New Sorbicillinoids with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus Hypocrea jecorina H8
Abstract
:1. Introduction
2. Results
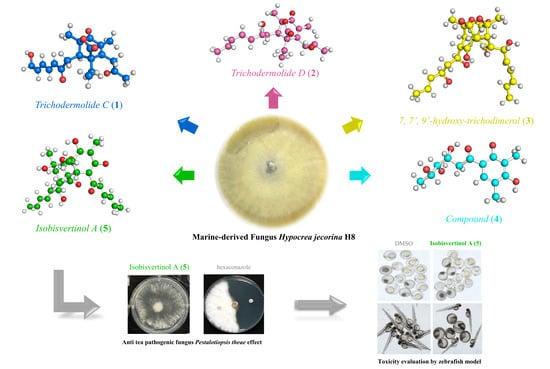
2.1. Structural Elucidation of New Compounds
2.2. Evaluation of Antifungal Activity
2.3. Evaluation of Toxicity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Eletronic Circular Dichroism (ECD) Calculations
4.3. Fungus Carbohydrate Fermentation
4.4. Extraction and Isolation
4.5. Structrural Elucidation of the New Compounds 1–5
4.6. Antifungal Activity Assay
4.7. Toxicity Evaluation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2021, 10, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.W.; Tang, X.X.; Fan, Z.W.; Xia, J.M.; Xie, C.L.; Yang, X.W. Fusarisolins A-E, Polyketides from the Marine-Derived Fungus Fusarium solani H918. Mar. Drugs 2019, 17, 408–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Pan, Y.M.; Dai, Y.L.; Gao, Z.M. First Report of Brown Blight Disease Caused by Colletotrichum gloeosporioides on Camellia sinensis in Anhui Province, China. Plant Dis. 2014, 98, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, K.; Ma, Q.P.; Chen, J.; Wang, L.; Yang, D.J.; Chen, X.; Li, X.H. Colletotrichum gloeosporioides-Contaminated Tea Infusion Blocks Lipids Reduction and Induces Kidney Damage in Mice. Front. Microbiol. 2017, 8, 2089–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.X.; Yan, X.; Fu, W.H.; Yi, L.Q.; Tang, B.W.; Yu, L.B.; Fang, M.J.; Wu, Z.; Qu, Y.K. New beta-Lactone with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus MCCC3A00957. J. Agric. Food Chem. 2019, 67, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Ponmurugan, P.; Baby, U.I.; Rajkumar, R. Growth, photosynthetic and biochemical responses of tea cultivars infected with various diseases. Photosynthetica 2007, 45, 143–146. [Google Scholar] [CrossRef]
- Sanjay, R.; Ponmurugan, P.; Baby, U.I. Evaluation of fungicides and biocontrol agents against grey blight disease of tea in the field. Crop Prot. 2008, 27, 689–694. [Google Scholar] [CrossRef]
- Lan, W.J.; Zhao, Y.; Xie, Z.L.; Liang, L.Z.; Shao, W.Y.; Zhu, L.P.; Yang, D.P.; Zhu, X.F.; Li, H.J. Novel sorbicillin analogues from the marine fungus Trichoderma sp. associated with the seastar Acanthaster planci. Nat. Prod. Commun. 2012, 7, 1337–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Zhu, T.; Li, L.; Cai, S.; Zhao, B.; Gu, Q. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem. Pharm. Bull. 2009, 57, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, N.; Ohshiro, T.; Tomoda, H.; Omura, S. Fungal isobisvertinol, a new inhibitor of lipid droplet accumulation in mouse macrophages. Org. Lett. 2007, 9, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Yang, L.J.; Zhang, Y.H.; Wu, J.S.; Shi, T.; Haider, W.; Shao, C.L.; Wang, C.Y. Sorbicillinoid Derivatives From Sponge-Derived Fungus Trichoderma reesei (HN-2016-018). Front. Microbiol. 2020, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Liu, X.H.; Li, X.N.; Ji, N.Y. Antifungal and Antimicroalgal Trichothecene Sesquiterpenes from the Marine Algicolous Fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food Chem. 2020, 68, 15440–15448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Deng, Y.; Lin, X.; Chen, B.; Li, J.; Liu, H.; Chen, S.; Liu, L. Anti-inflammatory Mono- and Dimeric Sorbicillinoids from the Marine-Derived Fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.R.; Hissa, D.C.; de Souza, T.M.; Sa, C.A.; Evaristo, J.A.M.; Nogueira, F.C.S.; Carvalho, A.F.U.; Farias, D.F. Proteomics analysis of zebrafish larvae exposed to 3,4-dichloroaniline using the fish embryo acute toxicity test. Environ. Toxicol. 2020, 35, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ou-Yang, H.; Yan, X.; Tang, B.W.; Fang, M.J.; Wu, Z.; Chen, J.W.; Qiu, Y.K. Open-Ring Butenolides from a Marine-Derived Anti-Neuroinflammatory Fungus Aspergillus terreus Y10. Mar. Drugs 2018, 16, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, A.; Saha, S.; Walia, S.; Shakil, N.A.; Kumar, J.; Annapurna, K. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Sci. Health B 2013, 48, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Z.; Yan, X.; Tang, X.-X.; Lin, J.-G.; Qiu, Y.-K. New Bis-Alkenoic Acid Derivatives from a Marine-Derived Fungus Fusarium solani H915. Mar. Drugs 2018, 16, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| NO. | 1 | Trichodermolide B | 2 | 4 | |||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | 174.9 | 174.7 | 176.4 | 113.6 | |||
| 3 | 55.7 | 55.9 | 56.2 | 159.9 | |||
| 4 | 149.2 | 150.9 | 152.1 | 111.7 | |||
| 5 | 134.4 | 133.9 | 133.8 | 160.3 | |||
| 6 | 191.3 | 191.2 | 191.9 | 7.34 | 118.4 | ||
| 7 | 86.8 | 86.5 | 87.1 | 125.1 | |||
| 8 | 1.77 s | 11.6 | 11.9 | 1.87 s | 12.1 | 2.89 dd (16.9, 13.7) 2.31 dd (16.9, 2.7) | 192.1 |
| 9 | 1.48 s | 16.4 | 16.5 | 1.56 s | 17.3 | 4.64 dt (13.7, 2.6) | 39.8 |
| 10 | 1.30 dq (13.7, 7.2) 2.08 dq (13.8, 7.2) | 20.5 | 20.5 | 2.21 dq (14.2, 7.3) 1.75 s | 20.9 | 3.09 td (8.5, 1.5) | 77.1 |
| 11 | 0.95 t (7.2) | 8.4 | 8.6 | 0.97 t (7.2) | 8.6 | 3.88 dq (13.6, 6.0) | 76.8 |
| 12 | 3.55 d (18.2) 3.63 d (18.2) | 44.5 | 44.7 | 2.60 dd (13.7, 9.6) 2.39 brd (13.6) | 39.2 | 1.19 d (6.0) | 65.9 |
| 13 | 204 | 205.0 | 4.10 m | 67.1 | 2.04 s | 21.5 | |
| 14 | 2.27 s | 30.3 | 30.5 | 1.29 t (7.2) | 24.1 | 2.12 s | 9.2 |
| 15 | 3.55 t (5.3) | 49.9 | 50.0 | 2.97 dd (6.4, 4.4) | 51.3 | 16.7 | |
| 16 | 2.43 dd (18.5, 5.2) 3.13 dd (18.5, 5.2) | 35.2 | 35.0 | 1.52 m 1.37 m | 32.3 | ||
| 17 | 196.5 | 197.2 | 4.17 m | 71.1 | |||
| 18 | 6.43 br dd (15.4, 10.2) | 127.9 | 127.6 | 6.16 dd (15.2, 10.5) | 132.1 | ||
| 19 | 7.25 dd (15.2, 10.8) | 143.0 | 143.9 | 5.44 dd (15.2, 7.2) | 131.9 | ||
| 20 | 6.20 d (15.4) | 128.8 | 130.6 | 5.75 dq (14.3, 6.8) | 131.7 | ||
| 21 | 6.35 dt (15.2, 4.8) | 143.2 | 141.9 | 5.99 dd (15.2, 10.7) | 130.0 | ||
| 22 | 4.32 d (4.2) | 62.7 | 19.1 | 1.76 br d (6.6) | 18.2 | ||
| NO. | 3 | 10 | NO. | 5 | 11 | ||
|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δC | δH (J in Hz) | δC | δC | ||
| 1 | 2.94, s | 58.3 | 57.5 | 1 | 191.8 | 194.3 | |
| 1′ | 3.31, s | 52.3 | 57.5 | 2 | 100.7 | 101.9 | |
| 2,2′ | 78.3 | 78.9 | 3 | 2.58 br d (14.1) 2.73 m | 35.9 | 35.4 | |
| 3,3′ | 108.7 | 104.1 | 4 | 73.9 | 72.7 | ||
| 4 | 59.0 | 58.8 | 5 | 168.7 | 168.8 | ||
| 4′ | 59.0 | 58.8 | 6 | 103.9 | 105.6 | ||
| 5,5′ | 199.0 | 198.0 | 7 | 191.7 | 191.6 | ||
| 6′ | 104.0 | 102.8 | 8 | 106.1 | 106.2 | ||
| 6 | 103.8 | 102.8 | 1′ | 179.7 | 178.0 | ||
| 7 | 172.5 | 175.9 | 2′ | 6.40 br d (14.9) | 120.3 | 121.4 | |
| 7′ | 175.2 | 175.9 | 3′ | 7.25 br s | 139.0 | 137.8 | |
| 8 | 6.18 d (14.7) | 118.6 | 118.5 | 4′ | 6.21 m | 130.8 | 131.4 |
| 8′ | 2.38 dd (16.9, 3.5) 2.54 dd (16.9,13.6) | 40.9 | 118.5 | 5′ | 6.10 br d (7.0) | 131.1 | 131.7 |
| 9 | 7.35 dd (14.8,10.9) | 143.0 | 143.6 | 6′ | 1.86 br d (6.4) | 18.8 | 18.9 |
| 9′ | 4.31 ddd (13.5, 7.1,3.2) | 80.7 | 143.6 | 1″ | 168.8 | 167.3 | |
| 10 | 6.31 dd (15.4, 11.0) | 130.8 | 130.9 | 2″ | 6.27 m | 120.2 | 121.3 |
| 10′ | 5.67 dq (15.2,6.6) | 131.8 | 130.9 | 3″ | 6.29 m | 140.3 | 140.1 |
| 11 | 6.22 dd (13.6,6.6) | 140.1 | 140.5 | 4″ | 6.23 br s | 131.8 | 131.4 |
| 11′ | 5.49 ddd (15.3,7.0,1.6) | 127.2 | 140.5 | 5″ | 6.18 m | 137.0 | 136.6 |
| 12 | 1.91 brd (6.6) | 18.9 | 18.8 | 6″ | 1.86 br d (6.4) | 18.9 | 19.1 |
| 12′ | 1.68 brd (6.4) | 17.8 | 18.8 | 1a | 58.4 | 59.1 | |
| 13 | 1.43 s | 21.5 | 21.3 | 1a-CH3 | 1.34 s | 19.2 | 19.9 |
| 13′ | 1.35 s | 20.7 | 21.3 | 4a | 110.2 | 108.4 | |
| 14 | 1.41 s | 18.2 | 18.9 | 4-CH3 | 1.29, s | 22.4 | 22.8 |
| 14′ | 1.41 s | 18.4 | 18.9 | 5a | 79.6 | 78.8 | |
| 5a-CH3 | 1.48 br s | 25.6 | 25.8 | ||||
| 6-CH3 | 1.53, s 1.86 br d (6.4) | 6.8 | 7.3 | ||||
| 8a | 3.63, s | 53.6 | 53.6 | ||||
| Compd. | Pestalotiopsis theae | Compd. | Pestalotiopsis theae |
|---|---|---|---|
| 1 | >100 | 8 | 18.22 ± 1.29 |
| 2 | >100 | 9 | 1.83 ± 1.37 |
| 3 | >100 | 10 | 4.68 ± 1.44 |
| 4 | >100 | 11 | >100 |
| 5 | 9.13 ± 1.25 | hexaconazole * | 24.25 ± 1.57 |
| 6 | 2.04 ± 1.91 | DMSO | None |
| 7 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-Z.; Xu, G.-X.; He, F.-M.; Zhang, W.-B.; Wu, Z.; Li, M.-Y.; Tang, X.-X.; Qiu, Y.-K. New Sorbicillinoids with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus Hypocrea jecorina H8. Mar. Drugs 2022, 20, 213. https://doi.org/10.3390/md20030213
Liu S-Z, Xu G-X, He F-M, Zhang W-B, Wu Z, Li M-Y, Tang X-X, Qiu Y-K. New Sorbicillinoids with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus Hypocrea jecorina H8. Marine Drugs. 2022; 20(3):213. https://doi.org/10.3390/md20030213
Chicago/Turabian StyleLiu, Shun-Zhi, Guang-Xin Xu, Feng-Ming He, Wei-Bo Zhang, Zhen Wu, Ming-Yu Li, Xi-Xiang Tang, and Ying-Kun Qiu. 2022. "New Sorbicillinoids with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus Hypocrea jecorina H8" Marine Drugs 20, no. 3: 213. https://doi.org/10.3390/md20030213
APA StyleLiu, S.-Z., Xu, G.-X., He, F.-M., Zhang, W.-B., Wu, Z., Li, M.-Y., Tang, X.-X., & Qiu, Y.-K. (2022). New Sorbicillinoids with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus Hypocrea jecorina H8. Marine Drugs, 20(3), 213. https://doi.org/10.3390/md20030213