Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Tests
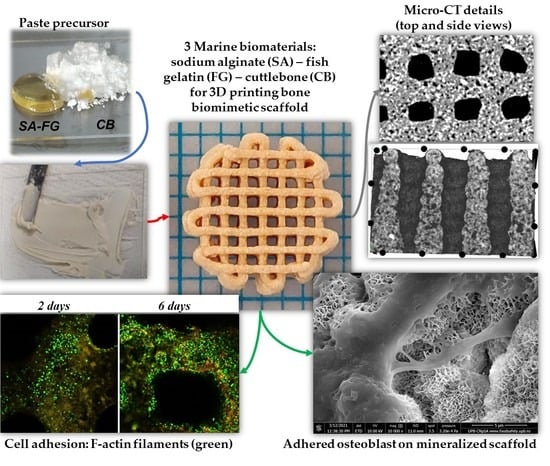
2.2. Scaffold’s Manufacturing Process
2.3. Crosslinking Efficiency Investigation
2.4. Structural and Dimensional Stability and Integrity
2.5. Morpho- and Microstructural Characterization
2.6. Mechanical Behavior
2.7. In Vitro Biocompatibility Investigation: Cell-Scaffolds Interactions
2.7.1. Biocompatibility Investigation
2.7.2. Cell Adhesion Investigation
2.7.3. Mineralization Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of CB Powder
3.2.2. Ink Formulation
3.2.3. Rheological Test
3.2.4. 3D Printing
3.2.5. Post-Printing Crosslinking Method
3.3. Characterization Methods
3.3.1. GF Analysis
3.3.2. Swelling Behavior Evaluation
3.3.3. Structural Stability Test in PBS
3.3.4. Stability Test in CM and PBS
3.3.5. Morpho-Structural Characterization of 3D Printed Scaffolds
3.3.6. Mechanical Properties Evaluation
3.3.7. In Vitro Assessment of Biocompatibility
Achievement of Three-Dimensional Cell-Scaffold System
Evaluation of In Vitro Biocompatibility
Cell Adhesion Investigation
3.3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macha, I.J.; Ben-Nissan, B. Marine skeletons: Towards hard tissue repair and regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.K.; Soker, S.; Atala, A. Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–35. [Google Scholar]
- Vajrabhaya, L.O.; Korsuwannawong, S.; Surarit, R. Cytotoxic and the proliferative effect of cuttlefish bone on MC3T3-E1 osteoblast cell line. Eur. J. Dent. 2017, 11, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Özçimen, D.; İnan, B.; Morkoç, O.; Efe, A. A Review on Algal Biopolymers. J. Chem. Eng. Res. Updat. 2017, 4, 7–14. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Yoon, H.S.; Yang, K.; Kim, Y.M.; Nam, K.; Roh, Y.H. Cellulose nanocrystals as support nanomaterials for dual droplet-based freeform 3D printing. Carbohydr. Polym. 2021, 272, 118469. [Google Scholar] [CrossRef]
- Datta, S.; Sarkar, R.; Vyas, V.; Bhutoria, S.; Barui, A.; Roy Chowdhury, A.; Datta, P. Alginate-honey bioinks with improved cell responses for applications as bioprinted tissue engineered constructs. J. Mater. Res. 2018, 33, 2029–2039. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced printable hydrogels from pre-crosslinked alginate as a new tool in semi solid extrusion 3D printing process. Carbohydr. Polym. 2022, 276, 118746. [Google Scholar] [CrossRef]
- Olmos-Juste, R.; Alonso-Lerma, B.; Pérez-Jiménez, R.; Gabilondo, N.; Eceiza, A. 3D printed alginate-cellulose nanofibers based patches for local curcumin administration. Carbohydr. Polym. 2021, 264, 118026. [Google Scholar] [CrossRef]
- Bertuola, M.; Aráoz, B.; Gilabert, U.; Gonzalez-Wusener, A.; Pérez-Recalde, M.; Arregui, C.O.; Hermida, É.B. Gelatin–alginate–hyaluronic acid inks for 3D printing: Effects of bioglass addition on printability, rheology and scaffold tensile modulus. J. Mater. Sci. 2021, 56, 15327–15343. [Google Scholar] [CrossRef]
- Raddatz, L.; Lavrentieva, A.; Pepelanova, I.; Bahnemann, J.; Geier, D.; Becker, T.; Scheper, T.; Beutel, S. Development and Application of an Additively Manufactured Calcium Chloride Nebulizer for Alginate 3D-Bioprinting Purposes. J. Funct. Biomater. 2018, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, S.J.; Lee, H.; Park, S.A.; Lee, J.Y. Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr. Polym. 2018, 196, 217–224. [Google Scholar] [CrossRef]
- Dogan, E.; Okumus, Z. Cuttlebone used as a bone xenograft in bone healing. Vet. Med. (Praha) 2014, 59, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, K.; Mohankumar, G.C. Sea coral-derived cuttlebone reinforced epoxy composites: Characterization and tensile properties evaluation with mathematical models. J. Compos. Mater. 2016, 50, 807–823. [Google Scholar] [CrossRef]
- Poompradub, S.; Ikeda, Y.; Kokubo, Y.; Shiono, T. Cuttlebone as reinforcing filler for natural rubber. Eur. Polym. J. 2008, 44, 4157–4164. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Dragusin, D.M.; Curti, F.; Cecoltan, S.; Sarghiuta, D.; Butac, L.M.; Vasile, E.; Marinescu, R.; Stancu, I.C. Biocomposites based on biogenous mineral for inducing biomimetic mineralization. Mater. Plast. 2017, 54, 207–213. [Google Scholar] [CrossRef]
- Curti, F.; Drăgușin, D.M.; Serafim, A.; Sorescu, A.; Stancu, I.C.; Iovu, H.; Marinescu, R. Cuttlefish Bone-Based Ink For 3d Printing Of Scaffolds For Orthopedic Applications. U.P.B. Sci. Bull 2021, 83, 3–14. [Google Scholar]
- Stancu, I.C.; Lungu, A.; Dragusin, D.M.; Vasile, E.; Damian, C.; Iovu, H. Porous gelatin-alginate-polyacrylamide scaffolds with interpenetrating network structure: Synthesis and characterization. Soft Mater. 2013, 11, 384–393. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Chen, X. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 299–306. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, G.; Kang, M.; Lee, J.; Seo, J.; Do, J.; Hong, K.; Cha, J.; Shin, S.; Bae, H. Marine Biomaterial-Based Bioinks for Generating 3D Printed Tissue Constructs. Mar. Drugs 2018, 16, 484. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tagami, T.; Ozeki, T. Fabrication of 3D-printed fish-gelatin-based polymer hydrogel patches for local delivery of pegylated liposomal doxorubicin. Mar. Drugs 2020, 18, 325. [Google Scholar] [CrossRef]
- Alonso, M.; Guerrero-Beltrán, C.E.; Ortega-Lara, W. Design and characterization of Gelatin/PVA hydrogels reinforced with ceramics for 3D printed prosthesis. In Proceedings of the Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 13, pp. 324–331. [Google Scholar]
- Curti, F.; Drăgușin, D.-M.M.; Serafim, A.; Iovu, H.; Stancu, I.-C.C. Development of thick paste-like inks based on superconcentrated gelatin/alginate for 3D printing of scaffolds with shape fidelity and stability. Mater. Sci. Eng. C 2021, 122, 111866. [Google Scholar] [CrossRef]
- Curti, F.; Stancu, I.-C.; Voicu, G.; Iovu, H.; Dobrita, C.-I.; Ciocan, L.T.; Marinescu, R.; Iordache, F. Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks. Polymers 2020, 12, 2420. [Google Scholar] [CrossRef]
- Serafim, A.; Cecoltan, S.; Lungu, A.; Vasile, E.; Iovu, H.; Stancu, I.C. Electrospun fish gelatin fibrous scaffolds with improved bio-interactions due to carboxylated nanodiamond loading. RSC Adv. 2015, 5, 95467–95477. [Google Scholar] [CrossRef]
- Cecoltan, S.; Serafim, A.; Dragusin, D.M.; Lungu, A.; Lagazzo, A.; Barberis, F.; Stancu, I.C. The potential of NDPs-loaded fish gelatin fibers as reinforcing agent for fish gelatin hydrogels. Key Eng. Mater. 2016, 695, 278–283. [Google Scholar] [CrossRef]
- Declet, A.; Reyes, E.; Suárez, O.M. Calcium carbonate precipitation: A review of the carbonate crystallization process and applications in bioinspired composites. Rev. Adv. Mater. Sci. 2016, 44, 87–107. [Google Scholar]
- Das, S.; Basu, B. An Overview of Hydrogel-Based Bioinks for 3D Bioprinting of Soft Tissues. J. Indian Inst. Sci. 2019, 99, 405–428. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2020, 8, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Nanocellulosic materials as bioinks for 3D bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef]
- Lee, J.M.; Ng, W.L.; Yeong, W.Y. Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing. Appl. Phys. Rev. 2019, 6, 011307. [Google Scholar] [CrossRef]
- Lee, J.M.; Yeong, W.Y. Design and Printing Strategies in 3D Bioprinting of Cell-Hydrogels: A Review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef]
- Colosi, C.; Shin, S.R.; Manoharan, V.; Massa, S.; Costantini, M.; Barbetta, A.; Dokmeci, M.R.; Dentini, M.; Khademhosseini, A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv. Mater. 2016, 28, 677–684a. [Google Scholar] [CrossRef] [Green Version]
- Contessi Negrini, N.; Celikkin, N.; Tarsini, P.; Farè, S.; Święszkowski, W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication 2020, 12, 025001. [Google Scholar] [CrossRef]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Shen, H.; Zhi, Y.; Si, J.; Shi, J.; Guo, L.; Shen, S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. B Biointerfaces 2019, 181, 1026–1034. [Google Scholar] [CrossRef]
- Hernández-Sosa, A.; Ramírez-Jiménez, R.A.; Rojo, L.; Boulmedais, F.; Aguilar, M.R.; Criado-Gonzalez, M.; Hernández, R. Optimization of the Rheological Properties of Self-Assembled Tripeptide/Alginate/Cellulose Hydrogels for 3D Printing. Polymers 2022, 14, 2229. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Xu, S.; Zheng, Q.; Cao, X. Preparation and properties of 3D printed alginate-chitosan polyion complex hydrogels for tissue engineering. Polymers 2018, 10, 664. [Google Scholar] [CrossRef] [Green Version]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous alginate scaffolds assembled using vaterite CaCO3 crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Cheng, L.; Yao, B.; Hu, T.; Cui, X.; Shu, X.; Tang, S.; Wang, R.; Wang, Y.; Liu, Y.; Song, W.; et al. Properties of an alginate-gelatin-based bioink and its potential impact on cell migration, proliferation, and differentiation. Int. J. Biol. Macromol. 2019, 135, 1107–1113. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wattanaanek, N.; Suttapreyasri, S.; Samruajbenjakun, B. 3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization. J. Funct. Biomater. 2022, 13, 47. [Google Scholar] [CrossRef]
- Khavari, A.; Nydén, M.; Weitz, D.A.; Ehrlicher, A.J. Composite alginate gels for tunable cellular microenvironment mechanics. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]









| Code | Mass Ratio in the Final Mixture | ||
|---|---|---|---|
| FG | SA | CB | |
| GCB | 27.78 | - | 72.22 |
| GA0.5CB | 27.74 | 0.14 | 72.12 |
| GA1CB | 27.7 | 0.28 | 72.02 |
| GA1.5CB | 27.66 | 0.42 | 71.92 |
| Samples Code | Pressure (kPa) | Feed Rate (mm/s) |
|---|---|---|
| GCB | 550 ± 50 | 3.3–3.5 |
| GA0.5CB | 2.9–3.1 | |
| GA1CB | 2.5–2.7 | |
| GA1.5CB | 1.8–2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curti, F.; Serafim, A.; Olaret, E.; Dinescu, S.; Samoila, I.; Vasile, B.S.; Iovu, H.; Lungu, A.; Stancu, I.C.; Marinescu, R. Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential. Mar. Drugs 2022, 20, 670. https://doi.org/10.3390/md20110670
Curti F, Serafim A, Olaret E, Dinescu S, Samoila I, Vasile BS, Iovu H, Lungu A, Stancu IC, Marinescu R. Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential. Marine Drugs. 2022; 20(11):670. https://doi.org/10.3390/md20110670
Chicago/Turabian StyleCurti, Filis, Andrada Serafim, Elena Olaret, Sorina Dinescu, Iuliana Samoila, Bogdan Stefan Vasile, Horia Iovu, Adriana Lungu, Izabela Cristina Stancu, and Rodica Marinescu. 2022. "Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential" Marine Drugs 20, no. 11: 670. https://doi.org/10.3390/md20110670
APA StyleCurti, F., Serafim, A., Olaret, E., Dinescu, S., Samoila, I., Vasile, B. S., Iovu, H., Lungu, A., Stancu, I. C., & Marinescu, R. (2022). Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential. Marine Drugs, 20(11), 670. https://doi.org/10.3390/md20110670