Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion
Abstract
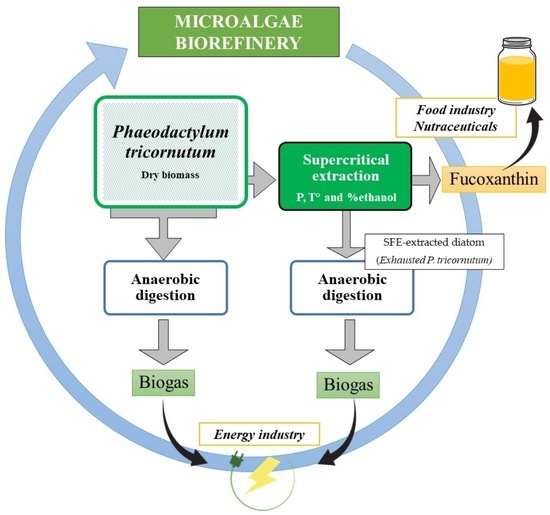
:1. Introduction
2. Results and Discussion
2.1. Effects of Pressure and Temperature on the Yield of Fucoxanthin from P. tricornutum
2.2. Effects of Ethanol as a Co-Solvent Factor on the Yield of Fucoxanthin from Phaeodactylum tricornutum
2.3. Biochemical Composition and the Effects of SFE on AD Using P. tricornutum
2.4. BMP of Diatom P. tricornutum and Extracted P. tricornutum by SFE
2.5. Characteristics of Effluents after AD
3. Materials and Methods
3.1. Biomass and Chemicals
3.2. Determination of Particle Size Distribution of the P. tricornutum Freeze-Dried Biomass
3.3. SFE
3.4. Fucoxanthin Quantification by HPLC
3.5. Characterization of Biochemical Composition
3.6. Analytical Methods
3.7. Anaerobic Inoculum and BMP
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, P.J.; Desclés, J.; Allen, A.E.; Bowler, C. Prospects in diatom research. Curr. Opin. Biotechnol. 2005, 16, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Villanova, V.; Fortunato, A.E.; Singh, D.; Bo, D.D.; Conte, M.; Obata, T.; Jouhet, J.; Fernie, A.R.; Marechal, E.; Falciatore, A. Investigating mixotrophic metabolism in the model diatom Phaeodactylum tricornutum. Philos. Trans. R. Soc. B-Biol. Sci. 2017, 372, 20160404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Dolch, L.-J.; Maréchal, E. Inventory of Fatty Acid Desaturases in the Pennate Diatom Phaeodactylum tricornutum. Mar. Drugs 2015, 13, 1317–1339. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, T.; Robert, J.-M. Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products. Appl. Microbiol. Biotechnol. 2003, 60, 624–632. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Soundharrajan, I.; Srigopalram, S.; Yusoff, M.M.; Maniam, G.P.; Govindan, N.; Choi, K.C. Potential pharmaceutical and biomedical applications of Diatoms microalgae-An overview. Indian J. Geo Mar. Sci. 2017, 46, 663–667. [Google Scholar]
- Sharma, N.; Simon, D.P.; Diaz-Garza, A.M.; Fantino, E.; Messaabi, A.; Meddeb-Mouelhi, F.; Germain, H.; Desgagné-Penix, I. Diatoms Biotechnology: Various Industrial Applications for a Greener Tomorrow. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Atalah, E.; Cruz, C.M.H.; Izquierdo, M.S.; Rosenlund, G.; Caballero, M.J.; Valencia, A.; Robaina, L. Two microalgae Crypthecodinium cohnii and Phaeodactylum tricornutum as alternative source of essential fatty acids in starter feeds for seabream (Sparus aurata). Aquaculture 2007, 270, 178–185. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Eilers, U.; Bikoulis, A.; Breitenbach, J.; Büchel, C.; Sandmann, G. Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. J. Appl. Phycol. 2016, 28, 123–129. [Google Scholar] [CrossRef]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, B.; Yang, B.; Sun, P.; Lu, X.; Liu, J.; Chen, F. Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Mar. Drugs 2016, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. Trac-Trends Anal. Chem. 2019, 116, 248–253. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trac-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sust. Energ. Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Conv. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Roadmap, U. Roadmap for Biomass Technologies in the United States. Biomass Research and Development Technical Advisory Committee. 2002. Available online: https://biomassboard.gov/sites/default/files/pdfs/final_biomass_roadmap_2002kw.pdf (accessed on 11 November 2021).
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion Processes in Anaerobic Digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, J.; Zhao, Q.; Laurens, L.; Jarvis, E.; Chen, S.; Frear, C. Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production. Bioresour. Technol. 2014, 161, 423–430. [Google Scholar] [CrossRef]
- Lage, S.; Willfors, A.; Hörnberg, A.; Gentili, F. Impact of organic solvents on lipid-extracted microalgae residues and wastewater sludge co-digestion. Bioresour. Technol. Reports 2021, 16, 100850. [Google Scholar] [CrossRef]
- Sardessai, Y.N.; Bhosle, S. Industrial potential of organic solvent tolerant bacteria. Biotechnology Progress 2004, 20, 655–660. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R. Thermodynamics of anaerobic digestion: Mechanism of suppression on biogas production during acidogenesis. INMATEH-Agric. Eng. 2019, 57, 287–301. [Google Scholar]
- Ruiz-Domínguez, M.C.; Marticorena, P.; Sepúlveda, C.; Salinas, F.; Cerezal, P.; Riquelme, C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Mar. Drugs 2020, 18, 528. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Uquiche, E.L. Particle size effects on supercritical CO2 extraction of oil-containing seeds. J. Am. Oil Chem. Soc. 2002, 79, 1261–1266. [Google Scholar] [CrossRef]
- Snyder, J.; Friedrich, J.; Christianson, D. Effect of moisture and particle size on the extractability of oils from seeds with supercritical CO2. J. Am. Oil Chem. Soc. 1984, 61, 1851–1856. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Modeling and simulation of the supercritical CO2 extraction of vegetable oils. J. Supercrit. Fluids 2001, 19, 161–175. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer Science & Business Media: Berlin, Germany, 2013; Volume 4. [Google Scholar]
- Vieira de Melo, S.; Costa, G.M.; Viana, A.C.; Pessoa, F.L. Computation of crossover pressure for synthesis of supercritical fluid separation systems. Comput. Chem. Eng. 2009, 27, 399–404. [Google Scholar]
- Vieira de Melo, S.; Costa, G.M.N.; Viana, A.; Pessoa, F. Solid pure component property effects on modeling upper crossover pressure for supercritical fluid process synthesis: A case study for the separation of Annatto pigments using SC-CO2. J. Supercrit. Fluids 2009, 49, 1–8. [Google Scholar] [CrossRef]
- Rad, H.B.; Sabet, J.K.; Varaminian, F. Study of solubility in supercritical fluids: Thermodynamic concepts and measurement methods-a review. Braz. J. Chem. Eng. 2020, 36, 1367–1392. [Google Scholar] [CrossRef] [Green Version]
- Foster, N.R.; Gurdial, G.S.; Yun, J.S.; Liong, K.K.; Tilly, K.D.; Ting, S.S.; Singh, H.; Lee, J.H. Significance of the crossover pressure in solid-supercritical fluid phase equilibria. Ind. Eng. Chem. Res. 1991, 30, 1955–1964. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibañez, E.; Łęska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Cerezal, P.; Salinas, F.; Medina, E.; Renato-Castro, G. Application of Box-Behnken Design and Desirability Function for Green Prospection of Bioactive Compounds from Isochrysis galbana. Appl. Sci.-Basel 2020, 10, 2789. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of Fucoxanthin and Polyphenol from Undaria pinnatifida Using Supercritical Carbon dioxide with Co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Shi, J.; Mittal, G.; Kim, E.; Xue, S.J. Solubility of Carotenoids in Supercritical CO2. Food Rev. Int. 2007, 23, 341–371. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Gao, B.; Huang, L.; Zhang, C. An integrated biorefinery process: Stepwise extraction of fucoxanthin, eicosapentaenoic acid and chrysolaminarin from the same Phaeodactylum tricornutum biomass. Algal Res. 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Partition behavior of fucoxanthin in ethanol-potassium phosphate two-phase systems. J. Chem. Technol. Biotechnol. 2014, 89, 1637–1645. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J. Appl. Phycol. 2015, 27, 957–964. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sust. Energ. Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; del Pulgar, J.S.; Aguzzi, A.; Caproni, R.; Gabrielli, P.; Lombardi-Boccia, G. Chemical characterization and nutritional evaluation of microalgal biomass from large-scale production: A comparative study of five species. Eur. Food Res. Technol. 2020, 246, 323–332. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Wang, G.; Dai, S.; He, H.; Xiang, W. A comparative analysis of fatty acid composition and fucoxanthin content in six Phaeodactylum tricornutum strains from diff erent origins. Chin. J. Oceanol. Limnol. 2016, 34, 391–398. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Rincón, B. Chapter 12—Biogas from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: London, UK, 2020; Volume 1, pp. 311–328. [Google Scholar] [CrossRef]
- Ramos-Suárez, J.L.; Carreras, N. Use of microalgae residues for biogas production. Chem. Eng. J. 2014, 242, 86–95. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Olkiewicz, M.; Torras, C.; Salvadó, J.; Clavero, E.; Bengoa, C. Effect of pre-treatments on the production of biofuels from Phaeodactylum tricornutum. J. Environ. Manag. 2016, 177, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.-C.; Abdou, R.H.; McGinn, P.J.; O’Leary, S.J.; Guiot, S.R. Fate of palmitic, palmitoleic and eicosapentaenoic acids during anaerobic digestion of Phaeodactylum tricornutum at varying lipid concentration. Algal Res. 2014, 6, 46–51. [Google Scholar] [CrossRef]
- Zamalloa, C.; Boon, N.; Verstraete, W. Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl. Energy 2012, 92, 733–738. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Biotecnología del Medio Ambiente, Principios y Aplicaciones; McGraw Hill: Madrid, Spain, 2001; 760 p, ISBN 9788448132804. [Google Scholar]
- Zhang, J.; Wang, S.; Lang, S.; Xian, P.; Xie, T. Kinetics of combined thermal pretreatment and anaerobic digestion of waste activated sludge from sugar and pulp industry. Chem. Eng. J. 2016, 295, 131–138. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ahring, B. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenerg. 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- American Society of Agricultural and Biological Engineers. Method of Determining and Expressing Particle Size of Chopped Forage Materials by Screening; ASABE: St. Joseph, MI, USA, 2007; Volume 424, pp. 663–665. [Google Scholar]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 104824. [Google Scholar] [CrossRef]
- Rosa, P.T.; Meireles, M.A.A. Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. J. Food Eng. 2005, 67, 235–240. [Google Scholar] [CrossRef]
- del Valle, J.M.; Núñez, G.A.; Aravena, R.I. Supercritical CO2 oilseed extraction in multi-vessel plants. 1. Minimization of operational cost. J. Supercrit. Fluids 2014, 92, 197–207. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A Single-Step Method for Rapid Extraction of Total Lipids from Green Microalgae. PLoS ONE 2014, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 17th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Rice, E.W., Franson, M.A.H., Eds.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- The US Department of Agriculture and the US Composting Council. Test Methods for the Examination of Composting and Compost (TMECC); Edaphos International: Houston, TX, USA, 2002. Available online: https://www.compostingcouncil.org/page/tmecc (accessed on 22 November 2021).






| SFE Conditions | CO2 | Fucoxanthin | |||
|---|---|---|---|---|---|
| P (MPa) | T (°C) | Density (g/mL) | Superficial Velocity (mm/s) | Purity (mg/g Extract) | Recovery (% w/w) |
| 20 | 30 | 0.890 | 0.426 | 11.07 ± 0.46 a | 2.87 ± 0.12 a |
| 20 | 50 | 0.784 | 0.484 | 8.56 ± 0.14 a | 2.38 ± 0.04 a |
| 30 | 30 | 0.948 | 0.401 | 60.62 ± 0.40 d | 18.82 ± 0.12 e |
| 30 | 50 | 0.870 | 0.436 | 37.89 ± 1.00 c | 11.59 ± 0.31 d |
| 40 | 30 | 0.988 | 0.384 | 29.06 ± 1.63 b | 4.76 ± 0.19 b |
| 40 | 50 | 0.923 | 0.411 | 26.67 ± 2.08 b | 8.53 ± 0.66 c |
| Co-Solvent (Ethanol, % v/v) | Ethanol + CO2 (g/min) | Fucoxanthin Purity (mg/g Extract) | Fucoxanthin Recovery (% w/w) |
|---|---|---|---|
| 10 | 3.406 | 22.06 ± 1.92 ab | 12.69 ± 1.14 a |
| 20 | 3.208 | 28.65 ± 0.49 b | 41.68 ± 0.71 b |
| 30 | 3.002 | 13.75 ± 0.65 a | 34.17 ± 1.61 b |
| 40 | 2.801 | 85.03 ± 7.67 c | 66.60 ± 6.00 c |
| 50 | 2.591 | 74.73 ± 2.45 c | 39.46 ± 1.29 b |
| Parameters | P. tricornutum | Supercritical Fluid Extracted P. tricornutum |
|---|---|---|
| TS (mg/kg) | 891,635 ± 1485 | 920,030 ± 1025 |
| MS (mg/kg) | 182,375 ± 2030 | 200,420 ± 4005 |
| VS (mg/kg) | 709,260 ± 3520 | 719,610 ± 1090 |
| VS (%) | 79.55 | 78.22 |
| Humidity (%) | 10.84 ± 0.07 | 8.00 ± 0.05 |
| N-TKN (mg/kg) | 56,167 ± 173 | 42,937 ± 4687 |
| PT –P2O5 (mg/g) | 1.692 ± 0.038 | 0.433 ± 0.004 |
| Cr (mg/kg) | 10.9 ± 0.25 | 16.36 ± 0.10 |
| Cd (mg/kg) | 2.05 ± 0.30 | 4.26 ± 0.65 |
| Pb (mg/kg) | 3.25 ± 0.50 | 5.11 ±0.70 |
| Ni (mg/kg) | 4.11 ± 0.15 | 6.48 ± 0.68 |
| Zn (mg/kg) | 180.30 ± 0.85 | 255.43 ± 0.68 |
| Cu (mg/kg) | 12.95 ± 0.17 | 18.84 ± 0.08 |
| Strain/Diatom | Proteins (% w/w) | Carbohydrates (% w/w) | Lipids (% w/w) | References |
|---|---|---|---|---|
| P. tricornutum | 36.20 ± 1.20 d | 18.80 ± 1.12 b | 16.15 ± 0.75 c | In this study |
| Supercritical fluid extracted P. tricornutum | 14.41 ± 0.62 a | 13.32 ± 0.45 a | 5.01 ± 0.01 a | In this study |
| P. tricornutum F&M-M4 | 38.8 ± 0.11 e | 11.0 ± 0.70 a | 20.5 ± 0.54 d | [57] |
| P. tricornutum | 29.4 ± 0.4 c | 10.5 ± 0.26 *a | 17.1 ± 0.9 c | [54] |
| P. tricornutum | 26.95 ± 0.05 b | 16.91 ± 1.61b | 12.73 ± 0.13 b | [53] |
| Variables | 1st Load P. Tricornutum | 2nd Load P. Tricornutum | 1st Load Supercritical Fluid Extracted P. Tricornutum | 2nd Load Supercritical Fluid Extracted P. Tricornutum |
|---|---|---|---|---|
| pH | 8.26 ± 0.01 | 9.05 ± 0.02 | 8.00 ± 0.02 | 8.29 ± 0.01 |
| Conductivity (mS/cm) | 18.09 ± 0.02 | 18.14 ± 0.03 | 17.89 ± 0.01 | 17.75 ± 0.01 |
| T-Alk (mg CO3Ca/L) | 9678 ± 90 | 10,098 ± 105 | 9815 ± 115 | 10,300 ± 95 |
| VFA (mg C/L) | 134 ± 10 | 87 ± 5 | 184 ± 8 | 154 ± 5 |
| VFA (mgCH3COOH/L) | 335 ± 15 | 218 ± 13 | 525 ± 20 | 385 ± 13 |
| TS (mg/L) | 17,952 ± 435 | 18,606 ± 105 | 17,900 ± 80 | 18,325 ± 160 |
| MS (mg/L) | 11,177 ± 210 | 11,311 ± 85 | 11,940 ± 250 | 11,898 ± 350 |
| VS (mg/L) | 6775 ± 225 | 7295± 40 | 6260 ± 100 | 6620 ± 430 |
| N-NH4+ (mg/L) | 1472 ± 60 | 1653± 50 | 1265 ± 5 | 1290 ± 10 |
| TKN (mg/L) | 2013 ± 25 | 2169 ± 25 | 1720 ± 10 | 1812 ± 15 |
| PT –P2O5 (mg/L) | 4.72 ± 0.02 | 5.56 ± 0.02 | 5.01 ± 0.02 | 5.83 ± 0.02 |
| Cu (mg/L) | 0.28 ± 0.01 | 0.17 ± 0.02 | 0.10 ± 0.01 | 0.05 ± 0.01 |
| Ni (mg/L) | 0.90 ± 0.01 | 0.22 ± 0.01 | 0.02 ± 0.01 | <DL (0.05 mg/L) |
| Zn (mg/L) | 0.98 ± 0.02 | 0.26 ± 0.03 | 0.30 ± 0.01 | <DL (0.05 mg/L) |
| Pb (mg/L) | 6.36 ± 0.06 | 5.46 ± 0.05 | 0.43 ± 0.01 | 0.21 ± 0.01 |
| Cr (mg/L) | 0.078 ± 0.002 | <DL (0.05 mg/L) | 2.96 ± 0.01 | <DL (0.05 mg/L) |
| Cd (mg/L) | 0.60 ± 0.01 | 0.22 ± 0.02 | 0.41 ± 0.01 | <DL (0.05 mg/L) |
| Acetic acid (mg/L) | 26.80 ± 2.56 | 28.4 ± 4.21 | 51.80± 5.20 | 27.95 ± 4.12 |
| Propionic acid (mg/L) | 6.12 ± 0.25 | 7.69 ± 2.10 | 14.48 ± 2.35 | 7.23 ± 1.98 |
| Isobutiric acid (mg/L) | 25.00 ± 1.00 | 27.54 ± 1.36 | 21.67 ± 1.38 | 4.87 ± 5.24 |
| Butiric acid (mg/L) | 8.35 ± 1.40 | 7.63 ± 1.02 | 25.89 ± 3.33 | 14.23 ± 3.07 |
| Isovaleric acid (mg/L) | 18.25 ± 3.15 | 25.08 ± 3.00 | <DL (0.5 mg/L) | <DL (0.5 mg/L) |
| Valeric acid (mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) |
| Caproic acid (mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) | <DL (0.5 mg/L) |
| Variables | Value | Variables | Value |
|---|---|---|---|
| pH | 7.70 ± 0.01 | TS (mg/L) | 17,565 ± 115 |
| Conductivity (mS/cm) | 18.01 ± 0.01 | MS (mg/L) | 11,855 ± 110 |
| T-Alk (mg CO3Ca/L) | 9496 ± 65 | VS (mg/L) | 5710 ± 95 |
| VFA (mg C/L) | 195 ± 10 | N-NH4+ (mg/L) | 1055 ± 6 |
| VFA (mg CH3COOH/L) | 485 ± 25 | N-TKN (mg/L) | 1579 ± 10 |
| PT –P2O5(mg/L) | 16.60 ± 0.08 | Acetic acid (mg/L) | 54.48 ± 8.60 |
| Cu (mg/L) | 0.310 ± 0.002 | Propionic acid (mg/L) | 8.64 ± 2.35 |
| Ni (mg/L) | 0.450 ± 0.002 | Isobutyric acid (mg/L) | 9.67 ± 5.25 |
| Zn (mg/L) | 2.750 ± 0.030 | Butyric acid (mg/L) | 19.40 ± 5.40 |
| Pb (mg/L) | 0.310 ± 0.025 | Isovaleric acid (mg/L) | n.d. |
| Cr (mg/L) | 0.078 ± 0.002 | Valeric acid (mg/L) | n.d. |
| Cd (mg/L) | 0.090 ± 0.002 | Caproic acid (mg/L) | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Domínguez, M.C.; Salinas, F.; Medina, E.; Rincón, B.; Martín, M.Á.; Gutiérrez, M.C.; Cerezal-Mezquita, P. Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion. Mar. Drugs 2022, 20, 127. https://doi.org/10.3390/md20020127
Ruiz-Domínguez MC, Salinas F, Medina E, Rincón B, Martín MÁ, Gutiérrez MC, Cerezal-Mezquita P. Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion. Marine Drugs. 2022; 20(2):127. https://doi.org/10.3390/md20020127
Chicago/Turabian StyleRuiz-Domínguez, Mari Carmen, Francisca Salinas, Elena Medina, Bárbara Rincón, Marí Ángeles Martín, Marí Carmen Gutiérrez, and Pedro Cerezal-Mezquita. 2022. "Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion" Marine Drugs 20, no. 2: 127. https://doi.org/10.3390/md20020127
APA StyleRuiz-Domínguez, M. C., Salinas, F., Medina, E., Rincón, B., Martín, M. Á., Gutiérrez, M. C., & Cerezal-Mezquita, P. (2022). Supercritical Fluid Extraction of Fucoxanthin from the Diatom Phaeodactylum tricornutum and Biogas Production through Anaerobic Digestion. Marine Drugs, 20(2), 127. https://doi.org/10.3390/md20020127