Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals
Abstract
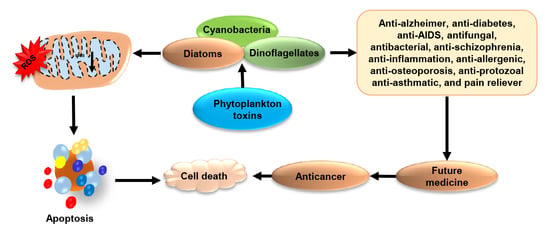
:1. Introduction
2. Phytoplankton: The Most Ingenious Source of Toxins
3. Phytoplankton Toxins Kills to Heal: A Cross Talk
4. Toxins Produced by Cyanobacteria and Their Potential Biomedical Applications
5. Diatom’s Toxins: The Legendary Furthest Effective Biological Properties
6. Dinoflagellate Biologically Active Toxins and Their Potential Biomedical Applications
6.1. Dinoflagellate Toxins: The Most Prevailing Source of Toxins with Biological Properties
6.2. Bioactive Compounds from Dinoflagellates and Their Potential Biomedical Applications
7. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.-S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Nayak, R.; Behera, C.; Jena, M. Evaluation of the anti-bacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Future J. Pharm. Sci. 2021, 7, 17. [Google Scholar] [CrossRef]
- Kathiresan, K.; Nabeel, M.; Manivannan, S. Bioprospecting of marine organisms for novel bioactive compounds. Sci. Trans. Environ. Technovation 2008, 1, 107–120. [Google Scholar] [CrossRef]
- Motuhi, S.-E.; Mehiri, M.; Payri, C.E.; La Barre, S.; Bach, S. Marine natural products from new caledonia—A review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, B.; Maharana, S.; Bhakta, S.; Jena, M. Marine phytoplankton diversity of Odisha coast, India with special reference to new record of diatoms and dinoflagellates. Vegetos 2021. [Google Scholar] [CrossRef]
- Behera, C.; Pradhan, B.; Panda, R.; Nayak, R.; Nayak, S.; Jena, M. Algal Diversity of Saltpans, Huma (Ganjam), India. J. Indian Bot. Soc. 2021, 101, 107–120. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C.; Nayak, R.; Jena, M. Algal Flora of Tampara Lake, Chhatrapur, Odisha, India. J. Indian Bot. Soc. 2021, 101, 1–15. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C.; Jena, M. Algal Diversity of Kanjiahata Lake, Nandankanan, Odisha, India. J. Indian Bot. Soc. 2020, 99, 11–24. [Google Scholar] [CrossRef]
- Behera, C.; Dash, S.R.; Pradhan, B.; Jena, M.; Adhikary, S.P. Algal Diversity of Ansupa lake, Odisha, India. Nelumbo 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A. Preliminary Investigation of the Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activity of Enteromorpha intestinalis Extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jena, M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020, 10, 1–8. [Google Scholar]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Dash, S.R.; Ki, J.-S.; Adhikary, S.P.; Ragusa, A.; Jena, M. Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak. Antioxidants 2022, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Wang, H.; Yoo, H.Y.; Park, J.; Ki, J.-S. Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium Catenella. Mar. Drugs 2021, 19, 291. [Google Scholar] [CrossRef] [PubMed]
- Bui, Q.T.N.; Kim, H.; Park, H.; Ki, J.-S. Salinity Affects Saxitoxins (STXs) Toxicity in the Dinoflagellate Alexandrium pacificum, with Low Transcription of SXT-Biosynthesis Genes sxtA4 and sxtG. Toxins 2021, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, H.; Park, H.; Ki, J.-S. Temperature influences the content and biosynthesis gene expression of saxitoxins (STXs) in the toxigenic dinoflagellate Alexandrium Pac. Sci. Total Environ. 2022, 802, 149801. [Google Scholar] [CrossRef]
- Wang, D.-Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rept. 2020, 47, 9567–9578. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. (Amst. Neth.) 2021, 30, e00633. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Maharana, S.; Pradhan, B.; Jena, M.; Misra, M.K. Diversity of Phytoplankton in Chilika Lagoon, Odisha, India. Environ. Ecol 2019, 37, 737–746. [Google Scholar]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role(s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef] [Green Version]
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 323–347. [Google Scholar]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. United Kingd. 2016, 96, 61–91. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [Green Version]
- Gehringer, M.M. Microcystin-LR and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2004, 557, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xie, P.; Li, G.; Hao, L.; Xiong, Q. In vivo study on the effects of microcystin extracts on the expression profiles of proto-oncogenes (c-fos, c-jun and c-myc) in liver, kidney and testis of male Wistar rats injected iv with toxins. Toxicon 2009, 53, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Matos, P.; Pereira, P.; Batoréu, M.; Silva, M.; Jordan, P. Microcystin-LR activates the ERK1/2 kinases and stimulates the proliferation of the monkey kidney-derived cell line Vero-E6. Toxicology In Vitro 2010, 24, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins—A review. Mutat. Res./Rev. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Burch, M.D. Effective doses, guidelines & regulations. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: New York, NY, USA, 2008; pp. 831–853. [Google Scholar]
- Merel, S.; Villarín, M.C.; Chung, K.; Snyder, S. Spatial and thematic distribution of research on cyanotoxins. Toxicon 2013, 76, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting marine plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [Green Version]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, J.; Brin, M.F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [Google Scholar]
- Ting, P.T.; Freiman, A. The story of Clostridium botulinum: From food poisoning to Botox. Clin. Med. 2004, 4, 258. [Google Scholar] [CrossRef]
- Liu, J.; Sidell, N. Anti-estrogenic effects of conjugated linoleic acid through modulation of estrogen receptor phosphorylation. Breast Cancer Res. Treat. 2005, 94, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, Y.; Kasahara, T.; Yamaguchi, Y.; Kuno, K.; Matsushima, K.; Mukaida, N. Stimulation of interleukin-8 production by okadaic acid and vanadate in a human promyelocyte cell line, an HL-60 subline: Possible role of mitogen-activated protein kinase on the okadaic acid-induced NF-κB activation. J. Biol. Chem. 1997, 272, 15366–15372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-S.; Ahn, K.-H.; Kim, S.-Y.; Jeong, J.-W. Okadaic acid promotes angiogenesis via activation of hypoxia-inducible factor-1. Cancer Lett. 2009, 276, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.i.; Tsuda, M. Amphidinolides, bioactive macrolides from symbiotic marine dinoflagellates. Nat. Prod. Rep. 2004, 21, 77–93. [Google Scholar] [CrossRef]
- Daranas, A.H.; Norte, M.; Fernández, J.J. Toxic marine microalgae. Toxicon 2001, 39, 1101–1132. [Google Scholar] [CrossRef]
- Ishibashi, M.; Kobayashi, J.i. Amphidinolides: Unique macrolides from marine dinoflagellates. Heterocycles 1997, 1, 543–572. [Google Scholar]
- Sasaki, M.; Tsukano, C.; Tachibana, K. Studies toward the Total Synthesis of Gymnocin A, a Cytotoxic Polyether: A Highly Convergent Entry to the F–N Ring Fragment. Org. Lett. 2002, 4, 1747–1750. [Google Scholar] [CrossRef]
- Ronzitti, G.; Callegari, F.; Malaguti, C.; Rossini, G.P. Selective disruption of the E-cadherin-catenin system by an algal toxin. Br. J. Cancer 2004, 90, 1100–1107. [Google Scholar] [CrossRef]
- Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin, a promising therapeutic tool. Mar. Drugs 2016, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Korsnes, M.S.; Korsnes, R. Mitotic catastrophe in BC3H1 cells following yessotoxin exposure. Front. Cell Dev. Biol. 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, M.; Yang, X.; Li, B.; Fairchild, C.R.; Shimizu, Y. Highly Cytotoxic Metabolites from the Culture Supernatant of the Temperate Dinoflagellate Protoceratium cf. r eticulatum. J. Nat. Prod. 2004, 67, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Sim, C.J.; Lee, C.-O. Cytotoxic compounds from a two-sponge association. J. Nat. Prod. 1995, 58, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Flowers, A.; Capra, M. Effects of ciguatoxin on nerve excitability in rats (Part I). J. Neurol. Sci. 1991, 101, 87–92. [Google Scholar] [CrossRef]
- Cameron, J.; Flowers, A.; Capra, M. Electrophysiological studies on ciguatera poisoning in man (Part II). J. Neurol. Sci. 1991, 101, 93–97. [Google Scholar] [CrossRef]
- Walsh, C.J.; Leggett, S.R.; Strohbehn, K.; Pierce, R.H.; Sleasman, J.W. Effects of in vitro brevetoxin exposure on apoptosis and cellular metabolism in a leukemic T cell line (Jurkat). Mar. Drugs 2008, 6, 291–307. [Google Scholar] [CrossRef]
- Murrell, R.N.; Gibson, J.E. Brevetoxins 2, 3, 6, and 9 show variability in potency and cause significant induction of DNA damage and apoptosis in Jurkat E6-1 cells. Arch. Toxicol. 2009, 83, 1009–1019. [Google Scholar] [CrossRef]
- Patocka, J.; Gupta, R.C.; Wu, Q.-h.; Kuca, K. Toxic potential of palytoxin. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2015, 35, 773–780. [Google Scholar] [CrossRef]
- Louzao, M.C.; Fraga, M.; Vilariño, N. Pharmacology of palytoxins and ostreocins. In Phycotoxins, Chemistry and Biochemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 113–135. [Google Scholar]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: Biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef] [Green Version]
- Twiner, M.J.; Hanagriff, J.C.; Butler, S.; Madhkoor, A.K.; Doucette, G.J. Induction of apoptosis pathways in several cell lines following exposure to the marine algal toxin azaspiracid. Chem. Res. Toxicol. 2012, 25, 1493–1501. [Google Scholar] [CrossRef]
- Twiner, M.J.; Ryan, J.C.; Morey, J.S.; Smith, K.J.; Hammad, S.M.; Van Dolah, F.M.; Hess, P.; McMahon, T.; Satake, M.; Yasumoto, T. Transcriptional profiling and inhibition of cholesterol biosynthesis in human T lymphocyte cells by the marine toxin azaspiracid. Genomics 2008, 91, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukano, C.; Sasaki, M. Structure—Activity relationship studies of gymnocin-A. Tetrahedron Lett. 2006, 47, 6803–6807. [Google Scholar] [CrossRef]
- Waters, A.L.; Hill, R.T.; Place, A.R.; Hamann, M.T. The expanding role of marine microbes in pharmaceutical development. Curr. Opin. Biotechnol. 2010, 21, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, A.L.; Oh, J.; Place, A.R.; Hamann, M.T. Stereochemical Studies of the Karlotoxin Class Using NMR Spectroscopy and DP4 Chemical-Shift Analysis: Insights into their Mechanism of Action. Angew. Chem. 2015, 127, 15931–15936. [Google Scholar] [CrossRef]
- Dragunow, M.; Trzoss, M.; Brimble, M.A.; Cameron, R.; Beuzenberg, V.; Holland, P.; Mountfort, D. Investigations into the cellular actions of the shellfish toxin gymnodimine and analogues. Environ. Toxicol. Pharmacol. 2005, 20, 305–312. [Google Scholar] [CrossRef]
- Kamat, P.K.; Rai, S.; Nath, C. Okadaic acid induced neurotoxicity: An emerging tool to study Alzheimer’s disease pathology. Neurotoxicology 2013, 37, 163–172. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [Green Version]
- López, L.M.B.; López, E.A.; González, C.V. Use of Gambierol for Treating and/or Preventing Neurodegenerative Diseases Related to Tau and Beta-Amyloid. U.S. Patent 13/504,825, 8 November 2012. [Google Scholar]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. The cholinergic antagonist gymnodimine improves Aβ and tau neuropathology in an in vitro model of Alzheimer disease. Cell. Physiol. Biochem. 2011, 27, 783–794. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Aβ and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef]
- Garrido, R.; Lagos, N.; Lattes, K.; Abedrapo, M.; Bocic, G.; Cuneo, A.; Chiong, H.; Jensen, C.; Azolas, R.; Henriquez, A. Gonyautoxin: New treatment for healing acute and chronic anal fissures. Dis. Colon Rectum 2005, 48, 335–343. [Google Scholar] [CrossRef]
- Lattes, K.; Venegas, P.; Lagos, N.; Lagos, M.; Pedraza, L.; Rodriguez-Navarro, A.; Garcia, C. Local infiltration of gonyautoxin is safe and effective in treatment of chronic tension-type headache. Neurol. Res. 2009, 31, 228–233. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Xu, H.; Zhang, X.; Li, X.-M. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology 2005, 30, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Braaten, D.; Franke, E.K.; Luban, J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 1995, 69, 6859–6864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Huang, J.; Yuan, X.; Peng, B.; Liu, W.; Han, S.; He, X. Toxins targeting the Kv1. 3 channel: Potential immunomodulators for autoimmune diseases. Toxins 2015, 7, 1749–1764. [Google Scholar] [CrossRef] [Green Version]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R. Kv1. 3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Satake, M.; Yasumoto, T. Antimicrobial activities of polyether compounds of dinoflagellate origins. J. Appl. Phycol. 1990, 2, 305–308. [Google Scholar] [CrossRef]
- Houdai, T.; Matsuoka, S.; Matsumori, N.; Murata, M. Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate. Biochim. Biophys. Acta-Biomembr. 2004, 1667, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Bachvaroff, T.R.; Adolf, J.E.; Squier, A.H.; Harvey, H.R.; Place, A.R. Characterization and quantification of karlotoxins by liquid chromatography–mass spectrometry. Harmful Algae 2008, 7, 473–484. [Google Scholar] [CrossRef]
- Deeds, J.R.; Hoesch, R.E.; Place, A.R.; Kao, J.P. The cytotoxic mechanism of karlotoxin 2 (KmTx 2) from Karlodinium veneficum (Dinophyceae). Aquat. Toxicol. 2015, 159, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Tobío, A.; Alfonso, A.; Madera-Salcedo, I.; Botana, L.M.; Blank, U. Yessotoxin, a marine toxin, exhibits anti-allergic and anti-tumoural activities inhibiting melanoma tumour growth in a preclinical model. PLoS ONE 2016, 11, e0167572. [Google Scholar] [CrossRef]
- George, J.; Baden, D.G.; Gerwick, W.H.; Murray, T.F. Bidirectional influence of sodium channel activation on NMDA receptor–dependent cerebrocortical neuron structural plasticity. Proc. Natl. Acad. Sci. USA 2012, 109, 19840–19845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, M.; Uemura, D. Bioactive heterocyclic alkaloids of marine origin. In Bioactive Heterocycles I.; Springer: Berlin, Germany, 2006; pp. 157–179. [Google Scholar] [CrossRef]
- Kita, M.; Inuzuka, T.; Maru, N.; Uemura, D. 12 Bioactive Molecules from Symbiotic Marine Dinoflagellates. In Marine Pharmacognosy: Trends and Applications; CRC Press: Boca Raton, FL, USA, 2012; pp. 137–151. [Google Scholar] [CrossRef]
- Cao, Z.; Cui, Y.; Busse, E.; Mehrotra, S.; Rainier, J.D.; Murray, T.F. Gambierol inhibition of voltage-gated potassium channels augments spontaneous Ca2+ oscillations in cerebrocortical neurons. J. Pharmacol. Exp. Ther. 2014, 350, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012, 2012, 179782. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Agrawal, M.; Raipuria, M.; Agrawal, M.K. Antimicrobial Activity of Nostoc calcicola (Cyanobacteria) isolated from central India against human pathogens. Asian J. Pharm. 2016, 10, S554–S559. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.-W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Mar. Drugs 2017, 15, 354. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, B.; Patra, S.; Dash, S.R.; Satapathy, Y.; Nayak, S.; Mandal, A.K.; Jena, M. In vitro antidiabetic, anti-inflammatory and antibacterial activity of marine alga Enteromorpha compressa collected from Chilika lagoon, Odisha, India. Vegetos 2022. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C. Marine cyanobacteria and microalgae metabolites—A rich source of potential anticancer drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar] [CrossRef]
- Nagarajan, M.; Maruthanayagam, V.; Sundararaman, M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 2012, 32, 153–185. [Google Scholar] [CrossRef]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Bhimba, B.V. Bioactive potency of cyanobacteria Oscillatoria spp. Int. J. Pharm. Pharm. Sci. 2013, 5, 611–612. [Google Scholar]
- Nagle, D.G.; Zhou, Y.-D. Marine natural products as inhibitors of hypoxic signaling in tumors. Phytochem. Rev. 2009, 8, 415–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oftedal, L.; Selheim, F.; Wahlsten, M.; Sivonen, K.; Døskeland, S.O.; Herfindal, L. Marine benthic cyanobacteria contain apoptosis-inducing activity synergizing with daunorubicin to kill leukemia cells, but not cardiomyocytes. Mar. Drugs 2010, 8, 2659–2672. [Google Scholar] [CrossRef]
- Mizuno, K.; Nakahata, N.; Ito, E.; Murakami, M.; Yamaguchi, K.; Ohizumi, Y. Goniodomin A, an antifungal polyether macrolide, increases the filamentous actin content of 1321N1 human astrocytoma cells. J. Pharm. Pharmacol. 1998, 50, 645–648. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Wu, J.; Fan, T.-T.; Zhang, H.-H.; Gong, X.-X.; Cao, Z.-Y.; Zhang, J.; Lin, H.-W.; Han, B.-N. Chemical and biological study of aplysiatoxin derivatives showing inhibition of potassium channel Kv1. 5. Rsc Adv. 2019, 9, 7594–7600. [Google Scholar] [CrossRef] [Green Version]
- White, J.D.; Xu, Q.; Lee, C.-S.; Valeriote, F.A. Total synthesis and biological evaluation of (+)-kalkitoxin, a cytotoxic metabolite of the cyanobacterium Lyngbya majuscula. Org. Biomol. Chem. 2004, 2, 2092–2102. [Google Scholar] [CrossRef]
- LePage, K.; Goeger, D.; Yokokawa, F.; Asano, T.; Shioiri, T.; Gerwick, W.; Murray, T. The neurotoxic lipopeptide kalkitoxin interacts with voltage-sensitive sodium channels in cerebellar granule neurons. Toxicol. Lett. 2005, 158, 133–139. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.; Vijayaraghavan, R.; Rao, P.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Sainis, I.; Fokas, D.; Vareli, K.; Tzakos, A.G.; Kounnis, V.; Briasoulis, E. Cyanobacterial cyclopeptides as lead compounds to novel targeted cancer drugs. Mar. Drugs 2010, 8, 629–657. [Google Scholar] [CrossRef]
- McDermott, C.; Nho, C.; Howard, W.; Holton, B. The cyanobacterial toxin, microcystin-LR, can induce apoptosis in a variety of cell types. Toxicon 1998, 36, 1981–1996. [Google Scholar] [CrossRef]
- Fladmark, K.; Brustugun, O.; Hovland, R.; Bøe, R.; Gjertsen, B.; Zhivotovsky, B.; Døskeland, S. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differ. 1999, 6, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankiewicz, J.; Tarczynska, M.; Fladmark, K.E.; Doskeland, S.O.; Walter, Z.; Zalewski, M. Apoptotic effect of cyanobacterial extract on rat hepatocytes and human lymphocytes. Environ. Toxicol. Int. J. 2001, 16, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Giordano, G.; Faustman, E.M. Domoic acid as a developmental neurotoxin. Neurotoxicology 2010, 31, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Dhar, B.C.; Cimarelli, L.; Singh, K.S.; Brandi, L.; Brandi, A.; Puccinelli, C.; Marcheggiani, S.; Spurio, R. Molecular detection of a potentially toxic diatom species. Int. J. Environ. Res. Public Health 2015, 12, 4921–4941. [Google Scholar] [CrossRef] [Green Version]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Bates, S.S. Domoic-acid-producing diatoms: Another genus added! J. Phycol. 2000, 36, 978–983. [Google Scholar] [CrossRef]
- Olesen, A.J.; Leithoff, A.; Altenburger, A.; Krock, B.; Beszteri, B.; Eggers, S.L.; Lundholm, N. First Evidence of the Toxin Domoic Acid in Antarctic Diatom Species. Toxins 2021, 13, 93. [Google Scholar] [CrossRef]
- Kotaki, Y.; Lundholm, N.; Onodera, H.; Kobayashi, K.; Bajarias, F.F.A.; Furio, E.F.; Iwataki, M.; Fukuyo, Y.; Kodama, M. Wide distribution of Nitzschia navis-varingica, a new domoic acid-producing benthic diatom found in Vietnam. Fish. Sci. 2004, 70, 28–32. [Google Scholar] [CrossRef]
- Holland, P.T.; Selwood, A.I.; Mountfort, D.O.; Wilkins, A.L.; McNabb, P.; Rhodes, L.L.; Doucette, G.J.; Mikulski, C.M.; King, K.L. Isodomoic acid C, an unusual amnesic shellfish poisoning toxin from Pseudo-Nitzschia australis. Chem. Res. Toxicol. 2005, 18, 814–816. [Google Scholar] [CrossRef]
- Romero, M.L.J.; Kotaki, Y.; Lundholm, N.; Thoha, H.; Ogawa, H.; Relox, J.R.; Terada, R.; Takeda, S.; Takata, Y.; Haraguchi, K. Unique amnesic shellfish toxin composition found in the South East Asian diatom Nitzschia Navis-Varingica. Harmful Algae 2011, 10, 456–462. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef] [Green Version]
- Ayed, Y.; Kouidhi, B.; Kassim, S.; Bacha, H. Proliferative effect of the phycotoxin domoic acid on cancer cell lines: A preliminary evaluation. J. Taibah Univ. Sci. 2018, 12, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Burkholder, J.M. Implications of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecol. Appl. 1998, 8, S37–S62. [Google Scholar] [CrossRef] [Green Version]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobó, P.; Franco, J.M.; Fernández, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef]
- Rhodes, L.; McNabb, P.; De Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Song, H.; Li, J.; Lu, C.L.; Kang, L.; Xie, L.; Zhang, Y.Y.; Zhou, X.B.; Zhong, S. Tetrodotoxin alleviates acute heroin withdrawal syndrome: A multicentre, randomized, double-blind, placebo-controlled study. Clin. Exp. Pharmacol. Physiol. 2011, 38, 510–514. [Google Scholar] [CrossRef]
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Inoue, D.; Matsunaga, K.; Ohizumi, Y.; Ueda, H.; Asano, T.; Murakami, M.; Sato, Y. Goniodomin A, an antifungal polyether macrolide, exhibits antiangiogenic activities via inhibition of actin reorganization in endothelial cells. J. Cell. Physiol. 2002, 190, 109–116. [Google Scholar] [CrossRef]
- Dechraoui, M.-Y.B.; Ramsdell, J.S. Type B brevetoxins show tissue selectivity for voltage-gated sodium channels: Comparison of brain, skeletal muscle and cardiac sodium channels. Toxicon 2003, 41, 919–927. [Google Scholar] [CrossRef]
- Lago, J.; Rodríguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Candenas, M.; Souto, M.; Trujillo, M.; Norte, M. Okadaic acid, useful tool for studying cellular processes. Curr. Med. Chem. 2002, 9, 229–262. [Google Scholar] [CrossRef] [PubMed]
- López, A.M.; Rodríguez, J.J.G.; Mirón, A.S.; Camacho, F.G.; Grima, E.M. Immunoregulatory potential of marine algal toxins yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicol. Lett. 2011, 207, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.i.; Shimbo, K.; Kubota, T.; Tsuda, M. Bioactive macrolides and polyketides from marine dinoflagellates. Pure Appl. Chem. 2003, 75, 337–342. [Google Scholar] [CrossRef]
- Murata, M.; Kumagai, M.; Lee, J.S.; Yasumoto, T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987, 28, 5869–5872. [Google Scholar] [CrossRef]
- Korsnes, M.S.; Espenes, A. Yessotoxin as an apoptotic inducer. Toxicon 2011, 57, 947–958. [Google Scholar] [CrossRef]
- Fernández-Araujo, A.; Alfonso, A.; Vieytes, M.; Botana, L. Key role of phosphodiesterase 4A (PDE4A) in autophagy triggered by yessotoxin. Toxicology 2015, 329, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Aune, T. Cleavage of tensin during cytoskeleton disruption in YTX-induced apoptosis. Toxicology In Vitro 2007, 21, 9–15. [Google Scholar] [CrossRef]
- Rubiolo, J.; López-Alonso, H.; Martínez, P.; Millán, A.; Cagide, E.; Vieytes, M.; Vega, F.; Botana, L. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell. Signal. 2014, 26, 419–432. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; Van den Top, H.J.; De Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espina, B.; Louzao, M.C.; Ares, I.R.; Fonfria, E.S.; Vilarino, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Impact of the pectenotoxin C-43 oxidation degree on its cytotoxic effect on rat hepatocytes. Chem. Res. Toxicol. 2010, 23, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-D.; Choi, T.-S.; Kim, B.-M.; Jung, J.H.; Bang, Y.-J.; Shin, D.Y. Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors. Oncogene 2005, 24, 4813–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, M. The origin of ciguatera—An update. Ciguatera Inf. Bull. Noumea 1992, 2, 8–9. [Google Scholar]
- Mattei, C.; Legros, C. The voltage-gated sodium channel: A major target of marine neurotoxins. Toxicon 2014, 91, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef]
- Nicolaou, K.; Aversa, R.J. Cover Picture: Maitotoxin: An Inspiration for Synthesis (Isr. J. Chem. 3-4/2011). Isr. J. Chem. 2011, 51, 305. [Google Scholar] [CrossRef]
- Xi, D.; Van Dolah, F.; Ramsdell, J. Maitotoxin induces a calcium-dependent membrane depolarization in GH4C1 pituitary cells via activation of type L voltage-dependent calcium channels. J. Biol. Chem. 1992, 267, 25025–25031. [Google Scholar] [CrossRef]
- Reyes, J.G.; Sánchez-Cárdenas, C.; Acevedo-Castillo, W.; Leyton, P.; López-González, I.; Felix, R.; Gandini, M.A.; Treviño, M.B.; Treviño, C.L. Maitotoxin: An enigmatic toxic molecule with useful applications in the biomedical sciences. Available online: https://www.researchgate.net/publication/260479283_Maitotoxin_An_Enigmatic_Toxic_Molecule_with_Useful_Applications_in_the_Biomedical_Sciences (accessed on 22 January 2022).
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marinedinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [CrossRef]
- Rhodes, L.; Harwood, T.; Smith, K.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Taglialatela, M.; Amoroso, S.; Yasumoto, T.; Di Renzo, G.; Annunziato, L. Maitotoxin and Bay-K-8644: Two putative calcium channel activators with different effects on endogenous dopamine release from tuberoinfundibular neurons. Brain Res. 1986, 381, 356–358. [Google Scholar] [CrossRef]
- Gusovsky, F.; Daly, J.W.; Yasumoto, T.; Rojas, E. Differential effects of maitotoxin on ATP secretion and on phosphoinositide breakdown in rat pheochromocytoma cells. FEBS Lett. 1988, 233, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Estacion, M.; Schilling, W.P. Maitotoxin-induced membrane blebbing and cell death in bovine aortic endothelial cells. BMC Physiol. 2001, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.A.; Kertesy, S.B.; Estacion, M.; Schilling, W.P.; Dubyak, G.R. Maitotoxin induces biphasic interleukin-1β secretion and membrane blebbing in murine macrophages. Mol. Pharmacol. 2004, 66, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Obara, Y.; Takahashi, M.; Nakahata, N.; Ohizumi, Y. Maitotoxin-induced nerve growth factor production accompanied by the activation of a voltage-insensitive Ca2+ channel in C6-BU-1 glioma cells. Br. J. Pharmacol. 1999, 127, 1577–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, P.L.; Rodríguez, E.; Zapata, E.; Carbó, R.; Farías, J.M.; Martínez, M. Maitotoxin Is a potential selective activator of the endogenous transient receptor potential canonical Type 1 channel in Xenopus laevis oocytes. Mar. Drugs 2017, 15, 198. [Google Scholar] [CrossRef] [Green Version]
- Baden, D.G. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar] [CrossRef]
- Plakas, S.M.; Dickey, R.W. Advances in monitoring and toxicity assessment of brevetoxins in molluscan shellfish. Toxicon 2010, 56, 137–149. [Google Scholar] [CrossRef]
- Hallegraeff, G. Harmful algal blooms: A global overview. Man. Harmful Mar. Microalgae 2003, 33, 1–22. [Google Scholar]
- Abraham, W.M.; Bourdelais, A.J.; Sabater, J.R.; Ahmed, A.; Lee, T.A.; Serebriakov, I.; Baden, D.G. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Hilderbrand, S.C.; Murrell, R.N.; Gibson, J.E.; Brown, J.M. Marine brevetoxin induces IgE-independent mast cell activation. Arch. Toxicol. 2011, 85, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.M.; Baatz, J.E. Brevetoxin-2 induces an inflammatory response in an alveolar macrophage cell line. Int. J. Hyg. Environ. Health 2010, 213, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G.; Abraham, W.M.; Bourdelais, A.J. Polyether Brevetoxin Derivatives as a Treatment for Cystic Fibrosis, Mucociliary Dysfunction, and Pulmonary Diseases. U.S. Patent 7,399,782, 15 July 2008. [Google Scholar]
- Gordon, C.J.; Ramsdell, J.S. Effects of marine algal toxins on thermoregulation in mice. Neurotoxicol. Teratol. 2005, 27, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Ohno, O.; Han, C.; Uemura, D. Bioactive secondary metabolites from symbiotic marine dinoflagellates: Symbiodinolide and durinskiols. Chem. Rec. 2010, 10, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Malagoli, D.; Ottaviani, E. Targets and effects of yessotoxin, okadaic acid and palytoxin: A differential review. Mar. Drugs 2010, 8, 658–677. [Google Scholar] [CrossRef] [Green Version]
- Assunção, J.; Guedes, A.C.; Malcata, F.X. Biotechnological and pharmacological applications of biotoxins and other bioactive molecules from dinoflagellates. Mar. Drugs 2017, 15, 393. [Google Scholar] [CrossRef] [Green Version]
- Pelin, M.; Florio, C.; Ponti, C.; Lucafò, M.; Gibellini, D.; Tubaro, A.; Sosa, S. Pro-inflammatory effects of palytoxin: An in vitro study on human keratinocytes and inflammatory cells. Toxicol. Res. 2016, 5, 1172–1181. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Fuwa, H.; Fukazawa, R.; Sasaki, M. Concise synthesis of the A/BCD-ring fragment of gambieric acid A. Front. Chem. 2015, 2, 116. [Google Scholar] [CrossRef] [Green Version]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.; Chan, L.L.; Lam, P.K. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Rubiolo, J.; Vale, C.; Martín, V.; Fuwa, H.; Sasaki, M.; Botana, L. Potassium currents inhibition by gambierol analogs prevents human T lymphocyte activation. Arch. Toxicol. 2015, 89, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Rubiolo, J.A. Therapeutics of marine toxins. Phycotoxins Chem. Biochem. 2015, 181–201. [Google Scholar]
- LePage, K.T.; Rainier, J.D.; Johnson, H.; Baden, D.G.; Murray, T.F. Gambierol acts as a functional antagonist of neurotoxin site 5 on voltage-gated sodium channels in cerebellar granule neurons. J. Pharmacol. Exp. Ther. 2007, 323, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Tillmann, U.; Elbrächter, M.; Krock, B.; John, U.; Cembella, A. Azadinium spinosum gen. et sp. nov.(Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol. 2009, 44, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Percopo, I.; Siano, R.; Rossi, R.; Soprano, V.; Sarno, D.; Zingone, A. A new potentially toxic A. zadinium species (D inophyceae) from the M editerranean Sea, A. dexteroporum sp. nov. J. Phycol. 2013, 49, 950–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, J.A.; Andree, K.B.; Diogène, J.; Fernández-Tejedor, M.; Toebe, K.; John, U.; Krock, B.; Tillmann, U.; Cembella, A.D. Toxigenic algae and associated phycotoxins in two coastal embayments in the Ebro Delta (NW Mediterranean). Harmful Algae 2016, 55, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Vilariño, N. Marine toxins and the cytoskeleton: Azaspiracids. FEBS J. 2008, 275, 6075–6081. [Google Scholar] [CrossRef]
- Twiner, M.J.; Hess, P.; Dechraoui, M.-Y.B.; McMahon, T.; Samons, M.S.; Satake, M.; Yasumoto, T.; Ramsdell, J.S.; Doucette, G.J. Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines. Toxicon 2005, 45, 891–900. [Google Scholar] [CrossRef]
- Cao, Z.; LePage, K.T.; Frederick, M.O.; Nicolaou, K.C.; Murray, T.F. Involvement of caspase activation in azaspiracid-induced neurotoxicity in neocortical neurons. Toxicol. Sci. 2010, 114, 323–334. [Google Scholar] [CrossRef]
- Vale, C.; Nicolaou, K.C.; Frederick, M.O.; Vieytes, M.R.; Botana, L.M. Cell volume decrease as a link between azaspiracid-induced cytotoxicity and c-Jun-N-terminal kinase activation in cultured neurons. Toxicol. Sci. 2010, 113, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, Y.; Alfonso, A.; Vieytes, M.R.; Ofuji, K.; Satake, M.; Yasumoto, T.; Botana, L.M. Effects of azaspiracids 2 and 3 on intracellular cAMP,[Ca2+], and pH. Chem. Res. Toxicol. 2004, 17, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- García-Altares, M. Structural diversity of microalgal marine toxins. Recent Adv. Anal. Mar. Toxins. Amst. Neth. Elsevier 2017, 78, 35–88. [Google Scholar]
- Adolf, J.E.; Bachvaroff, T.R.; Deeds, J.R.; Place, A.R. Ichthyotoxic Karlodinium veneficum (Ballantine) J Larsen in the upper Swan River estuary (Western Australia): Ecological conditions leading to a fish kill. Harmful Algae 2015, 48, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Cembella, A.; Lewis, N.; Quilliam, M. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef]
- Davidson, K.; Baker, C.; Higgins, C.; Higman, W.; Swan, S.; Veszelovszki, A.; Turner, A.D. Potential threats posed by new or emerging marine biotoxins in UK waters and examination of detection methodologies used for their control: Cyclic imines. Mar. Drugs 2015, 13, 7087–7112. [Google Scholar] [CrossRef]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. Neurotoxicology 2003, 24, 593–604. [Google Scholar] [CrossRef]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; de la Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Fabro, E.; Krock, B.; Torres, A.I.; Flavio, E.P.; Schloss, I.R.; Ferreyra, G.A.; Almandoz, G.O. Toxigenic dinoflagellates and associated toxins in San Jorge Gulf, Argentina. Oceanography 2018, 31, 145–153. [Google Scholar] [CrossRef]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, P.; DʼAuria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Nagai, H.; Torigoe, K.; Satake, M.; Murata, M.; Yasumoto, T.; Hirota, H. Gambieric acids: Unprecedented potent antifungal substances isolated from cultures of a marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1992, 114, 1102–1103. [Google Scholar] [CrossRef]
- Nagai, H.; Mikami, Y.; Yazawa, K.; Gonoi, T.; Yasumoto, T. Biological activities of novel polyether antifungals, gambieric acids A and B from a marine dinoflagellate Gambierdiscus toxicus. J. Antibiot. 1993, 46, 520–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Hirama, M.; Satake, M.; Sugiyama, K.; Yasumoto, T. Inhibition of brevetoxin binding to the voltage-gated sodium channel by gambierol and gambieric acid-A. Toxicon 2003, 41, 469–474. [Google Scholar] [CrossRef]
- Murakami, M.; Makabe, K.; Yamaguchi, K.; Konosu, S.; Wälchli, M.R. Goniodomin A, a novel polyether macrolide from the dinoflagellate Goniodoma pseudogoniaulax. Tetrahedron Lett. 1988, 29, 1149–1152. [Google Scholar] [CrossRef]
- Hsia, M.H.; Morton, S.L.; Smith, L.L.; Beauchesne, K.R.; Huncik, K.M.; Moeller, P.D. Production of goniodomin A by the planktonic, chain-forming dinoflagellate Alexandrium monilatum (Howell) Balech isolated from the Gulf Coast of the United States. Harmful Algae 2006, 5, 290–299. [Google Scholar] [CrossRef]
- Triki, H.Z.; Laabir, M.; Moeller, P.; Chomérat, N.; Daly-Yahia, O.K. First report of goniodomin A production by the dinoflagellate Alexandrium pseudogonyaulax developing in southern Mediterranean (Bizerte Lagoon, Tunisia). Toxicon 2016, 111, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espiña, B.; Cagide, E.; Louzao, M.C.; Vilariño, N.; Vieytes, M.R.; Takeda, Y.; Sasaki, M.; Botana, L.M. Cytotoxicity of goniodomin A and B in non contractile cells. Toxicol. Lett. 2016, 250, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.i. Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. 2016, 64, 1079–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usui, T.; Kazami, S.; Dohmae, N.; Mashimo, Y.; Kondo, H.; Tsuda, M.; Terasaki, A.G.; Ohashi, K.; Kobayashi, J.i.; Osada, H. Amphidinolide H, a potent cytotoxic macrolide, covalently binds on actin subdomain 4 and stabilizes actin filament. Chem. Biol. 2004, 11, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, J.i. Amphidinolides and its related macrolides from marine dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.; Belarbi, E.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Echigoya, R.; Rhodes, L.; Oshima, Y.; Satake, M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae 2005, 4, 383–389. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Sardo, A.; Fontana, A. The Missing piece in biosynthesis of amphidinols: First evidence of glycolate as a starter unit in New Polyketides from Amphidinium Carterae. Mar. Drugs 2017, 15, 157. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Cornelio, K.; Hanashima, S.; Malabed, R.; Murata, M.; Matsumori, N.; Zhang, H.; Hayashi, F.; Mori, S.; Kim, J.S. Structures of the largest amphidinol homologues from the dinoflagellate Amphidinium carterae and structure–activity relationships. J. Nat. Prod. 2017, 80, 2883–2888. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium Kleb. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Swasono, R.T.; Mouri, R.; Morsy, N.; Matsumori, N.; Oishi, T.; Murata, M. Sterol effect on interaction between amphidinol 3 and liposomal membrane as evidenced by surface plasmon resonance. Bioorganic Med. Chem. Lett. 2010, 20, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Akakabe, M.; Kumagai, K.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-5, a novel linear polyketide from a cultured marine dinoflagellate Amphidinium species with a potent cell proliferation-promoting activity. Tetrahedron Lett. 2014, 55, 3491–3494. [Google Scholar] [CrossRef]
- Minamida, M.; Kumagai, K.; Ulanova, D.; Akakabe, M.; Konishi, Y.; Tominaga, A.; Tanaka, H.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-4 with potent proliferation-promoting activity on bone marrow stromal cells from a marine dinoflagellate Amphidinium species. Org. Lett. 2014, 16, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Akakabe, M.; Kumagai, K.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Iriomoteolide-13a, a cytotoxic 22-membered macrolide from a marine dinoflagellate Amphidinium species. Tetrahedron 2014, 70, 2962–2965. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ishibashi, M.; Nakamura, H.; Hirata, Y.; Yamasu, T.; Sasaki, T.; Ohizumi, Y. Symbioramide, a novel Ca2+-ATPase activator from the cultured dinoflagellate Symbiodinium sp. Experientia 1988, 44, 800–802. [Google Scholar] [CrossRef]
- Kobayashi, J.i. Pharmacologically active metabolites from symbiotic microalgae in Okinawan marine invertebrates. J. Nat. Prod. 1989, 52, 225–238. [Google Scholar] [CrossRef]
- Nagai, H.; Murata, M.; Torigoe, K.; Satake, M.; Yasumoto, T. Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gamb. Toxicus. J. Org. Chem. 1992, 57, 5448–5453. [Google Scholar] [CrossRef]





| Disease | Toxin | Application | Reference |
|---|---|---|---|
| Cancer | Okadaic acid (OA) | Breast, intestinal, blood, brain, lungs, hepatic, human leukemia and human endothelial cancer cell lines | [42,43,44,45] |
| Amphidinolides and colopsinols | Murine lymphoma L1210 and human epidermoid carcinoma KB cells | [46] | |
| Caribenolide I | Human colon tumor cell line HCT 116 and HCT 116/VM 46 | [47] | |
| in vivo against the mouse tumor P388 | [48] | ||
| Gymnocin-A | P388 murine leukemia cells | [49] | |
| Yessotoxins (YTXs) | Epithelial cancer cells | [50] | |
| YTX and its analogues | In BC3H1 myoblast cells, primary cortical neurons, and glioma cells | [51] | |
| Melanoma tumor cells | [52] | ||
| Protoceratins I, II, III, and IV | Human colon cancer cell lines | [53] | |
| Pectenotoxin (PTX) | Lung, colon, and breast cancer cells | [54] | |
| Ciguatoxin (CTX) | Gastrointestinal cell lines | [55,56] | |
| Brevetoxin (BTX) | Jurkat E6-1 cell lines | [57,58] | |
| Palytoxin (PLTX) | Lymphoblastic or myelogenous leukemia cell lines | [59] | |
| Palytoxin (PLTX) and Ostreocin-D | Intestinal and neuroblastoma cell lines | [60,61] | |
| Azaspiracid (AZA) | T-lymphocyte cell lines | [62,63] | |
| Gymnocin-A (GYMA) | P388 murine leukemia cell lines | [64] | |
| Karlotoxin (KmTx) | Breast and prostate cancer cell lines | [65,66] | |
| Combination of GYM and OA | Several cancer cell lines | [67] | |
| GYM | Neuroblastoma cell line | [67] | |
| Alzheimer | Okadaic acid (OA) YTX and its analogues Gambierol GYM Spirolides | Inhibits the level of t- and β-amyloid | [68] [69] [70] [71] [72] |
| Pain | Gonyautoxins (GTX) | - | [73] |
| GTX2, GTX3 and TTX | - | [74] | |
| Schizophrenia | Okadaic acid (OA) | - | [75] |
| Diabetes | Okadaic acid (OA) Gambierol | - | [76] |
| [77,78] | |||
| AIDS | Okadaic acid (OA) | - | [76] |
| Fungal disease | Okadaic acid (OA) Karlotoxin (KmTx) | Suppress Candida albicans growth | [79] |
| [65,80,81,82] | |||
| Allergy and Asthma | YTX and its analogues | - | [83] |
| Brain disorder | BTX-2 | - | [84] |
| Osteoporosis | Symbioimine | Postmenopausal women | [85] |
| Inflammation | Symbioimine | Treatment of cyclooxygenase-2-related disorders | [86] |
| Brain injury, autoimmune disorders, multiple sclerosis, and rheumatoid arthritis | Gambierol | - | [87] |
| [77,78] | |||
| [77,78] | |||
| Coronary heart disease (CHD) | Karlotoxin (KmTx) | - | [65,66] |
| Pain, | Gonyautoxins (GTX) | - | [73] |
| Fungal, bacterial, and protozoal disease | Saxitoxin (STXs) | - | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, B.; Ki, J.-S. Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Mar. Drugs 2022, 20, 271. https://doi.org/10.3390/md20040271
Pradhan B, Ki J-S. Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Marine Drugs. 2022; 20(4):271. https://doi.org/10.3390/md20040271
Chicago/Turabian StylePradhan, Biswajita, and Jang-Seu Ki. 2022. "Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals" Marine Drugs 20, no. 4: 271. https://doi.org/10.3390/md20040271
APA StylePradhan, B., & Ki, J. -S. (2022). Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Marine Drugs, 20(4), 271. https://doi.org/10.3390/md20040271