakam, P., & Khan, A. (2021). Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Marine Drugs, 19(8), 467. https://doi.org/10.3390/md19080467