Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing
Abstract
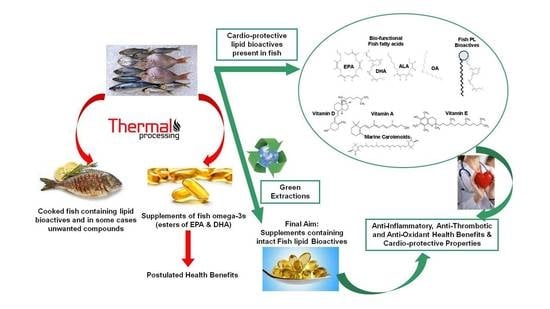
:1. Introduction
2. Fish Lipid Bioactives and Health Benefits
2.1. Fatty Acid Content of Fish
2.1.1. Saturated Fatty Acids (SFA) in Fish
2.1.2. Monounsaturated Fatty Acids (MUFA) in Fish
2.1.3. Polyunsaturated Fatty Acids (PUFA) of Fish and the Importance of the n-6/n-3 PUFA Ratio
2.2. Fish Polar Lipids (Phospholipids and Glycolipids)
2.3. Fish Alkylacylglycerols
2.4. Lipid Vitamins in Fish
2.4.1. The Lipid Vitamin D
2.4.2. The Lipid Vitamin E
2.4.3. The Lipid Vitamin A and Marine Carotenoids
3. The Effects of Thermal Processing—Cooking on the Bio-Functionality of Fish Lipid Content
4. Current and Future Perspectives on Green Extraction Methodologies for the Recovery of High-Quality Fish Lipid Bioactives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Jamioł-Milc, D.; Biernawska, J.; Liput, M.; Stachowska, L.; Domiszewski, Z. Seafood Intake as a Method of Non-Communicable Diseases (NCD) Prevention in Adults. Nutrients 2021, 13, 1422. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed] [Green Version]
- Nesheim, M.C.; Oria, M.; Yih, P.T.; National Research Council; Institute of Medicine; Food and Nutrition Board; Board on Agriculture and Natural Resources. Committee on a Framework for Assessing the Health, Environmental, and Social Effects of the Food System. In Dietary Recommendations for Fish Consumption; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Kiczorowska, B.; Samolińska, W.; Grela, E.R.; Bik-Małodzińska, M. Nutrient and Mineral Profile of Chosen Fresh and Smoked Fish. Nutrients 2019, 11, 1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gormley, R. Fish as a functional food. Food Sci. Technol. 2006, 20, 27. [Google Scholar]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Gil, F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015, 113, S58–S67. [Google Scholar] [CrossRef] [Green Version]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for Antithrombotic Polar Lipids from Salmon, Herring, and Boarfish By-Products. Foods 2019, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254. [Google Scholar]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Ruthu Murthy, P.S.; Rai, A.K.; Bhaskar, N. Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. J. Food Sci. Technol. 2014, 51, 1884–1892. [Google Scholar] [CrossRef] [Green Version]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Adv. Food Nutr. Res. 2012, 65, 31–46. [Google Scholar] [PubMed]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of Valuable Compounds and Bioactive Metabolites from By-Products of Fish Discards Using Chemical Processing, Enzymatic Hydrolysis, and Bacterial Fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Pothineni, N.V.; Singhal, M.; Paydak, H.; Saldeen, T.; Mehta, J.L. Fish, Fish Oils and Cardioprotection: Promise or Fish Tale? Int. J. Mol. Sci. 2018, 19, 3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorngren, M.; Gustafson, A. Effects of 11-week increase in dietary eicosapentaenoic acid on bleeding time, lipids, and platelet aggregation. Lancet 1981, 318, 1190–1193. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Enns, J.E.; Yeganeh, A.; Zarychanski, R.; Abou-Setta, A.M.; Friesen, C.; Zahradka, P.; Taylor, C.G. The impact of omega-3 polyunsaturated fatty acid supplementation on the incidence of cardiovascular events and complications in peripheral arterial disease: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walz, C.P.; Barry, A.R.; Koshman, S.L. Omega-3 polyunsaturated fatty acid supplementation in the prevention of cardiovascular disease. Can. Pharm. J. 2016, 149, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Myung, S.; Lee, Y.; Seo, H.; Korean Meta-Analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Int. Med. 2012, 172, 686–694. [Google Scholar]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar] [CrossRef] [Green Version]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating paf catabolism. Lipids Health Dis. 2011, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural Elucidation of Irish Organic Farmed Salmon (Salmo salar) Polar Lipids with Antithrombotic Activities. Mar. Drugs 2018, 16, 176. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouinard-Watkins, R.; Lacombe, R.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA esterified to phosphatidylserine or phosphatidylcholine is more efficient at targeting the brain than DHA esterified to triacylglycerol. Mol. Nutr. Food Res. 2019, 63, 1801224. [Google Scholar] [CrossRef] [PubMed]
- Merdzhanova, A.; Stancheva, M.; Dobreva, D.A.; Makedonski, L. Fatty acid and fat soluble vitamins composition of raw and cooked Black Sea horse mackerel. Ovidius Univ. Ann. Chem. 2013, 24, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.S.; Pan, A.; Wang, R.; Odegaard, A.O.; Pereira, M.A.; Yuan, J.M.; Koh, W.P. The association between dietary omega-3 fatty acids and cardiovascular death: The Singapore Chinese Health Study. Eur. J. Prev. Cardiol. 2015, 22, 364–372. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Varzakas, T.; Munekata, P.E.S.; Movilla Fierro, E.; Lorenzo, J.M. Omega-3-Rich Oils from Marine Side Streams and Their Potential Application in Food. Mar. Drugs 2021, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Cropotova, J.; Mozuraitytė, R.; Standal, I.B.; Rustad, T. Assessment of lipid oxidation in Atlantic mackerel (Scomber scombrus) subjected to different antioxidant and sous-vide cooking treatments by conventional and fluorescence microscopy methods. Food Control 2019, 104, 1–8. [Google Scholar] [CrossRef]
- Kundam, D.N.; Acham, I.O.; Girgih, A.T. Bioactive compounds in fish and their health benefits. Asian Food Sci. J. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, K.; Iso, H.; Date, C.; Fukui, M.; Wakai, K.; Kikuchi, S.; Inaba, Y.; Tanabe, N.; Tamakoshi, A.; Group, J.S. Fish, ω-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based co-hort of Japanese men and women: The JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J. Am. Coll. Cardiol. 2008, 52, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, S.; Haddad, E.H.; Mejia, A.; Sabaté, J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: A randomized controlled study. Am. J. Clin. Nutr. 2009, 89, 1657S–1663S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, M.L.; Gilbert, J.; Holliday, R.A.; Elwood, P.; Fehily, A.; Rogers, S.; Sweetnam, P.; Deadman, N. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 334, 757–761. [Google Scholar] [CrossRef]
- Singh, R.B.; Niaz, M.A.; Sharma, J.P.; Kumar, R.; Rastogi, V.; Moshiri, M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: The Indian experiment of infarct survival—4. Cardiovasc. Drugs Ther. 1997, 11, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Investigators, G.P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial in-farction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Von Schacky, C.; Angerer, P.; Kothny, W.; Theisen, K.; Mudra, H. The effect of dietary Ω-3 fatty acids on coronary atherosclerosis: A randomized, double-blind, placebo-controlled trial. Anna. Intern. Med. 1999, 130, 554–562. [Google Scholar] [CrossRef]
- Bucher, H.C.; Hengstler, P.; Schindler, C.; Meier, G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am. J. Med. 2002, 112, 298–304. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Harris, W.S.; Garg, S.; Na, B.; Whooley, M.A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 2009, 205, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- He, K.; Liu, K.; Daviglus, M.L.; Jenny, N.S.; Mayer-Davis, E.; Jiang, R.; Steffen, L.; Siscovick, D.; Tsai, M.; Her-Rington, D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Turunen, A.W.; Jula, A.; Suominen, A.L.; Männistö, S.; Marniemi, J.; Kiviranta, H.; Tiittanen, P.; Karanko, H.; Moilanen, L.; Nieminen, M.S.; et al. Fish consumption, omega-3 fatty acids, and environmental contaminants in relation to low-grade inflammation and early atherosclerosis. Environ. Res. 2013, 120, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Das Undurti, N.; Chrysohoou, C.; Skoumas, Y.; Stefanadis, C. Fish Consumption Among Healthy Adults Is Associated with Decreased Levels of Inflammatory Markers Related to Cardiovascular Disease. J. Am. Coll. Cardiol. 2005, 46, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Xin, W.; Wei, W.; Li, X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2012, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- König, A.; Bouzan, C.; Cohen, J.T.; Connor, W.E.; Kris-Etherton, P.M.; Gray, G.M.; Lawrence, R.S.; Savitz, D.A.; Teutsch, S.M. A Quantitative Analysis of Fish Consumption and Coronary Heart Disease Mortality. Am. J. Prev. Med. 2005, 29, 335–346. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Horn, L.V.; Dyer, A.R.; Greenland, P. Accumulated Evidence on Fish Consumption and Coronary Heart Disease Mortality. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papamichael, M.M.; Katsardis, C.; Lambert, K.; Tsoukalas, D.; Koutsilieris, M.; Erbas, B.; Itsiopoulos, C. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: A randomised controlled trial. J. Hum. Nutr. Diet 2019, 32, 185–197. [Google Scholar] [CrossRef]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HEL-FIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Tedeschi, S.K.; Bathon, J.M.; Giles, J.T.; Lin, T.C.; Yoshida, K.; Solomon, D.H. Relationship Between Fish Consumption and Disease Activity in Rheumatoid Arthritis. Arthritis Care Res. 2018, 70, 327–332. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bønaa, K.H.; Hansen, J.B.; Myhre, E. Effects of highly purified eicosapentaenoic acid and doco-sahexaenoic acid on hemodynamics in humans. Am. J. Clin. Nutr. 1998, 68, 52–59. [Google Scholar] [CrossRef]
- Redfern, S.; Dermiki, M.; Fox, S.; Lordan, R.; Shiels, K.; Kumar Saha, S.; Tsoupras, A.; Zabetakis, I. The effects of cooking salmon sous-vide on its antithrombotic properties, lipid profile and sensory characteristics. Food Res. Int. 2021, 139, 109976. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Demopoulos, C.A.; Zabetakis, I. In vivo anti-atherogenic properties of cultured gilthead sea bream (Sparus aurata) polar lipid extracts in hypercholesterolaemic rabbits. Food Chem. 2010, 120, 831–836. [Google Scholar] [CrossRef]
- Morphis, G.; Kyriazopoulou, A.; Nasopoulou, C.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. Assessment of the in Vitro Antithrombotic Properties of Sardine (Sardina pilchardus) Fillet Lipids and Cod Liver Oil. Fishes 2016, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.A.; Zabetakis, I. Comparison of antiatherogenic properties of lipids obtained from wild and cultured sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M.; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Marchioli, R.; Gardner, T.; Ferrazzi, P.; O’Gara, P.; Latini, R.; Libby, P.; Lombardi, F.; Macchia, A.; Page, R.; et al. The ω-3 fatty acids for Prevention of Post-Operative Atrial Fibrillation trial—Rationale and design. Am. Heart J. 2011, 162, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ORIGIN Trial Investigators. Cardiovascular and Other Outcomes Postintervention with Insulin Glargine and Omega-3 Fatty Acids (ORIGINALE). Diabetes Care 2016, 39, 709–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Zabetakis, I. Comment on “optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections”. Nutrients 2020, 12, 1181, Comment: Nutrients 2020, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [Green Version]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]
- Iggman, D.; Risérus, U. Role of different dietary saturated fatty acids for cardiometabolic risk. Clin. Lipidol. 2011, 6, 209–223. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.H.; Ciçek, E.; Polat, A.; Kuley, E. Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int. J. Food Sci. Nutr. 2009, 60, 464–475. [Google Scholar] [CrossRef]
- Perdomo, L.; Beneit, N.; Otero, Y.F.; Escribano, O.; Diaz-Castroverde, S.; Gómez-Hernández, A.; Benito, M. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc. Diabetol. 2015, 14, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, M.G.; Selmin, O.I.; Stillwaterm, B.J.; Neumayerm, L.A.; Romagnolo, D.F. Do Olive and Fish Oils of the Mediterranean Diet Have a Role in Triple Negative Breast Cancer Prevention and Therapy? An Exploration of Evidence in Cells and Animal Models. Front. Nutr. 2020, 7, 571455. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; Von Schacky, C.; Kleber, M.E. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135. [Google Scholar] [CrossRef]
- Nunez, D.; Randon, J.; Gandhi, C.; Siafaka-Kapadai, A.; Olson, M.S.; Hanahan, D.J. The inhibition of platelet-activating factor-induced platelet activation by oleic acid is associated with a decrease in polyphosphoinositide metabolism. J. Biol. Chem. 1990, 265, 18330–18338. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation and Cardiovascular Diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 53–117. [Google Scholar]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, K.S. Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 2003, 38, 336–344. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. (Eds.) Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Academic Press: London, UK, 2019. [Google Scholar]
- Das, U.N. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: But, why and how? Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; McFadden, G. Modulation of NF-κB signalling by microbial pathogens. Nat. Rev. Microbiol. 2011, 9, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Barlera, S.; Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; Tognoni, G.; et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar]
- Mahaffey, K.R.; Sunderland, E.M.; Chan, H.M.; Choi, A.L.; Grandjean, P.; Mariën, K.; Oken, E.; Sakamoto, M.; Schoeny, R.; Weihe, P.; et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmer-cury exposure from fish consumption. Nutr. Rev. 2011, 69, 493–508. [Google Scholar] [CrossRef]
- Bang, H.O. Lipid research in Greenland. Preventive and therapeutic consequences. Scand. J. Soc. Med. 1990, 18, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Dolecek, T.A.; Grandits, G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev. Nutr. Diet. 1991, 66, 205–216. [Google Scholar] [PubMed]
- Menotti, A.; Keys, A.; Blackburn, H.; Kromhout, D.; Karvonen, M.; Nissinen, A.; Pekkanen, J.; Punsar, S.; Fidanza, F.; Giampaoli, S.; et al. Comparison of multivariate predictive power of major risk factors for coronary heart diseases in different countries: Results from eight nations of the Seven Countries Study, 25-year follow-up. J. Cardiovasc. Risk. 1996, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.L.; Stamler, J.; Orencia, A.J.; Dyer, A.R.; Liu, K.; Greenland, P.; Walsh, M.K.; Morris, D.; She-Kelle, R.B. Fish consumption and the 30-year risk of fatal myocardial infarction. N. Engl. J. Med. 1997, 336, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sasaki, S.; Amano, K.; Kesteloot, H. Fish consumption and mortality from all causes, ischemic heart disease, and stroke: An ecological study. Prev. Med. 1999, 28, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, S.; Moriguchi, E.H.; Ishikawa, P.; Hekman, P.; Nara, Y.; Mimura, G.; Moriguchi, Y.; Yamori, Y. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J. Cardiovasc. Risk. 1997, 4, 191–199. [Google Scholar] [CrossRef]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Albert, C.M.; Hennekens, C.H.; O’donnell, C.J.; Ajani, U.A.; Carey, V.J.; Willett, W.C.; Ruskin, J.N.; Manson, J.E. Fish consumption and risk of sudden cardiac death. JAMA 1998, 279, 23–28. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, H.; Yang, X.; Luo, X.; Wang, X.; Shao, C.; He, J. Fish Consumption and Stroke Risk: A Meta-Analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2019, 28, 604–611. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Raghunathan, T.; King, I.; Weinmann, S.; Wicklund, K.G.; Albright, J.; Bovbjerg, V.; Arbo-Gast, P.; Smith, H.; Kushi, L.H. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA 1995, 274, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Korup, E.; Aarøe, J.; Toft, E.; Møller, J.; Rasmussen, K.; Dyerberg, J.; Schmidt, E.B. Fish consumption, n-3 fatty acids in cell membranes, and heart rate variability in survivors of myocardial infarction with left ventricular dysfunction. Am. J. Cardiol. 1997, 79, 1670–1673. [Google Scholar] [CrossRef]
- Kinoshita, I.; Itoh, K.; Nishida-Nakai, M.; Hirota, H.; Otsuji, S.; Shibata, N. Antiarrhythmic effects of eicosa-pentaenoic acid during myocardial infarction: Enhanced cardiac microsomal (Ca2+-Mg2+)-ATPase activity. Jpn. Circ. J. 1994, 58, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Black, K.L.; Culp, B.; Madison, D.; Randall, O.S.; Lands, W.E. The protective effects of dietary fish oil on focal cerebral infarction. Prostaglandins Med. 1979, 3, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.X.; Leaf, A. Antiarrhythmic effects of polyunsaturated fatty acids: Recent studies. Circulation 1996, 94, 1774–1780. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Kromhout, D.; Bosschieter, E.B.; Coulander, C.D.L. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Ciubotaru, I.; Lee, Y.S.; Wander, R.C. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J. Nutr. Biochem. 2003, 14, 513–521. [Google Scholar] [CrossRef]
- Trebble, T.; Arden, N.K.; Stroud, M.A.; Wootton, S.A.; Burdge, G.C.; Miles, E.A.; Ballinger, A.B.; Thompson, R.L.; Calder, P.C. Inhibition of tumour necrosis factor-α and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br. J. Nutr. 2003, 90, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoso, A.; González, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, F.; Cheng-Sánchez, I. Chemistry and Biology of Bioactive Glycolipids of Marine Origin. Mar. Drugs 2018, 16, 294. [Google Scholar]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive Lipids of Marine Microalga Chlorococcum sp. SABC 012504 with Anti-Inflammatory and Anti-Thrombotic Activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Hayashi, H.; Tanaka, Y.; Hibino, H.; Umeda, Y.; Kawamitsu, H.; Fujimoto, H.; Amakawa, T. Beneficial effect of salmon roe phosphatidylcholine in chronic liver disease. Curr. Med. Res. Opin. 1999, 15, 177–184. [Google Scholar] [CrossRef]
- Taylor, L.A.; Pletschen, L.; Arends, J.; Unger, C.; Massing, U. Marine phospholipids—A promising new dietary approach to tumor-associated weight loss. Support. Care Cancer 2010, 18, 159–170. [Google Scholar] [CrossRef]
- Hung, M.C.; Shibasaki, K.; Yoshida, R.; Sato, M.; Imaizumi, K. Learning behaviour and cerebral protein kinase C, anti-oxidant status, lipid composition in senescence-accelerated mouse: Influence of a phosphatidylcholine–vitamin B12 diet. Br. J. Nutr. 2001, 86, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Scholey, A.B.; Camfield, D.A.; Hughes, M.E.; Woods, W.; Stough, C.K.K.; White, D.J.; Gondalia, S.V.; Frederiksen, P.D. A randomized controlled trial investigating the neurocognitive effects of Lacprodan® PL-20, a phospholipid-rich milk protein concentrate, in elderly participants with age-associated memory impairment: The Phospholipid Intervention for Cognitive Ageing Reversal (PLICAR): Study protocol for a randomized controlled trial. Trials 2013, 14, 404. [Google Scholar]
- Zhang, K. Omega-3 phospholipids. In Polar Lipids: Biology, Chemistry, and Technology; Ahmad, M.U., Xu, X., Eds.; AOCS Press: Urbana, IL, USA, 2015; pp. 463–493. [Google Scholar]
- Jung, Y.Y.; Nam, Y.; Park, Y.S.; Lee, H.S.; Hong, S.A.; Kim, B.K.; Park, E.S.; Chung, Y.H.; Jeong, J.H. Protective effect of phosphatidylcholine on lipopolysaccharide-induced acute inflammation in multiple organ injury. Korean J. Physiol. Pharmacol. 2013, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Rementzis, J.; Antonopoulou, S.; Argyropoulos, D.; Demopoulos, C.A. Biologically active lipids from S. scombrus. Adv Exp Med Biol. 1996, 416, 65–72. [Google Scholar] [PubMed]
- Nasopoulou, C.; Psani, E.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. Evaluation of sensory and in vitro cardio protective properties of sardine (Sardina pilchardus): The effect of grilling and brining. Food Nutr. Sci. 2013, 4, 940–949. [Google Scholar]
- Panayiotou, A.; Samartzis, D.; Nomikos, T.; Fragopoulou, E.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Lipid fractions with aggregatory and antiaggregatory activity toward platelets in fresh and fried cod (Gadus morhua): Correlation with platelet-activating factor and atherogenesis. J. Agric. Food Chem. 2000, 48, 6372–6379. [Google Scholar] [CrossRef]
- Lewkowicz, N.; Lewkowicz, P.; Kurnatowska, A.; Tchórzewski, H. Biological action and clinical application of shark liver oil. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2006, 20, 598–601. [Google Scholar]
- Deniau, A.L.; Mosset, P.; Pédrono, F.; Mitre, R.; Bot, D.L.; Legrand, A.B. Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Mar. Drugs 2010, 8, 2175–2184. [Google Scholar] [CrossRef] [Green Version]
- Iannitti, T.; Palmieri, B. An update on the therapeutic role of alkylglycerols. Mar. Drugs 2010, 8, 2267–2300. [Google Scholar] [CrossRef] [Green Version]
- Ngwenya, B.Z.; Foster, D.M. Enhancement of antibody production by lysophosphatidylcholine and alkylglycerol. Proc. Soc. Exp. Biol. Med. 1991, 196, 69–75. [Google Scholar] [CrossRef]
- Brohult, A.; Brohult, J.; Brohult, S. Regression of tumour growth after administration of alkoxyglycerols. Acta Obstetr Gynecol. Scand. 1978, 57, 79–83. [Google Scholar] [CrossRef]
- Pedrono, F.; Martin, B.; Leduc, C.; Le Lan, J.; Saïag, B.; Legrand, P.; Moulinoux, J.P.; Legrandm, A.B. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr. Cancer 2004, 48, 64–69. [Google Scholar] [CrossRef]
- Molina, S.; Moran-Valero, M.I.; Martin, D.; Vázquez, L.; Vargas, T.; Torres, C.F.; De Molina, A.R.; Reglero, G. Antiproliferative effect of alkylglycerols as vehicles of butyric acid on colon cancer cells. Chem. Phys. Lipids 2013, 175, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Malesa-Ciećwierz, M.; Usydus, Z. Vitamin D: Can fish food–based solutions be used for reduction of vitamin D deficiency in Poland? Nutrition 2015, 31, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Recent developments in intestinal calcium absorption. Nutr. Rev. 2009, 67, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, S.; Katai, K.; Miyamoto, K.I.; Kishida, S.; Segawa, H.; Nii, T.; Tanaka, H.; Tani, Y.; Arai, H.; Morita, K.; et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1, 25-dihydroxyvitamin D3. Biochem. J. 1999, 343, 705–712. [Google Scholar]
- Grant, W.B. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog. Biophys. Mol. Biol. 2006, 92, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. What is new in vitamin D: 2006–2007. Curr. Opin. Rheumatol. 2007, 19, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Atkins, G.J. The skeleton as an intracrine organ for vitamin D metabolism. Mol. Asp. Med. 2008, 29, 397–406. [Google Scholar] [CrossRef]
- De la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol effects on activities and metabolism of platelet activating factor and on inflammatory cytokines in hemodialysis patients. Int. J. Artif. Organs 2013, 36, 87–96. [Google Scholar] [CrossRef]
- Ross, A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011, 14, 938–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential Role of Nutrition. Front. Nutr. 2020, 7, 583080. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Janjusevic, M.; Gagno, G.; Fluca, A.L.; Padoan, L.; Beltrami, A.P.; Sinagra, G.; Moretti, R.; Aleksova, A. The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci. 2022, 289, 120193. [Google Scholar] [CrossRef]
- Mendivil, C.O. Fish Consumption: A Review of Its Effects on Metabolic and Hormonal Health. Nutr. Metab. Insights 2021, 14, 11786388211022378. [Google Scholar] [CrossRef]
- González-Rodríguez, L.G.; Estaire, P.; Peñas-Ruiz, C.; Ortega, R.M.; UCM Research Group VALORNUT (920030). Vitamin D intake and dietary sources in a representative sample of Spanish adults. J Hum Nutr Diet. 2013, 26 (Suppl. S1), 64–72. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitamin D and the skin: What should a dermatologist know? G. Ital. Dermatol. Venereol. 2019, 154, 669–680. [Google Scholar] [CrossRef]
- Lock, E.J.; Waagbø, R.; Wendelaar Bonga, S.; Flik, G. The significance of vitamin D for fish: A review. Aquac. Nutr. 2010, 16, 100–116. [Google Scholar] [CrossRef]
- Soto-Dávila, M.; Valderrama, K.; Inkpen, S.M.; Hall, J.R.; Rise, M.L.; Santanderm, J. Effects of Vitamin D2 (Ergocalciferol) and D3 (Cholecalciferol) on Atlantic Salmon (Salmo salar) Primary Macrophage Immune Response to Aeromonas salmonicida subsp. salmonicida Infection. Front. Immunol. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birgisdottir, B.E.; Brantsaeter, A.L.; Kvalem, H.E.; Knutsen, H.K.; Haugen, M.; Alexander, J.; Hetland, R.B.; Aksnes, L.; Meltzer, H.M. Fish liver and seagull eggs, vitamin D-rich foods with a shadow: Results from the Norwegian Fish and Game Study. Mol. Nutr. Food Res. 2012, 56, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Muñoz, I.; Muñoz, S.; Díaz, N.F.; Medina, A.; Bazaes, J.; Riquelme, C. Nutritional Enhancement of Farmed Salmon Meat via Non-GMO Nannochloropsis Gaditana: Eicosapentaenoic Acid (EPA, 20:5 n-3), Docosapentaenoic Acid (DPA, 22:5 n-3) and Vitamin D3 for Human Health. Molecules 2020, 25, 4615. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.; Smith, C.; Bysted, A.; Cashman, K.D. Vitamin D in Wild and Farmed Atlantic Salmon (Salmo salar)—What Do We Know? Nutrients 2019, 11, 982. [Google Scholar] [CrossRef] [Green Version]
- De Roos, B.; Wood, S.; Bremner, D.; Bashir, S.; Betancor, M.B.; Fraser, W.D.; Duthie, S.J.; Horgan, G.W.; Sneddon, A.A. The nutritional and cardiovascular health benefits of rapeseed oil-fed farmed salmon in humans are not decreased compared with those of traditionally farmed salmon: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 2063–2075. [Google Scholar] [CrossRef]
- Kjerstad, M.; Larssen, W.E.; Midtbø, L.K. Belly flap from Norwegian spring-spawning herring (Clupea harengus L.): A potentially new product with high content of vitamin D, EPA and DHA. Heliyon 2020, 6, e05239. [Google Scholar] [CrossRef]
- Dovnik, A.; Mujezinović, F. The Association of Vitamin D Levels with Common Pregnancy Complications. Nutrients 2018, 10, 867. [Google Scholar] [CrossRef] [Green Version]
- Woon, F.C.; Chin, Y.S.; Ismail, I.H.; Batterham, M.; Abdul Latiff, A.H.; Gan, W.Y.; Appannah, G.; Mohammed Hussien, S.H.; Edi, M.; Tan, M.L.; et al. Vitamin D deficiency during pregnancy and its associated factors among third trimester Malaysian pregnant women. PLoS ONE 2019, 14, e0216439. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.F.; Chan, H.M. Seafood Consumption and Its Contribution to Nutrients Intake among Canadians in 2004 and 2015. Nutrients 2020, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; Zhou, A.; Leach, M.J.; Hyppönen, E. Differences and determinants of vitamin D deficiency among UK biobank participants: A cross-ethnic and socioeconomic study. Clin. Nutr. 2021, 40, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.; Bhatia, V.; George, B. Vitamin D status of children in Kerala, southern India. Public Health Nutr. 2020, 23, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fu, S.; Li, N.; Hu, F.; Zhang, H.; Zhu, Q.; Luan, F.; Zhang, F.; Zhao, Y.; He, Y. Sex, Residence and Fish Intake Predict Vitamin D Status in Chinese Centenarians. J. Nutr. Health Aging 2019, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Godala, M.; Sewerynek, E.; Gaszyńska, E. Vitamin D status in Polish women with endocrine and osteoporotic disorders in relation to diet, supplement use and exposure to ultraviolet radiation. Adv. Clin. Exp. Med. 2022, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Utri, Z.; Głąbska, D. Vitamin D Intake in a Population-Based Sample of Young Polish Women, Its Major Sources and the Possibility of Meeting the Recommendations. Foods 2020, 9, 1482. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Itsiopoulos, C.; Lambert, K.; Katsardis, C.; Tsoukalas, D.; Erbas, B. Sufficient vitamin D status positively modified ventilatory function in asthmatic children following a Mediterranean diet enriched with fatty fish intervention study. Nutr. Res. 2020, 82, 99–109. [Google Scholar] [CrossRef]
- Díaz-Rizzolo, D.A.; Serra, A.; Colungo, C.; Sala-Vila, A.; Sisó-Almirall, A.; Gomis, R. Type 2 diabetes preventive effects with a 12-months sardine-enriched diet in elderly population with prediabetes: An interventional, randomized and controlled trial. Clin. Nutr. 2021, 40, 2587–2598. [Google Scholar] [CrossRef]
- Filgueiras, M.S.; Suhett, L.G.; Silva, M.A.; Rocha, N.P.; de Novaes, J.F. Lower vitamin D intake is associated with low HDL cholesterol and vitamin D insufficiency/deficiency in Brazilian children. Public Health Nutr. 2018, 21, 2004–2012. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Bassuk, S.S.; Cook, N.R.; Lee, I.M.; Mora, S.; Albert, C.M.; Buring, J.E.; VITAL Research Group. Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circ. Res. 2020, 126, 112–128. [Google Scholar] [CrossRef]
- Okereke, O.I.; Reynolds, C.F., 3rd; Mischoulon, D.; Chang, G.; Cook, N.R.; Copeland, T.; Friedenberg, G.; Buring, J.E.; Manson, J.E. The VITamin D and OmegA-3 TriaL-Depression Endpoint Prevention (VITAL-DEP): Rationale and design of a large-scale ancillary study evaluating vitamin D and marine omega-3 fatty acid supplements for prevention of late-life depression. Contemp. Clin. Trials 2018, 68, 133–145. [Google Scholar] [CrossRef]
- Okereke, O.I.; Vyas, C.M.; Mischoulon, D.; Chang, G.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; Lee, I.M.; et al. Effect of Long-term Supplementation with Marine Omega-3 Fatty Acids vs. Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA 2021, 326, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.M.; Sanders, T.A. Vitamin D and risk of CVD: A review of the evidence. Proc. Nutr Soc. 2015, 74, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, U.; Gjessing, H.R.; Hirche, F.; Mueller-Belecke, A.; Gudbrandsen, O.A.; Ueland, P.M.; Mellgren, G.; Lauritzen, L.; Lindqvist, H.; Hansen, A.L.; et al. Efficacy of fish intake on vitamin D status: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2015, 102, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bays, H.E. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007, 99, 35C–43C. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q.; Liu, Y. Vitamin E deficiency depressed fish growth, disease resistance, and the immunity and structural integrity of immune organs in grass carp (Ctenopharyngodon idella): Referring to NF-κB, TOR and Nrf2 signaling. Fish Shellfish Immunol. 2017, 60, 219–236. [Google Scholar] [CrossRef]
- Head, B.; La Du, J.; Tanguay, R.L.; Kioussi, C.; Traber, M.G. Vitamin E is necessary for zebrafish nervous system development. Sci. Rep. 2020, 10, 15028. [Google Scholar] [CrossRef]
- Guerriero, G.; Ferro, R.; Russo, G.L.; Ciarciam, G. Vitamin E in early stages of sea bass (Dicentrarchus labrax) development. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 435–439. [Google Scholar] [CrossRef]
- Ushkalova, V.N. Content, antioxidant activity and stability of tocopherols in dietary lipids. Vopr. Pitan. 1986, 3, 10–17. [Google Scholar]
- Xu, J.; Zhang, J.; Cai, Z.; Zheng, Y.; Huang, B. Eight vitamin E congeners in seafood and aquatic products in Zhejiang Province. Wei Sheng Yan Jiu 2020, 49, 990–997. [Google Scholar]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in Seafood and Aquaculture Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Gieseg, S.P.; Cuddihy, S.; Hill, J.V.; Davison, W. A comparison of plasma vitamin C and E levels in two Antarctic and two temperate water fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 125, 371–378. [Google Scholar] [CrossRef]
- Suárez-Jiménez, G.M.; López-Saiz, C.M.; Ramírez-Guerra, H.E.; Ezquerra-Brauer, J.M.; Ruiz-Cruz, S.; Torres-Arreola, W. Role of Endogenous and Exogenous Tocopherols in the Lipid Stability of Marine Oil Systems: A Review. Int. J. Mol. Sci. 2016, 17, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.S.; Nielsen, N.S.; Timm-Heinrich, M.; Jacobsenm, C. Oxidative stability of marine phospholipids in the liposomal form and their applications. Lipids 2011, 46, 3–23. [Google Scholar]
- Kagan, V.E.; Serbinova, E.A.; Forte, T.; Scita, G.; Packer, L. Recycling of vitamin E in human low density lipoproteins. J. Lipid Res. 1992, 33, 385–397. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1716–1723. [Google Scholar] [CrossRef] [Green Version]
- Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Giovannucci, E.; Colditz, G.A.; Willett, W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. 1993, 328, 1450–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violi, F.; Pratico, D.; Ghiselli, A.; Alessandri, C.; Iuliano, L.; Cordova, C.; Balsano, F. Inhibition of cyclooxygenase-independent platelet aggregation by low vitamin E concentration. Atherosclerosis 1990, 82, 247–252. [Google Scholar] [CrossRef]
- Kakishita, E.; Suehiro, A.; Oura, Y.; Nagai, K. Inhibitory effect of vitamin E (α-tocopherol) on spontaneous platelet aggregation in whole blood. Thromb. Res. 1990, 60, 489–499. [Google Scholar] [CrossRef]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, phytochemicals and mediterranean diet: A potential protective role against COVID-19 through modulation of paf actions and metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Rumore, S.; McGrath, K.; Scott, A.; Sexton, E.; Wong, T. Fat soluble vitamin status in children on home parenteral nutrition in a tertiary paediatric intestinal rehabilitation unit. Clin. Nutr. ESPEN 2021, 46, 240–245. [Google Scholar] [CrossRef]
- Chen, M.F.; Hsu, H.C.; Liau, C.S.; Lee, Y.T. The role of vitamin E on the anti-atherosclerotic effect of fish oil in diet-induced hypercholesterolemic rabbits. Prostaglandins Other Lipid Mediat. 1999, 57, 99–111. [Google Scholar] [CrossRef]
- Nestel, P.J. Fish oil and cardiovascular disease: Lipids and arterial function. Am. J. Clin. Nutr. 2000, 71, 228S–231S. [Google Scholar] [CrossRef]
- Bessell, E.; Jose, M.D.; McKercher, C. Associations of fish oil and vitamin B and E supplementation with cardiovascular outcomes and mortality in people receiving haemodialysis: A review. BMC Nephrol. 2015, 16, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidow-Kebritchi, S.; Mobarhan, S. Effects of diets containing fish oil and vitamin E on rheumatoid arthritis. Nutr. Rev. 2001, 59, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Paknezhad, F.; Rashidi Nooshabadi, M.; Kavianpour, M.; Jafari Rad, S.; Khadem Haghighian, H. Vitamin E and fish oil, separately or in combination, on treatment of primary dysmenorrhea: A double-blind, randomized clinical trial. Gynecol. Endocrinol. 2018, 34, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. Vitamin E requirement in relation to dietary fish oil and oxidative stress in elderly. EXS 1992, 62, 411–418. [Google Scholar]
- Wu, D.; Han, S.N.; Meydani, M.; Meydani, S.N. Effect of concomitant consumption of fish oil and vitamin E on production of inflammatory cytokines in healthy elderly humans. Ann. N.Y. Acad. Sci. 2004, 1031, 422–424. [Google Scholar] [CrossRef]
- Chea, E.P.; Lopez, M.J.; Milstein, H. Vitamin A. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Elmadfa, I.; Majchrzak, D. Carotinoide und Vitamin A in Fischproben [Carotenoids and vitamin A in fish]. Z. Ernahr. 1998, 37, 207–210. [Google Scholar]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Changes provoked by boiling, steaming and sous-vide cooking in the lipid and volatile profile of European sea bass. Food Res. Int. 2017, 99, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, L.A.; Gisbert, E.; Moriceau, J.; Cahu, C.L.; Zambonino Infante, J.L. Intake of high levels of vitamin A and polyunsaturated fatty acids during different developmental periods modifies the expression of morphogenesis genes in European sea bass (Dicentrarchus labrax). Br. J. Nutr. 2006, 95, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Hodge, C.; Taylor, C. Vitamin A Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Roos, N.; Wahab, M.A.; Chamnan, C.; Thilsted, S.H. The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. J. Nutr. 2007, 137, 1106–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Frano, M.R.; Cai, Y.; Burri, B.J.; Thilsted, S.H. Discovery and biological relevance of 3,4-didehydroretinol (vitamin A2) in small indigenous fish species and its potential as a dietary source for addressing vitamin A deficiency. Int. J. Food Sci. Nutr. 2018, 69, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kawarazuka, N.; Béné, C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: Building evidence. Public Health Nutr. 2011, 14, 1927–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, C.; Domangé, B.; Torrents, R.; de Haro, L.; Simon, N. Hypervitaminosis A Following the Ingestion of Fish Liver: Report on 3 Cases from the Poison Control Center in Marseille. Wilderness Environ. Med. 2020, 31, 454–456. [Google Scholar] [CrossRef]
- Wold, H.L.; Wake, K.; Higashi, N.; Wang, D.; Kojima, N.; Imai, K.; Blomhoff, R.; Senoo, H. Vitamin A distribution and content in tissues of the lamprey, Lampetra japonica. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 276, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rice, R. Fish and healthy pregnancy: More than just a red herring! Prof. Care Mother Child. 1996, 6, 171–173. [Google Scholar]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.S.; Galano, J.M.; Durand, T.; Lee, J.C.Y. Profiling of omega-polyunsaturated fatty acids and their oxidized products in salmon after different cooking methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Sioen, I.; Haak, L.; Raes, K.; Hermans, C.; De Henauw, S.; De Smet, S.; Van Camp, J. Effects of pan-frying in margarine and olive oil on the fatty acid composition of cod and salmon. Food Chem. 2006, 98, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Ansorena, D.; Guembe, A.; Mendizábal, T.; Astiasarán, I. Effect of Fish and Oil Nature on Frying Process and Nutritional Product Quality. J. Food Sci. 2010, 75, H62–H67. [Google Scholar] [CrossRef]
- Schellekens, M. New research issues in sous-vide cooking. Trends Food Sci. Technol. 1996, 7, 256–262. [Google Scholar] [CrossRef]
- Al-Saghir, S.; Thurner, K.; Wagner, K.; Frisch, G.; Luf, W.; Razzazi-Fazeli, E.; Elmadfa, I. Effects of Different Cooking Procedures on Lipid Quality and Cholesterol Oxidation of Farmed Salmon Fish (Salmo salar). J. Agric. Food Chem. 2004, 52, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Bhouri, A.M.; Harzallah, H.J.; Dhibi, M.; Bouhlel, I.; El Cafsi, M.; Hammami, M.; Chaouch, A. Effects of different cooking treatments on flesh fatty acid composition of total lipids in farmed Sea bass Dicentrarchus labrax (Moronidae). Cybium 2010, 34, 29–36. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, T.; Basso, V.; Goethals, S.; Vossen, E.; De Smet, S. The formation of 4-HHE and 4-HNE during cooking and in vitro gastroduodenal digestion of meat and fish. Free Radic. Biol. Med. 2018, 124, 576. [Google Scholar] [CrossRef]
- Karlsdottir, M.G.; Sveinsdottir, K.; Kristinsson, H.G.; Villot, D.; Craft, B.D.; Arason, S. Effect of thermal treatment and frozen storage on lipid decomposition of light and dark muscles of saithe (Pollachius virens). Food Chem. 2014, 164, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Parisi, G.; Dasilva, G.; Medina, I. Stress during slaughter increases lipid metabolites and decreases oxidative stability of farmed rainbow trout (Oncorhynchus mykiss) during frozen storage. Food Chem. 2016, 190, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. The influence of frying technique, cooking oil and fish species on the changes occurring in fish lipids and oil during shallow frying, studied by 1H NMR. Food Res. Int. 2016, 84, 150–159. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two Toxic Lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), Accumulate in Patients with Chronic Kidney Disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef]
- Mason, R.P.; Sherratt, S.C. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem. Biophys. Res. Commun. 2017, 483, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Jacob, R.F.; Bjork, R.E.; Jeffers, B.; Buch, J.; Mizuno, Y.; Mason, R.P.; PREVENT Investigators. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: The PREVENT study. J. Am. Coll. Cardiol. 2008, 51, 1196–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamharzi, N.; Renard, C.B.; Kramer, F.; Pennathur, S.; Heinecke, J.W.; Chait, A.; Bornfeldt, K.E. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized LDL. Diabetes 2004, 53, 3217–3225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, M.; Berneis, K. Low-density lipoprotein size and cardiovascular risk assessment. QJM 2006, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Cao, A.; Cai, L. Effects of vacuum or sous-vide cooking methods on the quality of largemouth bass (Micropterus salmoides). Int. J. Gastron. Food Sci. 2019, 18, 100181. [Google Scholar] [CrossRef]
- Pavlicevic, N.; Baltic, M.Z.; Dimitrijevic, M.; Karabasil, N.; Djordjevic, V.; Markovic, R.; Grbic, S. Polyunsaturated fatty acids in the fish meat and their significance for human health. Meat Technol. 2014, 55, 1–7. [Google Scholar] [CrossRef]
- Kilibarda, N.; Brdar, I.; Baltić, B.; Marković, V.; Mahmutović, H.; Karabasil, N.; Stanišić, S. The safety and quality of sous vide food. Meat Technol. 2018, 59, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Ghazala, S.; Aucoin, J.; Alkanani, T. Pasteurization effect on fatty acid stability in a sous vide product containing seal meat (Phoca groenlandica). J. Food Sci. 1996, 61, 520–523. [Google Scholar] [CrossRef]
- Aberoumand, A.; Ziaeinejad, S. Effect of Cooking on Quality Commonly Consumed Marine Fish Platycephalidae (Platycephalus indicus) in Iran. Turk. J. Agric. Food Sci. Technol. 2015, 3, 891–893. [Google Scholar]
- Neff, M.R.; Bhavsar, S.P.; Braekevelt, E.; Arts, M.T. Effects of different cooking methods on fatty acid profiles in four freshwater fishes from the Laurentian Great Lakes region. Food Chem. 2014, 164, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.; Poppe, S.; Bondan, E. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulska-llow, B.; Ilow, R. Comparison of the effects of microwave cooking and conventional cooking methods on the composition of fatty acids and fat quality indicators in herring. Nahrung 2002, 46, 383–388. [Google Scholar] [CrossRef]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Szlinder-Richert, J.; Malesa-Ciećwierz, M. Effect of household cooking methods on nutritional value of cod and salmon-twin fillet approach. Carpath. J. Food Sci. Technol. 2018, 10, 142–157. [Google Scholar]
- Ložnjak, P.; Jakobsen, J. Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after household cooking. Food Chem. 2018, 254, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Al Khawli, F.; Ferrer, E.; Berrada, H.; Barba, F.J.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K. Innovative Green Technologies of Intensification for Valorization of Seafood and Their by-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [Green Version]
- Haq, M.; Ahmed, R.; Cho, Y.J.; Chun, B.S. Quality Properties and Bio-potentiality of Edible Oils from Atlantic Salmon Byproducts Extracted by Supercritial Carbon Dioxide and Conventional Methods. Waste Biomass Valoz. 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Impact of pretreatment and atmosphere on quality of lipids extracted from cephalothorax of Pacific white shrimp by ultrasonic assisted process. Food Chem. 2020, 309, 125732. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasonic assisted process. LWT 2019, 100, 106–113. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Raju, N.; Chandragiri Nagarajarao, R.; Benjakul, S. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application—A review. Trends Food Sci. Technol. 2020, 100, 307–319. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Impact of pulsed electric field pretreatment on yield and quality of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasound-assisted process. Int. J. Food Sci. Technol. 2020, 55, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Avellone, G.; Pagliaro, M. A circular economy approach to fish oil extraction. ChemistrySelect 2019, 4, 5106–5109. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lino, C.; Pagliaro, M. Omeg@Silica: Entrapment and Stabilization of Sustainably Sourced Fish Oil. ChemistryOpen 2021, 10, 581–586. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Fabiano-Tixier, A.S.; Lino, C.; Avellone, G.; Chemat, F.; Pagliaro, M. Omega-3 Extraction from Anchovy Fillet Leftovers with Limonene: Chemical, Economic, and Technical Aspects. ACS Omega 2019, 4, 15359–15363. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.; Gustafsson, H.; Prestidge, C.A. Enhancing the lipase-mediated bioaccessibility of omega-3 fatty acids by microencapsulation of fish oil droplets within porous silica particles. J. Funct. Foods 2018, 47, 491–502. [Google Scholar]
- Bastías, J.; Balladares, P.; Acuña, S.; Quevedo, R.; Muñoz, O. Determining the effect of different cooking methods on the nutritional composition of salmon (Salmo salar) and chilean jack mackerel (Trachurus murphyi) fillets. PLoS ONE 2017, 12, e0180993. [Google Scholar] [CrossRef]
- Larsen, D.; Quek, S.Y.; Eyres, L. Effect of cooking method on the fatty acid profile of New Zealand King Salmon (Oncorhynchus tshawytscha). Food Chem. 2010, 119, 785–790. [Google Scholar] [CrossRef]
- Garcia-Linares, M.C.; Gonzalez-Andos, E.; García-Fernández, M.C.; Garcia-Arias, M.T. Microbiological and nutritional quality of sous vide or traditionally processed fish: Influence of fat content. J. Food Qual. 2004, 27, 371–387. [Google Scholar] [CrossRef]
- Bakar, J.; Rahimabadi, E.; Che Man, Y. Lipid characteristics in cooked, chill-reheated fillets of Indo-Pacific king mackerel (Scomberomorous guttatus). LWT—Food Sci. Technol. 2008, 41, 2144–2150. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Alagoz, S. Fatty acid profiles and fat contents of commercially important seawater and freshwater fish species of Turkey: A comparative study. Food Chem. 2007, 103, 217–223. [Google Scholar] [CrossRef]
- Türkkan, A.U.; Cakli, S.; Kilinc, B.E.R.N.A. Effects of cooking methods on the proximate composition and fatty acid composition of seabass (Dicentrarchus labrax, Linnaeus, 1758). Food Bioprod. Process. 2008, 86, 163–166. [Google Scholar] [CrossRef]
- Lenas, D.; Chatziantoniou, S.; Nathanailides, C.; Triantafillou, D. Comparison of wild and farmed sea bass (Dicentrarchus labrax L.) lipid quality. Procedia Food Sci. 2011, 1, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Zotos, A.; Kotaras, A.; Mikras, E. Effect of baking of sardine (Sardina pilchardus) and frying of anchovy (Engraulis encrasicholus) in olive and sunflower oil on their quality. Food Sci. Technol. Int. 2013, 19, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.D.; Kitts, D.D.; Hu, C.; Trites, A.W. Comparison of fatty acid profiles of spawning and non-spawning Pacific herring, Clupea harengus pallasi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 504–511. [Google Scholar] [CrossRef]
- Jensen, I.J.; Larsen, R.; Rustad, T.; Eilertsen, K.E. Nutritional content and bioactive properties of wild and farmed cod (Gadus morhua L.) subjected to food preparation. J. Food Compos. Anal. 2013, 31, 212–216. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of way of cooking on content of essential polyunsaturated fatty acids in muscle tissue of humpback salmon (Oncorhynchus gorbuscha). Food Chem. 2006, 96, 446–451. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem. 2007, 101, 1694–1700. [Google Scholar] [CrossRef]
- Moussa, E.R.W.; Shereen, A.N.; Manal, A.; Mehanni, A.H.E.; Rasha, A.E. Nutritional value and fatty acid composition of household cooking on fish fatty acids profile using atherogenicity and thrombogenicity indices. J. Food Chem. Nutr. 2014, 2, 27–41. [Google Scholar]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty acids, mercury, and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: Effect of culinary treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Şengör, G.F.Ü.; Alakavuk, D.Ü.; Tosun, Ş.Y. Effect of cooking methods on proximate composition, fatty acid composition, and cholesterol content of Atlantic Salmon (Salmo salar). J. Aquat. Food Prod. Technol. 2013, 22, 160–167. [Google Scholar] [CrossRef]
- Puwastien, P.; Judprasong, K.; Kettwan, E.; Vasanachitt, K.Y.; Nakngamanong Bhattacharjee, L. Proximate Composition of Raw and Cooked Thai Freshwater and Marine Fish. J. Food Compos. Anal. 1999, 12, 9–16. [Google Scholar] [CrossRef]
- Mai, J.; Shimp, J.; Weihrauch, J.; Kinsella, J.E. Lipids of fish fillets: Changes following cooking by different methods. J. Food Sci. 1975, 42, 1669–1674. [Google Scholar] [CrossRef]
- Echarte, M.; Zulet, M.A.; Astiasaran, I. Oxidation process affecting fatty acids and cholesterol in fried and roasted salmon. J. Agric. Food Chem. 2001, 49, 5662–5667. [Google Scholar] [CrossRef]
- Badiani, A.; Stipa, S.; Bitossi, F.; Pirini, M.; Bonaldo, A.; Gatta, P.; Rotolo, M.; Testi, S. True retention of nutrients upon household cooking of farmed portion-size European sea bass (Dicentrarchus labrax L.). LWT—Food Sci. Technol. 2013, 50, 72–77. [Google Scholar] [CrossRef]
- Schneedorferová, I.; Tomčala, A.; Valterová, I. Effect of heat treatment on the n-3/n-6 ratio and content of polyunsaturated fatty acids in fish tissues. Food Chem. 2015, 176, 205–211. [Google Scholar] [CrossRef]
- Yanar, Y.; Kuecuekguelmez, A.; Ersoy, B.; Celik, M. Cooking effects on fatty acid composition of cultured sea bass (Dicentrarchus labrax) fillets. J. Muscle Foods 2007, 18, 88–94. [Google Scholar] [CrossRef]
- Saldanha, T.; Benassi, M.T.; Bragagnolo, N. Fatty acid contents evolution and cholesterol oxides formation in Brazilian sardines (Sardinella brasiliensis) as a result of frozen storage followed by grilling. LWT—Food Sci. Technol. 2008, 41, 1301–1309. [Google Scholar] [CrossRef]
- Sanchez-Muniz, F.J.; Viejo, J.M.; Medina, R. Deep-frying of sardines in different culinary fats. Changes in the fatty acid composition of sardines and frying fats. J. Agric. Food Chem. 1992, 40, 2252–2256. [Google Scholar] [CrossRef]
- Garcıa-Arias, M.T.; Pontes, E.Á.; Garcıa-Linares, M.C.; Garcıa-Fernandez, M.C.; Sánchez-Muniz, F.J. Cooking–freezing–reheating (CFR) of sardine (Sardina pilchardus) fillets. Effect of different cooking and reheating procedures on the proximate and fatty acid compositions. Food Chem. 2003, 83, 349–356. [Google Scholar] [CrossRef]
- Lira, G.M.; Cabral, C.C.V.Q.; de Oliveira, Í.B.A.; Figueirêdo, B.C.; Simon, S.J.G.B.; Bragagnolo, N. Changes in the lipid fraction of king mackerel pan fried in coconut oil and cooked in coconut milk. Food Res. Int. 2017, 101, 198–202. [Google Scholar] [CrossRef] [PubMed]


| Reference | Study Design | Fish/Fish Oil/Fish Lipid Bioactives (Dose/Amount per Day in Cases of In Vivo Trials) | Health Effects Studied | Cell-Models (In Vitro)—Participants (In Vivo) (Duration) | Main Effects on Health | Other Health Observations—Benefits |
|---|---|---|---|---|---|---|
| [41] | Prospective study | n-3 PUFA | Risk of CVD | 57,972 participants (12.7 years) | Reduced risk of mortality | Lowered blood pressure and inflammatory markers |
| [42] | Randomized crossover feeding trial | Salmon (113 g, twice/wk) | Incident of CHD | 25 participants (4 weeks) | Lower cholesterol and triglyceride conc. | Increased HDL-cholesterol |
| [43] | Randomised controlled trial | Fish | Secondary prevention of MI | 2033 participants (2 years) | 29% reduction all-cause mortality | 3–4% lower serum cholesterol |
| [44] | Randomised, placebo-controlled trial | Fish oil | Prevention of MI | 122, 120, and 118 patients (1 year) | Decrease in total cardiac events | Reduced left ventricular enlargement and angina pectoris |
| [45] | Randomised controlled trial | n-3 PUFA | Prevention of MI | 11,324 participants (3.5 years) | Lowered risk of primary endpoint | Reduced cholesterol and triglyceride |
| [46] | Randomized, double- blind, placebo-controlled clinically controlled trial | Fish oil concentrate (6 g/d for 3 months and 3 g/d for 21 months) | Effect on CHD | 223 patients (2 years) | Lowering in CHD events | Loss in minimal luminal diameter |
| [47] | Meta-analysis | Dietary and non-dietary intake of n-3 PUFA | Effect on CHD | 7951 participants in the intervention, 7855 participants controlled (1966–1999) | Reduction in overall mortality | Reduction in MI and sudden death |
| [48] | Cross-sectional study | n-3 PUFA | Effect on inflammatory biomarkers | 1024 patients (2 years) | Inverse association of n-3 intake and levels of inflammatory biomarkers | |
| [49] | Epidemiological study | n-3 PUFA | Effect on inflammatory markers | 1123 patients | Intake associated with lower levels of pro-inflammatory markers | Intake associated with high levels of anti-inflammatory markers |
| [50] | Cross-sectional study | n-3 PUFA and fish | Effect on inflammation and its related markers | 5677 men and women | Lowered levels of inflammation and endothelial activation | Intake inversely associated with IL-6 levels |
| [51] | Cross-sectional study | n-3 PUFA and fish | Effect on low-grade inflammation, atheroclerosis and CVD | 2000 participants | Inverse association with inflammatory marker levels | Triglycerides decreased across n-3 tertiles |
| [52] | Cross-sectional study | Fish | Inflammatory markers | 3042 men and women | Associated with lower inflammatory marker levels | Significant results attained even in lower quantities on fish consumed |
| [53] | Meta-analysis | Fish oil | Inflammatory markers | 7 trials included | Decreased levels of TNF-a and IL-6 | C-reactive protein not significantly affected |
| [54] | Quantitative analysis | Fish | CHD mortality | 8 studies | Reduced risk of CHD | 3.9% reduction associated with each additional serving per week |
| [55] | Meta-analysis | Fish | CHD mortality | 11 eligible and 13 cohort studies (11.5 years average follow up) | Inverse association with CHD mortality | Benefits achieved by consuming fish just once per week |
| [56] | Randomised controlled trial | Mediterranean diet supplemented with fatty fish | Inflammation in paediatric asthma | 64 children (effects noticed after 6 months) | Reduced airway inflammation in childhood asthma | |
| [57] | Randomised controlled trial | Mediterranean diet supplemented with fish oil | Mental health | 95 participants (6 months) | Improved mental health in people with depression | At 3 months significant inverse correlation between Med-scores and depression |
| [58] | Cross-sectional analysis | Fish | Rheumatoid arthritis | 176 participants | Lowered disease activity and risk for CVD in RA patients | |
| [59] | Meta-analysis | EPA+DHA | Blood pressure | 7 RCTs (2012–2014) | Reduced systolic blood pressue | >2 g reduces diastolic blood pressure |
| [60] | In vitro study | Polar lipids from salmon under thermal treatment (cooking) versus raw untreated salmon | Anti-inflammatory and anti-thrombotic properties | Human platelets | Εffects of thermal treatment on the anti-inflammatory and antithrombotic potency of salmon polar lipids | Salmon PL rich in n-3 PUFA retain their ability to inhibit human platelet aggregation induced by the inflammatory and thrombotic mediators PAF and thrombin, but also by well-established platelet agonists such as ADP and collagen, after heat treatment |
| [9] | In vitro study | Fish by-products | Anti-inflammatory and anti-thrombotic properties | Human platelets | PL from fish by-products inhibited human platelet aggregation induced by the inflammatory and thrombotic mediators PAF and thrombin, but also by well-established platelet agonists such as ADP and collagen | PL bioactives from fish by-products are putative candidates for the sustainable development of novel supplements and nutraceuticals with cardio-protective properties |
| [30,31] | In vitro study | Salmon PL | Anti-inflammatory and anti-thrombotic cardio-protective properties | Human platelets | Food grade extracted salmon PL bioactives inhibited human platelet aggregation induced by the inflammatory and thrombotic mediators PAF and thrombin, at the same levels as the conventional extracted salmon PL | Food grade extracted PL bioactives rich in n-3 PUFA from fish sources are putative candidates for developing novel supplements and nutraceuticals with cardio-protective properties, according to EFSA and EU legislations, in contrast to conventional extracted salmon PL |
| [26,61] | Ex vivo trial in hypercholesterolaemic rabbits | Fish polar lipids | Formation of Atherosclerotic plaques Serum Lipid profile Inflammatory levels and metabolism of PAF | 12 rabbits (fish polar lipids were included in the diet of 66 rabbits versus another 6 that were not administered fish polar lipids (control) (45 days) | Evaluation of anti-atherogenic properties of fish PL: rabbits fed with hypercholesterolemic diet with fish PL developed atherosclerotic lessions of lower degree than the control ones, which were fed a hypercholesterolemic diet without the presence of fish PL. The inclusion of fish PL in the diet of these rabbits increased HDL levels as well | Fish PL modulated the metabolism of the inflammatory and thrombotic mediator, PAF, towards a reduction of PAF-levels to homeostatic lower levels in rabbits fed with hypercholesterolemic diet with fish PL, which reduced inflammation and thus reduced atherosclerosis progression |
| [62] | In vitro study | Sardine lipid bioactives and cod liver oil | Anti-platelet properties | Evaluation of the anti-platelet properties of an oily fish (sardines) and of a fish oil (cod liver oil) lipid bioactives as putative candidates for anti-atherogenic agents | Inhibition of rabbit platelet aggregation induced by the inflammatory and thrombotic mediator PAF | |
| [63] | In vitro study | Fish lipids | Anti-platelet properties | Rabbit platelets | Evaluation of anti-platelet properties of fish lipid bioactives as putative candidates for anti-atherogenic agents | Inhibition of rabbit platelet aggregation induced by the inflammatory and thrombotic mediator PAF |
| [27] | In vitro study | Fish polar lipids | Inflammatory levels and metabolism of PAF | Human mesangial cells | Reduction of inflammatory activation of mesangial cells and thus reduction of risk for glomerulosclerosis and other kidney disorders | Effect on PAF metabolism towards reduction of PAF-levels to homeostatic ones, which reduced inflammation |
| [21] | Systematic review, Meta-analysis | Supplementation of n-3 PUFA | Risk of major cardiovascular disease events | 20 studies—randomized trials that enrolled 68,680 patients throughout 2012 | Lack of evidence to suggest the beneficial effect of n-3 PUFA supplementation in respect of cardiovascular events and other measurable changes in health | n-3 PUFA supplementation was not associated with a lower risk of all-cause mortality, cardiac death, sudden death, myocardial infarction, or stroke based on relative and absolute measures of association |
| [22] | Meta-analysis | Supplementation of n-3 PUFA | Risk of major cardiovascular disease events and complications in peripheral arterial disease (PAD) | Randomized trials throughout 2013 that enrolled 396 individuals and lasted more than 12 weeks in adults with PAD | Insufficient evidence exists to suggest a beneficial effect of n-3 PUFA supplementation in adults with PAD with regard to cardiovascular events and other serious clinical outcomes | There was no evidence of a protective association of n-3 PUFA supplementation against major adverse cardiac events or other serious clinical outcomes. Any adverse events and compliance were poorly reported |
| [23] | Systematic review | Supplementation of n-3 PUFA | Prevention of cardiovascular disease | 2 meta-analysis studies on RCTs and 8 placebo-controlled RCTs, with more than 1000 patients and follow-up of more than a year, between 1999 and 2015, were included | There is currently a lack of evidence to support the routine use of omega-3 PUFAs in both the primary and secondary prevention of CVD. Safety of omega-3 PUFA supplementation should be considered and it was proposed that Pharmacists are ideally situated to engage patients in the discussion of the lack of benefit and possible risk of omega-3 PUFA | No reduction in CV events with n-3 PUFAs, in addition to standard, evidence-based therapy in patients after myocardial infarction. While data from RCTs have not demonstrated serious safety concerns, omega-3 PUFAs can increase the risk of bleeding and may interact with other medications that affect hemostasis, such as antiplatelet agents and warfarin |
| [24] | Meta-analysis | Supplementation of n-3 PUFA | Secondary prevention of cardiovascular disease | 14 randomized, double-blind, placebo-controlled trials (involving 20,485 patients with a history of CVD) since April 2011 | Insufficient evidence of a secondary preventive effect of n-3 PUFA supplements against overall cardiovascular events among patients with a history of cardiovascular disease | No reduction of the risk of overall cardiovascular events, all-cause mortality, sudden cardiac death, myocardial infarction, congestive heart failure, or transient ischemic attack and stroke |
| [25] | Systematic review, Meta-analysis | Consumption of fish and long chain n-3 PUFA | Risk of cerebrovascular disease | 26 prospective cohort studies and 12 randomised controlled trials with aggregate data on 794,000 non-overlapping people and 34,817 cerebrovascular outcomes, were included | Μoderate, inverse associations of fish consumption and long chain omega 3 fatty acids with cerebrovascular risk. The beneficial effect of fish intake on cerebrovascular risk is likely to be mediated through the interplay of a wide range of nutrients abundant in fish | Long chain n-3 PUFA measured as circulating biomarkers in observational studies or supplements in primary and secondary prevention trials were not associated with cerebrovascular disease |
| [20] | Randomized, placebo-controlled trial, VITAL (Vitamin D and Omega-3 Trial) | A two-by-two factorial design, of vitamin D3 (at a dose of 2000 IU per day) and fish n-3 PUFA (at a dose of 1 g per day) | Primary prevention of cardiovascular disease and cancer | A total of 25,871 participants of men 50 years of age or older and women 55 years of age or older in the United States | Supplementation with n-3 PUFA did not result in a lower incidence of major cardiovascular events or cancer than placebo | |
| [35] | A population-based cohort study (the Singapore Chinese Health Study) | Dietary n−3 PUFA | Association with cardiovascular death | 63,257 Chinese adults aged 45–74 years from 1993 to 1998 | Higher intakes of marine (EPA/DHA) and plant (ALA) omega-3 fatty acids are both associated with reduced risk of cardiovascular mortality in a Chinese population. The associations were similar for deaths from CHD and stroke and persisted in participants who were free of CVD at the baseline | High dietary intake of both marine and non-marine-based omega-3 fatty acids is associated with reduced risk of cardiovascular death in the Chinese population, particularly for deaths from coronary heart disease and in individuals without cardiovascular disease at baseline The beneficial effects of fish consumption of CVD risk and other chronic disease is due to the interplay of an array of different lipid nutrients instead of just n-3 PUFA in their neutral form |
| [64] | A 2-by-2 factorial randomised control trial (Alpha Omega Trial), and no significant effect was found for either EPA/DHA or ALA | 2 g ALA or 400 mg EPA/DHA as the interventions | Cardiovascular events after myocardial infarction | 4837 post-myocardial infarction patients | No significant beneficial effect was found for all n-3 PUFA assessed (either EPA/DHA or ALA) | |
| [65] | Randomised control trial for Prevention of Post-operative Atrial Fibrillation (OPERA) | Peri-operative oral n-3 PUFA supplementation (8–10g of n-3 PUFA or placebo divided over 2–5 days followed by 2 g per day until discharged from hospital or post-operative day 10) | Reduction of the occurrence of post-operative atrial fibrillation | 1516 patients receiving cardiac surgery | n-3 PUFA administration did not reduce the risk of post-operative atrial fibrillation, in comparison to the placebo | |
| [66] | Randomised control trial, ORIGIN (Outcome Reduction with an Initial Glargine Intervention) | Long term supplementation of n-3 PUFA (1g capsules per day, containing at least 900 mg of ethyl esters of n-3 PUFA) | Reduction of the rate of cardiovascular events in patients with either Type II diabetes, impaired fasting glucose or impaired glucose intolerance | 12,537 participants during an average follow-up of 6.2 years | Incidences of death from cardiovascular causes did not decrease significantly amongst patients that received n-3 PUFA, in comparison to the control group that received the placebo | During >6 years of treatment followed by >2.5 years of observation, omega-3 fatty acid supplementation had no effect on health outcomes and salutary effects on metabolic control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. https://doi.org/10.3390/md20030187
Tsoupras A, Brummell C, Kealy C, Vitkaitis K, Redfern S, Zabetakis I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Marine Drugs. 2022; 20(3):187. https://doi.org/10.3390/md20030187
Chicago/Turabian StyleTsoupras, Alexandros, Chloe Brummell, Ciara Kealy, Karolis Vitkaitis, Shane Redfern, and Ioannis Zabetakis. 2022. "Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing" Marine Drugs 20, no. 3: 187. https://doi.org/10.3390/md20030187
APA StyleTsoupras, A., Brummell, C., Kealy, C., Vitkaitis, K., Redfern, S., & Zabetakis, I. (2022). Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Marine Drugs, 20(3), 187. https://doi.org/10.3390/md20030187