Changes in the Molecular Characteristics of Bovine and Marine Collagen in the Presence of Proteolytic Enzymes as a Stage Used in Scaffold Formation
Abstract
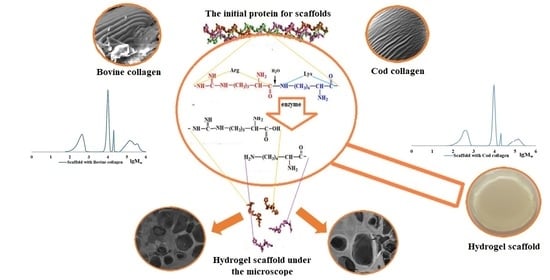
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Native Collagen—CC and BC
2.2. Characteristics of the CC and BC Hydrolysis Products
2.3. The Role of Collagen Hydrolysates in the Formation of the Spatial Structure of Scaffolds
2.4. Features of the Supramolecular Structure of Structure-Forming Biopolymers and Scaffolds Produced from Them
3. Materials and Methods
3.1. Enzymatic Hydrolysis of Collagen of Various Origins
3.2. Combined Hydrolysis of CC and Fg by Thrombin
3.3. Formation of Hybrid Hydrogel Scaffolds Based on Fg and Collagen
3.4. Freeze Drying
3.5. Scaffold Dissolution
3.6. Reading IR Spectra of CC and BC (See Article in “Inorganic”)
3.7. Determination of the Molecular Weight Characteristics of Proteins Using Gel-Penetrating Chromatography (GPC)
3.8. Scanning Electron Microscopy
3.9. Dynamic Light Scattering Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Mahija, J.S.R. Collagen and its therapeutical applications in regenerative medicine collagen and its therapeutical applications in regenerative medicine. Int. J. Sci. Res. Dev. 2018, 6, 268–277. [Google Scholar]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Sreekumar, G.; Sastry, T.; Chamundeeswari, M. Collagen as a Potential Biomaterial in Biomedical Applications. Rev. Adv. Mater. Sci. 2018, 53, 29–39. [Google Scholar] [CrossRef]
- Suzuki, A.; Kodama, Y.; Miwa, K.; Kishimoto, K.; Hoshikawa, E.; Haga, K.; Sato, T.; Mizuno, J.; Izumi, K. Manufacturing mi-cropatterned collagen scaffolds with chemical-crosslinking for development of biomimetic tissue-engineered oral mucosa. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Parisi, C.; Salvatore, L.; Veschini, L.; Serra, M.P.; Hobbs, C.; Madaghiele, M.; Sannino, A.; Di Silvio, L. Biomimetic gradient scaffold of collagen–hydroxyapatite for osteochondral regeneration. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.H.; Ahn, S.H.; Yang, G.-H.; Kim, G.H. A MSCs-laden polycaprolactone/collagen scaffold for bone tissue regeneration. RSC Adv. 2016, 6, 6259–6265. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Lausch, A.J.; Chong, L.C.; Uludag, H.; Sone, E.D. Multiphasic collagen scaffolds for engineered tissue interfaces. Adv. Funct. Mater. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Kourgiantaki, A.; Tzeranis, D.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalirajan, C.; Palanisamy, T. Bioengineered Hybrid Collagen Scaffold Tethered with Silver-Catechin Nanocomposite Modulates Angiogenesis and TGF-β Toward Scarless Healing in Chronic Deep Second Degree Infected Burns. Adv. Health Mater. 2020, 9, e2000247. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Esquivel, M.; Madrigal-Carballo, S. Collagen/chitosan hybrid 3D-scaffolds as potential biomaterials for tissue engineering. Int. J. Nano Biomater. 2018, 7, 163–175. [Google Scholar] [CrossRef]
- Rowe, S.L.; Stegemann, J.P. Interpenetrating Collagen-Fibrin Composite Matrices with Varying Protein Contents and Ratios. Biomacromolecules 2006, 7, 2942–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brougham, C.M.; Levingstone, T.J.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Incorporation of fibrin into a collagen-glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015, 26, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, T.A.; Shambat, S.M.; Haunreiter, V.D.; Mestres, C.A.; Weber, A.; Maisano, F.; Zinkernagel, A.S.; Hasse, B. Polyester Vascular Graft Material and Risk for Intracavitary Thoracic Vascular Graft Infection1. Emerg. Infect. Dis. 2020, 26, 2448–2452. [Google Scholar] [CrossRef] [PubMed]
- Kocen, R.; Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2017, 3, 129–138. [Google Scholar] [CrossRef]
- Al Kayal, T.; Losi, P.; Pierozzi, S.; Soldani, G. A New Method for Fibrin-Based Electrospun/Sprayed Scaffold Fabrication. Sci. Rep. 2020, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Poly. Res. 2020, 27, 73. [Google Scholar] [CrossRef] [Green Version]
- Castilho, M.; Hochleitner, G.; Wilson, W.; Van Rietbergen, B.; Dalton, P.D.; Groll, J.; Malda, J.; Ito, K. Mechanical behavior of a soft hydrogel reinforced with three-dimensional printed microfibre scaffolds. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Vedhanayagam, M.; Ananda, S.; Nair, B.U.; Sreeram, K.J. Polymethyl methacrylate (PMMA) grafted collagen scaffold re-inforced by PdO-TiO2 nanocomposites. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 108, 110378. [Google Scholar] [CrossRef]
- Carrion, B.; Souzanchi, M.F.; Wang, V.T.; Tiruchinapally, G.; Shikanov, A.; Putnam, A.J.; Coleman, R.M. The synergistic effects of matrix stiffness and composition on the response of chondroprogenitor cells in a 3D precondensation microenvironment. Adv. Healthcare Mater. 2015, 5, 1192–1202. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Chasova, V.O.; Fukina, D.G.; Koryagin, A.V.; Valetova, N.B.; Suleimanov, E.V. Synthesis of polymethyl methacrylate—Collagen graft copolymer using a photocatalyst—Complex oxide RbTe1.5W0.5O6. All materials. Encyclop. Ref. 2021, 7, 15–23. [Google Scholar]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng.-Part B 2019, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; Olivera-Castillo, L.; Cortes-Santiago, Y.; López, L.M. Comparison of collagen characteristic from the skin and swim bladder of Gulf corvina (Cynoscion othonopterus). Tissue Cell 2021, 72, 101593. [Google Scholar] [CrossRef]
- Sun, B.; Li, C.; Mao, Y.; Qiao, Z.; Jia, R.; Huang, T.; Xu, D.; Yang, W. Distinctive characteristics of collagen and gelatin extracted from Dosidicus gigas skin. Int. J. Food Sci. Technol. 2021, 56, 3443–3454. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Proc. Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Sharifi, E.; Azami, M.; Kajbafzadeh, A.M.; Moztarzadeh, F.; Faridi-Majidi, R.; Shamousi, A.; Karimi, R.; Ai, J. Preparation of a biomimetic composite scaffold from gelatin/collagen andbioactive glass fibers for bone tissue engineering. Mater. Sci. Eng. 2016, 59, 533–541. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Liu, Y.; Zhu, C.; Fan, D. Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int. J. Biol. Macromol. 2020, 155, 625–635. [Google Scholar] [CrossRef]
- Shah, B.M.; Palakurthi, S.S.; Khare, T.; Khare, S.; Palakurthi, S. Natural proteins and polysaccharides in the development of micro/nano delivery systems for the treatment of inflammatory bowel disease. Int. J. Biol. Macromol. 2020, 165, 722–737. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [Green Version]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.D.M.; Assis, C.R.D.; Costa, B.D.A.M.; Neri, R.C.D.A.; Monte, F.T.D.; Freitas, H.M.S.D.C.V.; França, R.C.P.; Santos, J.F.; Bezerra, R.D.S.; Porto, A.L.F. Physical, Biochemical, Densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2020, 1224, 129023. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Valetova, N.B.; Chasova, V.O.; Podguzkova, M.V.; Zakharycheva, N.S.; Egorikhina, M.; Astanina, M.V.; Kuznetsova, Y.L. Molecular Weight Parameters of Collagen from Different Feedstock and Dynamics of Their Change upon Enzymatic Hydrolysis by Pancreatin. Polym. Sci. Ser. D 2020, 13, 235–239. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Marova, I.; Matouskova, P.; Obruca, S.; Miloslav, P. Use of Ultrasonic Spectroscopy and Viscosimetry for the Characterization of Chicken Skin Collagen in Comparison with Collagens from other Animal Tissues. Prep. Biochem. Biotechnol. 2014, 44, 761–771. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a bio-material for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Guzmán, F.; Valencia, P.; Almonacid, S.; Cárdenas, C. Collagen as a source of bioactive peptides: A bioinformatics approach. Electron. J. Biotechnol. 2020, 48, 101–108. [Google Scholar] [CrossRef]
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J. Mater. Sci. Mater. Med. 2021, 32, 1–12. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. Part A 2016, 104, 2685–2692. [Google Scholar] [CrossRef] [Green Version]
- Egorikhina, M.N.; Aleynik, D.Y.; Rubtsova, Y.P.; Levin, G.Y.; Charykova, I.N.; Semenycheva, L.L.; Bugrova, M.L.; Zakharychev, E.A. Hydrogel scaffolds based on blood plasma cryoprecipitate and collagen derived from various sources: Structural, mechanical and biological characteristics. Bioact. Mater. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Egorikhina, M.N.; Levin, G.Y.; Charykova, I.N.; Alejnik, D.Y.; Sosnina, L.N. Method for Creating a Bioresorbable Cellular Scaffold Based on Fibrin of Blood Plasma. Patent 2653434 RU; Int. Cl. C12N 5/00, 8 May 2018. [Google Scholar]
- Arulmolia, J.; Wrightb, H.J.; Phanc, D.T.T.; Shethb, U.; Quea, R.A.; Bottend, G.A.; Keatinga, M. Combination scaffolds of salmon fibrin, hyaluronic acid, and laminin for human neural stem cell and vascular tissue engineering. Acta Biomater. 2016, 46, 122–138. [Google Scholar] [CrossRef] [Green Version]
- Sproul, E.P.; Hannan, R.T.; Brown, A.C. Controlling Fibrin Network Morphology, Polymerization, and Degradation Dynamics in Fibrin Gels for Promoting. Tissue Repair. 2018, 1758, 85–99. [Google Scholar] [CrossRef]
- Solovieva, E.V.; Fedotov, A.Y.; E Mamonov, V.; Komlev, V.S.; A Panteleyev, A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2017, 13, 025007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen-sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, H.; Mohd, R.N.; Yaakob, M.C.; Amin, I.; Noorfaizan, A. Chemical and functional properties of bovine and porcine skin gelatin. Int. Food Res. J. 2011, 18, 813–817. [Google Scholar]
- Sotelo, C.G.; Comesaña, M.B.; Ariza, P.R.; Ricardo, I. Characterization of collagen from different discarded fish species of the west coast of the iberian peninsula characterization of collagen from different discarded fish species. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, M. Species identification of bovine, ovine and porcine type 1 collagen; comparing peptide mass fingerprinting and LC-based proteomics methods. Int. J. Mol. Sci. 2016, 17, 445. [Google Scholar] [CrossRef] [Green Version]
- Semenycheva, L.L.; Kuznetsova, J.L.; Valetova, N.B.; Geras’kina, E.V.; Tarankova, O.A. Method for Producing of Acetic Dispersion of High Molecular Fish Collagen. Patent RF No. RU 2567171 C1, 10 November 2015. [Google Scholar]
- Available online: https://www.sigmaaldrich.com/RU/ru/product/sigma/c9791 (accessed on 25 August 2021).
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B.G. Thermal stability of calf skin collagen type I in salt solutions. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1996, 1297, 171–181. [Google Scholar] [CrossRef]
- Shtykova, E.V.; Bogacheva, E.N.; Dadinova, L.A.; Jeffries, C.M.; Fedorova, N.V.; Golovko, A.O.; Baratova, L.A.; Batishchev, O. Small-angle X-Ray analysis of macromolecular structure: The structure of protein NS2 (NEP) in solution. Crystallogr. Rep. 2017, 62, 894–902. [Google Scholar] [CrossRef]
- Sartuqui, J.; Elía, N.D.; Gravina, A.N.; Messina, P.V. Analyzing the hydrodynamic and crowding evolution of aqueous hydroxyapatite-gelatin networks: Digging deeper into bone scaffold design variables. Biopolymers 2015, 103, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, G.; Steen, P.V.D.; Cohen, S.; Bitler, A.; Brand, D.; Opdenakker, G.; Sagi, I. Direct Visualization of Protease Action on Collagen Triple Helical Structure. PLoS ONE 2010, 5, e11043. [Google Scholar] [CrossRef] [Green Version]
- Semenycheva, L.L.; Egorikhina, M.; Chasova, V.O.; Valetova, N.B.; Podguzkova, M.V.; Astanina, M.V.; Kuznetsova, Y.L. Enzymatic hydrolysis of collagen by pancreatin and thrombin as a step in the formation of scaffolds. Russ. Chem. Bull. 2020, 69, 164–168. [Google Scholar] [CrossRef]
- Semenycheva, L.L.; Egorikhina, M.N.; Chasova, V.O.; Valetova, N.B.; Kuznetsova, Y.L.; Mitin, A.V. Enzymatic Hydrolysis of Marine Collagen and Fibrinogen Proteins in the Presence of Thrombin. Mar. Drugs 2020, 18, 208. [Google Scholar] [CrossRef] [Green Version]
- Bender, M.; Bergeron, R.; Komiyama, M. Bioorganic Chemistry of Enzymatic Catalysis; Mir: Moscow, Russian, 1987; p. 352. [Google Scholar]
- Klein, T.; Eckhard, U.; Dufour, A.; Solis, N.; Overall, C.M. Proteolytic cleavage—Mechanisms, function, and “omic” approaches for a near-ubiquitous posttranslational modification. Chem. Rev. 2018, 118, 1137–1168. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J. Fragmentation of Protein Using Trypsin. Cold Spring Harb. Protoc. 2006, 282, 2612–2626. [Google Scholar] [CrossRef]
- Momot, A.P.; Taranenko, I.A. Method for Determination of Fibrin Monomer Self-Assembly Time. Patent RF No. 2,366,955, 10 September 2009. [Google Scholar]
- Tumurova, T.B.; Shalbuev, D.V. Study of the effect of the fermented milk composition on the degree of destruction of the collagen macrostructure. In Proceedings of the XIV International Scientific and Practical Conference “Leather and fur in the XXI century: Technology, Quality, Ecology, Education”, Ulan-Ude, Russia, 5–8 September 2018; pp. 12–17. [Google Scholar]
- Masuelli, M.A. Mark-Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2, 37–43. [Google Scholar]
- Sparrow, R.L.; Simpson, R.; Greening, D. A Protocol for the Preparation of Cryoprecipitate and Cryo-depleted Plasma for Proteomic Studies. In Serum/Plasma Proteomics; Humana Press: New York, NY, USA, 2017; Volume 1619, pp. 23–30. [Google Scholar] [CrossRef]
- Nascimento, B.; Goodnough, L.T.; Levy, J.H. Cryoprecipitate therapy. Br. J. Anaesth. 2014, 113, 922–934. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, A.; Karuri, N. Fibronectin alters the rate of formation and structure of the fibrin matrix. Biochem. Biophys. Res. Commun. 2014, 443, 395–399. [Google Scholar] [CrossRef]
- Falvo, M.R.; Gorkun, O.V.; Lord, S.T. The molecular origins of the mechanical properties of fibrin. Biophys. Chem. 2010, 152, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Shuman, H.; Ledger, R.E.; Lee, S.; Weisel, J.W. The elasticity of an individual fibrin fiber in a clot. Proc. Natl. Acad. Sci. USA 2005, 102, 9133–9137. [Google Scholar] [CrossRef] [Green Version]
- Bryant, S.J.; Durand, K.L.; Anseth, K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J. Biomed. Mater. Res. Part A 2003, 67, 1430–1436. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 2015, 4, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Egorikhina, M.N.; Rubtsova, Y.P.; Charykova, I.N.; Bugrova, M.L.; Bronnikova, I.I.; Mukhina, P.A.; Sosnina, L.N.; Aleynik, D.Y. Biopolymer Hydrogel Scaffold as An Artificial Cell Niche for Mesenchymal Stem Cells. Polymers 2020, 12, 2550. [Google Scholar] [CrossRef]











| Item No. | Nature of Collagen | Mw × 10−3, kDa | Mw/Mn | Content of HMC in the Dry Residue of Collagen, % |
|---|---|---|---|---|
| 1 | CC | 250–300 | 1.2–1.3 | 95–97 |
| 2 | 17–20 | 1.1–1.2 | 3–5 | |
| 3 | 9–10 | 1.1–1.2 | trace amounts | |
| 4 | BC | 600–950 | 1.2–1.6 | 92–93 |
| 5 | 17–20 | 1.1–1.2 | 7–8 | |
| 6 | 9–10 | 1.1–1.2 | trace amounts |
| Item No. | Sample | Parameter Values during Hydrolysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Min | 3 Days | |||||||
| 1 | 60 | ||||||||
| Mw, kDa | Mw, kDa | Mw, kDa | Mw, kDa | ||||||
| Value | Fraction, % | Value | Fraction, % | Value | Fraction, % | Value | Fraction, % | ||
| 1 | CC | 300 | 96 | 125–127 | 15 | 124 | 16 | 121 | 16 |
| 2 | 17–18 | 4 | 17–18 | 2 | 17–18 | 2 | 17–18 | 8 | |
| 3 | - | - | 9–10 | 83 | 9–10 | 82 | 9–10 | 76 | |
| 4 | BC | 950 | 93 | 990 | 7 | 980 | 8 | ˃103 | 10 |
| 5 | 17 | 7–8 | 17 | 5 | 17 | 1–2 | 17 | 3 | |
| 6 | - | - | 9–10 | 88 | 9–10 | 90–91 | 9–10 | 87 | |
| Item No. | Scaffold Based on | Mw × 10−3, kDa | Mw/Mn | Content, % |
|---|---|---|---|---|
| 1 | CC | 120–150 | 1.2–1.3 | 15 |
| 20 | 1.0 | 12 | ||
| 10 | 1.0 | 58 | ||
| oligomers | 1.2 | 15 | ||
| 2 | BC | 200–240 | 1.4–1.6 | 30 |
| 20 | 1.0 | 14 | ||
| 10 | 1.0 | 46 | ||
| oligomers | 1.2 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorikhina, M.N.; Semenycheva, L.L.; Chasova, V.O.; Bronnikova, I.I.; Rubtsova, Y.P.; Zakharychev, E.A.; Aleynik, D.Y. Changes in the Molecular Characteristics of Bovine and Marine Collagen in the Presence of Proteolytic Enzymes as a Stage Used in Scaffold Formation. Mar. Drugs 2021, 19, 502. https://doi.org/10.3390/md19090502
Egorikhina MN, Semenycheva LL, Chasova VO, Bronnikova II, Rubtsova YP, Zakharychev EA, Aleynik DY. Changes in the Molecular Characteristics of Bovine and Marine Collagen in the Presence of Proteolytic Enzymes as a Stage Used in Scaffold Formation. Marine Drugs. 2021; 19(9):502. https://doi.org/10.3390/md19090502
Chicago/Turabian StyleEgorikhina, Marfa N., Ludmila L. Semenycheva, Victoria O. Chasova, Irina I. Bronnikova, Yulia P. Rubtsova, Evgeniy A. Zakharychev, and Diana Ya. Aleynik. 2021. "Changes in the Molecular Characteristics of Bovine and Marine Collagen in the Presence of Proteolytic Enzymes as a Stage Used in Scaffold Formation" Marine Drugs 19, no. 9: 502. https://doi.org/10.3390/md19090502
APA StyleEgorikhina, M. N., Semenycheva, L. L., Chasova, V. O., Bronnikova, I. I., Rubtsova, Y. P., Zakharychev, E. A., & Aleynik, D. Y. (2021). Changes in the Molecular Characteristics of Bovine and Marine Collagen in the Presence of Proteolytic Enzymes as a Stage Used in Scaffold Formation. Marine Drugs, 19(9), 502. https://doi.org/10.3390/md19090502