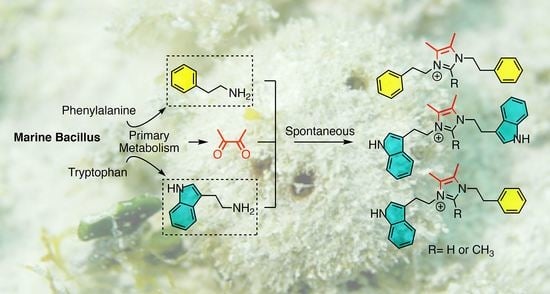
Bacillimidazoles A−F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus
Abstract
:1. Introduction
2. Results & Discussion
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material
4.3. Fermentation, Extraction and Isolation
4.4. Antibacterial Testing
4.5. Sequencing and Identification of Candidate Bacillimidazole Biosynthetic Genes
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, J.R. Amaryllidaceae, Sceletium, imidazole, oxazole, thiazole, peptide and miscellaneous alkaloids. Nat. Prod. Rep. 2001, 18, 95–128. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, Z.; Huang, R. Muscarine, imidazole, oxazole, thiazole, Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2002, 19, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z. Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2016, 33, 1268–1317. [Google Scholar] [CrossRef] [PubMed]
- Ziar, N.; Montalvão, S.; Hodnik, Z.; Nawrot, D.A.; Žula, A.; Ilaš, J.; Kikelj, D.; Tammela, P.; Mašič, L.P. Antimicrobial activity of the marine alkaloids clathrodin and oroidin, and their synthetic analogues. Mar. Drugs 2014, 12, 940–963. [Google Scholar]
- Hassan, W.; Edrada, R.; Ebel, R.; Wray, V.; Berg, A.; van Soest, R.; Wiryowidagdo, S.; Proksch, P. New imidazole alkaloids from the Indonesian sponge Leucetta chagosensis. J. Nat. Prod. 2004, 67, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Dyson, L.; Wright, A.D.; Young, K.A.; Sakoff, J.A.; McCluskey, A. Synthesis and anticancer activity of focused compound libraries form the natural product lead, oroidin. Bioorg. Med. Chem. 2014, 22, 1690–1699. [Google Scholar] [CrossRef]
- Jin, Z. Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2013, 30, 869–915. [Google Scholar] [CrossRef]
- Jin, Z. Muscarine, imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2011, 28, 1143–1191. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kusama, T.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J. Nagelamides X-Z, dimeric bromopyrrole alkaloids from a marine sponge Agelas sp. Org. Lett. 2013, 15, 3262–3265. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Prasad, P.; Capon, R.J.; Jia, Y. Asymmetric total synthesis of (+)-dragmacidin D reveals unexpected stereocomplexity. Org. Lett. 2015, 17, 1529–1532. [Google Scholar] [CrossRef]
- Gong, K.-K.; Tang, X.-L.; Liu, Y.-S.; Li, P.-L.; Li, G.-Q. Imidazole alkaloids form the South China Sea Sponge Pericharax heteroraphis and their cytotoxi and antiviral activities. Molecules 2016, 21, 150. [Google Scholar] [CrossRef] [Green Version]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Guyot, M. A New—Carboline Alkaloid Isolated from the Marine Sponge Hyrtios erecta. Tetrahedron Lett. 1996, 37, 3457–3460. [Google Scholar] [CrossRef]
- Pedpradab, S.; Edrada, R.; Ebel, R.; Wray, V.; Proksch, P. New beta-carboline alkaloids form the Andaman Sea sponge Dragmacidon sp. J. Nat. Prod. 2004, 67, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Avery, V.M. Leptoclinidamines A-C, indole alkaloids from the Australian ascidian Leptoclinides durus. J. Nat. Prod. 2009, 72, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.V.; Kumar, V.A. A profile of the in vitro anti-tumor activity of imidazolium-based ionic liquids. Bioorg. Med. Chem. Lett. 2010, 20, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-P.; Zong, M.-H.; Linhardt, R.J.; Lou, W.-Y.; Li, N.; Huang, C.; Wu, H. Mechanistic insights into the effect of imidazolium ionic liquid on liquid production by Geotrichum fermentas. Biotechnol. Biofuels 2016, 9, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, B.D.; Deblock, M.C.; Wagers, P.O.; Duah, E.; Robishaw, N.K.; Shelton, K.L.; Southerland, M.R.; DeBord, M.A.; Kersten, K.M.; McDonald, L.J.; et al. Anti-tumor activity of lipophilic imidazolium salts on select NSCLC cell lines. J. Med. Chem. Res. 2015, 24, 2838–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyche, T.P.; Piotrowski, J.S.; Hou, Y.; Braun, D.; Deshpande, R.; Mcllwain, S.; Ong, I.M.; Myers, C.L.; Guzei, I.A.; Westler, W.M.; et al. Forazoline A: Marine-derived polyketide with antifungal in vivo activity. Angew. Chem. Int. Ed. 2014, 53, 11583–11586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Braun, D.R.; Michel, C.R.; Klassen, J.L.; Adnani, N.; Wyche, T.P.; Bugni, T.S. Microbial strain prioritization using metabolomics tools for the discovery of natural products. Anal. Chem. 2012, 84, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Barns, K.; Hoffmann, F.M.; Braun, D.R.; Andes, D.R.; Bugni, T.S. Thalassosamide, a Siderophore Discovered from the Marine-Derived Bacterium Thalassospira profundimaris. J. Nat. Prod. 2017, 80, 2551–2555. [Google Scholar] [CrossRef]
- Cui, B.; Zheng, B.L.; He, K.; Zheng, Q.Y. Imidazole alkaloids from Lepidium meyenii. J. Nat. Prod. 2003, 66, 1101–1103. [Google Scholar] [CrossRef]
- Wu, Q.; Throckmorton, K.; Maity, M.; Chevrette, M.G.; Braun, D.R.; Rajski, S.R.; Currie, C.R.; Thomas, M.G.; Bugni, T.S. Bacillibactins E and F from a Marine Sponge-Associated Bacillus sp. J. Nat. Prod. 2021, 84, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Potts, M.B.; Colosimo, D.; Herrera-Herrera, M.L.; Legako, A.G.; Yousufuddin, M.; White, M.A.; MacMillan, J.B. Discoipyrroles A–D: Isolation, structure determination, and synthesis of potent migration inhibitors from Bacillus hunanensis. J. Am. Chem. Soc. 2013, 135, 13387–13392. [Google Scholar] [CrossRef] [Green Version]
- Mevers, E.; Saurí, J.; Helfrich, E.J.N.; Henke, M.; Barns, K.J.; Bugni, T.S.; Andes, D.; Currie, C.R.; Clardy, J. Pyronitrins A–D: Chimeric Natural Products Produced by Pseudomonas protegens. J. Am. Chem. Soc. 2019, 141, 17098–17101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Li, S.W.; Xu, H.; Wang, H.; Hu, P.; Zhang, H.; Luo, C.; Chen, K.X.; Nay, B.; Guo, Y.W.; et al. Complex Polypropionates from a South China Sea Photosynthetic Mollusk: Isolation and Biomimetic Synthesis Highlighting Novel Rearrangements. Angew. Chem. Int. Ed. 2020, 59, 12105–12112. [Google Scholar] [CrossRef]
- Wyche, T.P.; Hou, Y.; Braun, D.; Cohen, H.C.; Xiong, M.; Bugni, T.S. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp. J. Org. Chem. 2011, 76, 6542–6547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic. Acids. Res. 2019, 47, 81–87. [Google Scholar] [CrossRef] [Green Version]
- For related approach see: Kerkhof, L.J.; Dillon, K.P.; Häggblom, M.M.; McGuinnes, L.R. Profiling bacterial communities by MinION sequencing of ribosomal operons. Microbiome 2017, 5, 116. [Google Scholar]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassiano, M.H.A.; Silva-Rocha, R. Benchmarking Bacterial Promoter Prediction Tools: Potentialities and Limitations. mSystems 2020, 5, e00438-20. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1, 1′ | 137.8, qC | 138.2, qC | ||||||
| 2, 2′ | 130.0, CH | 7.12, dd (8.0, 1.8) | 130.2, CH | 7.11, dd (8.0, 1.8) | 124.4, CH | 6.96, s | 124.5, CH | 6.98, s |
| 3, 3′ | 130.0, CH | 7.34, t (7.4) | 130.1, CH | 7.34, t (7.8) | 110.5, qC | 110.8, qC | ||
| 4, 4′ | 128.4, CH | 7.30, t (7.4) | 128.6, CH | 7.32, t (7.8) | 128.3, qC | 128.4, qC | ||
| 5, 5′ | 130.0, CH | 7.34, t (7.4) | 130.1, CH | 7.34, t (7.8) | 118.4, CH | 7.32, d (7.6) | 118.2, CH | 7.24, d (8.0) |
| 6, 6′ | 130.0, CH | 7.12, dd (8.0, 1.8) | 130.2, CH | 7.11, dd (8.0, 1.8) | 120.1, CH | 7.03, t (7.6) | 120.2, CH | 7.03, t (8.0) |
| 7, 7′ | 37.1, CH2 | 3.06, t (6.6) | 36.3, CH2 | 3.02, t (6.8) | 122.8, CH | 7.15, t (7.6) | 122.9, CH | 7.14, t (8.0) |
| 8, 8′ | 49.3, CH2 | 4.35, t (6.6) | 47.9, CH2 | 4.31, t (6.8) | 112.6, CH | 7.39, d (7.8) | 112.7, CH | 7.39, d (8.0) |
| 9, 9′ | 138.1, qC | 138.0, qC | ||||||
| 10, 10′ | 128.5, qC | 127.1, qC | 26.8, CH2 | 3.07, t (6.8) | 26.0, CH2 | 3.03, t (6.0) | ||
| 11, 11′ | 135.5 CH | 8.51, s | 144.0, qC | 48.7, CH2 | 4.25, t (6.8) | 47.5, CH2 | 4.19, t (6.0) | |
| 12, 12′ | 7.9, CH3 | 2.08, s | 8.2, CH3 | 2.08, s | ||||
| 13, 13′ | 9.7, CH3 | 2.01, s | 128.2, qC | 127.0, qC | ||||
| 14 | 135.4, CH | 8.12, s | 144.0, qC | |||||
| 15, 15′ | 7.9, CH3 | 2.09, s | 8.3, CH3 | 2.18, s | ||||
| 16 | 9.3, CH3 | 1.64, s | ||||||
| Position | 5 | 6 | ||
|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | ||||
| 2 | 124.6, CH | 7.03, s | 124.6, CH | 7.02, s |
| 3 | 110.4, qC | 110.8, qC | ||
| 4 | 128.4, qC | 128.5, qC | ||
| 5 | 112.7, CH | 7.40, dd (8.2, 1.0) | 112.7, CH | 7.37, d (7.8) |
| 6 | 120.2, CH | 7.04, dt (7.6, 1.2) | 120.3, CH | 7.00, t (7.8) |
| 7 | 122.9, CH | 7.15, dt (8.0, 1.0) | 123.9, CH | 7.12, t (7.8) |
| 8 | 118.4, CH | 7.34, dd (8.0, 1.0) | 118.2, CH | 7.23, d (7.8) |
| 9 | 138.1, qC | 138.1, qC | ||
| 10 | 26.5, CH2 | 3.23, t (6.6) | 26.0, CH2 | 3.17, t (6.2) |
| 11 | 48.9, CH2 | 4.38, t (6.6) | 47.7, CH2 | 4.30, t (6.2) |
| 12 | ||||
| 13 | 128.3, qC | 127.1, qC | ||
| 14 | 128.3, qC | 127.0, qC | ||
| 15 | ||||
| 16 | 135.4, CH | 8.27, s | 144.0, qC | |
| 17 | 8.0, CH3 | 2.12, s | 8.3, CH3 | 2.15, s |
| 18 | 7.9, CH3 | 2.04, s | 8.2, CH3 | 2.07, s |
| 19 | 48.7, CH2 | 4.18, t (7.2) | 9.4, CH3 | 1.77, s |
| 20 | 36.7, CH2 | 2.84, t (7.2) | 47.6, CH2 | 4.11, t (6.8) |
| 21 | 137.8, qC | 36.3, CH2 | 2.77, t (6.8) | |
| 22 | 130.0, CH | 7.04, d (7.2) | 138.0, qC | |
| 23 | 129.9, CH | 7.29, m | 130.1, CH | 7.03, dd (7.3, 1.6) |
| 24 | 128.3, CH | 7.28, m | 130.0, CH | 7.30, m |
| 25 | 129.9, CH | 7.29, m | 128.5, CH | 7.26, m |
| 26 | 130.0, CH | 7.04, d (7.2) | 130.0, CH | 7.30, m |
| 27 | 130.1, CH | 7.03, dd (7.3, 1.6) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.-X.; Wu, Q.; Helfrich, E.J.N.; Chevrette, M.G.; Braun, D.R.; Heyman, H.; Ananiev, G.E.; Rajski, S.R.; Currie, C.R.; Clardy, J.; et al. Bacillimidazoles A−F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus. Mar. Drugs 2022, 20, 43. https://doi.org/10.3390/md20010043
Yan J-X, Wu Q, Helfrich EJN, Chevrette MG, Braun DR, Heyman H, Ananiev GE, Rajski SR, Currie CR, Clardy J, et al. Bacillimidazoles A−F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus. Marine Drugs. 2022; 20(1):43. https://doi.org/10.3390/md20010043
Chicago/Turabian StyleYan, Jia-Xuan, Qihao Wu, Eric J. N. Helfrich, Marc G. Chevrette, Doug R. Braun, Heino Heyman, Gene E. Ananiev, Scott R. Rajski, Cameron R. Currie, Jon Clardy, and et al. 2022. "Bacillimidazoles A−F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus" Marine Drugs 20, no. 1: 43. https://doi.org/10.3390/md20010043
APA StyleYan, J. -X., Wu, Q., Helfrich, E. J. N., Chevrette, M. G., Braun, D. R., Heyman, H., Ananiev, G. E., Rajski, S. R., Currie, C. R., Clardy, J., & Bugni, T. S. (2022). Bacillimidazoles A−F, Imidazolium-Containing Compounds Isolated from a Marine Bacillus. Marine Drugs, 20(1), 43. https://doi.org/10.3390/md20010043