Dual Role of Chitin as the Double Edged Sword in Controlling the NLRP3 Inflammasome Driven Gastrointestinal and Gynaecological Tumours
Abstract
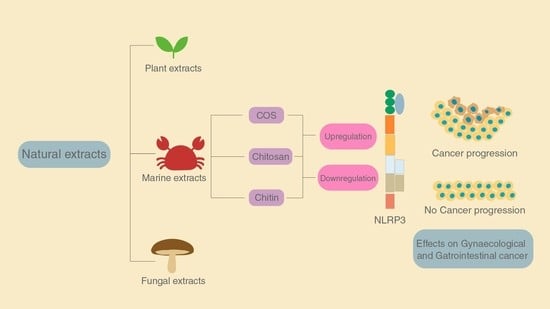
:1. Introduction
2. NLRP3 Inflammasome and Its Importance in Gastrointestinal and Gynaecological Cancers
2.1. Gastrointestinal Cancers
2.1.1. Colorectal Cancer (CRC)
2.1.2. Pancreatic Cancer
2.1.3. Gastric Cancer
2.1.4. Hepatic Cancer
2.2. Gynaecological Cancers
2.2.1. Endometrial Cancer
2.2.2. Ovarian Cancer
2.2.3. Cervical Cancer
3. Effects of Cancer Treatment
3.1. Chemical Treatments
3.2. Natural Extracts for Treatment
4. The Role of Chitin and Its Derivatives
4.1. Effect of Chitin
4.2. Effect of Chitosan
4.3. Effect of Chitooligosaccharides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Missiroli, S.; Missiroli, S.; Perrone, M.; Boncompagni, C.; Borghi, C.; Campagnaro, A.; Marchetti, F.; Anania, G.; Greco, P.; Fiorica, F.; et al. Targeting the nlrp3 inflammasome as a new therapeutic option for overcoming cancer. Cancers 2021, 13, 2297. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of nlrpinflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitvogel, L.; Kepp, O.; Galluzzi, L.; Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 2012, 13, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and anti-Inflammatory properties of chitin and chitosan oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 2009, 7, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Itani, S.; Watanabe, T.; Nadatani, Y.; Sugimura, N.; Shimada, S.; Takeda, S.; Otani, K.; Hosomi, S.; Nagami, Y.; Tanaka, F.; et al. NLRP3 inflammasome has a protective effect against oxazolone-induced colitis: A possible role in ulcerative colitis. Sci. Rep. 2016, 6, 39075. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M.; et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Afaq, F.; Mukhtar, H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010, 293, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tariq, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Shi, F.; Wei, B.; Lan, T.; Xiao, Y.; Quan, X.; Chen, J.; Zhao, C.; Gao, J. Low nlrp3 expression predicts a better prognosis of colorectal cancer. Biosci. Rep. 2021, 41, BSR20210280. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Wang, X.; Zhu, X. The association of aberrant expression of NLRP3 and p-S6K1 in colorectal cancer. Pathol. Res. Pract. 2020, 216, 152737. [Google Scholar] [CrossRef]
- Marandi, Y.; Hashemzade, S.; Tayebinia, H.; Karimi, J.; Zamani, A.; Khodadadi, I. NLRP3-inflammasome activation is associated with epithelial-mesenchymal transition and progression of colorectal cancer. Iran. J. Basic Med. Sci. 2021, 24, 483–492. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Q.; Zhao, K.; Zhou, Y.; Li, W.; Pan, C.; Qiang, L.; Li, Z.; Lu, N. Small molecule GL-V9 protects against colitis-associated colorectal cancer by limiting NLRP3 inflammasome through autophagy. OncoImmunology 2018, 7, e1375640. [Google Scholar] [CrossRef] [Green Version]
- Ushio, J.; Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; et al. Pancreatic ductal adenocarcinoma: Epidemiology and risk factors. Diagnostics 2021, 11, 562. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Banerjee, S.; Li, Y. Pancreatic cancer: Pathogenesis, prevention and treatment. Toxicol. Appl. Pharmacol. 2017, 224, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Pandian, G.S.B.; Savadkar, S.; Lee, K.B.; Torres-Hernandez, A.; Aykut, B.; Diskin, B.; et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J. Exp. Med. 2017, 214, 1711–1724. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Wang, Y.; Ding, X.; He, Y.; Lu, Z.; Wu, P.; Tian, L.; Yuan, H.; Liu, D.; Shi, G.; et al. Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3. Mol. Cancer 2018, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Boone, B.A.; Murthy, P.; Miller-Ocuin, J.L.; Liang, X.; Russell, K.L.; Loughran, P.; Gawaz, M.; Lotze, M.T.; Zeh, H.J.; Vogel, S. The platelet NLRP3 inflammasome is upregulated in a murine model of pancreatic cancer and promotes platelet aggregation and tumor growth. Ann. Hematol. 2019, 98, 1603–1610. [Google Scholar] [CrossRef]
- Yaw, A.C.K.; Chan, E.W.L.; Yap, J.K.Y.; Mai, C.W. The effects of NLRP3 inflammasome inhibition by MCC950 on LPS-induced pancreatic adenocarcinoma inflammation. J. Cancer Res. Clin. Oncol. 2020, 146, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Liang, K.; Liu, R. Immune cells combined with nlrp3 inflammasome inhibitor exert better antitumor effect on pancreatic ductal adenocarcinoma. Front. Oncol. 2020, 10, 1378. [Google Scholar] [CrossRef]
- Semper, R.P.; Mejías-Luque, R.; Groß, C.; Anderl, F.; Müller, A.; Vieth, M.; Busch, D.H.; da Costa, C.P.; Ruland, J.; Groß, O.; et al. Helicobacter pylori—induced il-1β secretion in innate immune cells is regulated by the nlrp3 inflammasome and requires the cag pathogenicity island. J. Immunol. 2014, 193, 3566–3576. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, S.; Luo, J.; Liu, A.; Tang, S.; Liu, S.; Yu, M.; Zhang, Y. Helicobacter pylori induces IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway. Pathog. Dis. 2015, 73, ftu024. [Google Scholar] [CrossRef]
- Pachathundikandi, S.K.; Blaser, N.; Bruns, H.; Backert, S. Helicobacter pylori avoids the critical activation of NLRP3 inflammasome-mediated production of oncogenic mature il-1β in human immune cells. Cancers 2020, 12, 803. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Zhu, R.; Zhu, J.; Zhao, R.; Li, M. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol. Res. 2019, 27, 827–834. [Google Scholar] [CrossRef]
- Wei, Q.; Mu, K.; Li, T.; Zhang, Y.; Yang, Z.; Jia, X.; Zhao, W.; Huai, W.; Guo, P.; Han, L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab. Investig. 2014, 94, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Saber, S.; Ghanim, A.M.H.; El-Ahwany, E.; El-Kader, E.M.A. Novel complementary antitumour effects of celastrol and metformin by targeting IκBκB, apoptosis and NLRP3 inflammasome activation in diethylnitrosamine-induced murine hepatocarcinogenesis. Cancer Chemother. Pharmacol. 2020, 85, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.H.; Wang, Y.Y.; Lu, J.; Zheng, Y.L.; Wu, D.M.; Li, M.Q.; Hu, B.; Zhang, Z.F.; Cheng, W.; Shan, Q. Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS ONE 2014, 9, e89961. [Google Scholar] [CrossRef] [PubMed]
- Ledford, L.R.C.; Lockwood, S. Scope and epidemiology of gynecologic cancers: An overview. Semin. Oncol. Nurs. 2019, 35, 147–150. [Google Scholar] [CrossRef]
- Liu, S.G.; Wu, X.X.; Hua, T.; Xin, X.Y.; Feng, D.L.; Chi, S.Q.; Wang, X.X.; Wang, H.B. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. OncoTargets Ther. 2019, 12, 6927–6936. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, P.Y.; Bao, W.; Chen, S.J.; Wu, F.S.; Zhu, P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 2020, 20, 28. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Luborsky, J.; Barua, A.; Edassery, S.; Bahr, J.M.; Edassery, S.L. Inflammasome expression is higher in ovarian tumors than in normal ovary. PLoS ONE 2020, 15, e227081. [Google Scholar] [CrossRef]
- Cervical Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/cervical-cancer/statistics#:~:text=Cervical%20cancer%20is%20most%20often,cancer%20screenings%20before%20age%2065 (accessed on 29 May 2022).
- Pontillo, A.; Bricher, P.; Leal, V.N.C.; Lima, S.; Souza, P.R.E.; Crovella, S. Role of inflammasome genetics in susceptibility to HPV infection and cervical cancer development. J. Med. Virol. 2016, 88, 1646–1651. [Google Scholar] [CrossRef]
- He, A.; Shao, J.; Zhang, Y.; Lu, H.; Wu, Z.; Xu, Y. cd200fc Reduces lps-Induced il-1β Activation in Human Cervical Cancer Cells by Modulating TLR4-NF-κB and nlrp3 Inflammasome Pathway. 2017. Available online: www.impactjournals.com/oncotarget/ (accessed on 7 March 2022).
- Types of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types (accessed on 22 June 2022).
- Chemotherapy Side Effects. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html (accessed on 29 May 2022).
- Hermelink, K.; Bühner, M.; Sckopke, P.; Neufeld, F.; Kaste, J.; Voigt, V.; Münzel, K.; Wuerstlein, R.; Ditsch, N.; Hellerhoff, K.; et al. Chemotherapy and post-traumatic stress in the causation of cognitive dysfunction in breast cancer patients. J. Natl. Cancer Inst. 2017, 109, djx057. [Google Scholar] [CrossRef] [Green Version]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, A.S.; Niu, J.; Giordano, S.H.; Zhao, H.; Wolff, A.C.; Chavez-MacGregor, M. Acute myeloid leukemia and myelodysplastic syndrome after adjuvant chemotherapy: A population-based study among older breast cancer patients. Cancer 2018, 124, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Keikhaei, B.; Bahadoram, M.; Keikha, A.; Bahadoram, S.; Hassanzadeh, S.; Mahmoudian-Sani, M.-R. Late side effects of cancer treatment in childhood cancer survivors. J. Oncol. Pharm. Pract. 2022, 10781552221087612. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, S.; Ni, S.; Zhang, B.; Kung, A.C.F.; Gao, J.; Lu, A.; Zhang, G. Targeting strategies for enhancing paclitaxel specificity in chemotherapy. Front. Cell Dev. Biol. 2021, 9, 626910. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yang, L.; Xia, X.; Zhu, R.; Chen, S.; Wang, M.; Cheng, L.; Wu, X.; Wang, S. Curcumin-loaded TPGS/F127/P123 mixed polymeric micelles for cervical cancer therapy: Formulation, characterization, and in vitro and in vivo evaluation. J. Biomed. Nanotechnol. 2017, 13, 1631–1646. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic potential of natural products in treatment of cervical cancer: A review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.; et al. Cyanobacteria—From the oceans to the potential biotechnological and biomedical applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Su, J.-H.; Chiang, M.Y.; Wen, Z.-H.; Dai, C.-F.; Hsu, C.-H.; Sheu, J.-H. Sesquiterpenoids from the Formosan Soft Coral Sinularia leptoclados. Chem. Pharm. Bull. 2010, 58, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Shih, H.J.; Tseng, Y.J.; Huang, C.Y.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2012, 68, 244–249. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. molecular mechanisms of inflammation. anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Li, W.; Xie, J.; Wang, F.; Peng, X.; Song, Y.; Tan, G. The role of Caspase-1/GSDMD-mediated pyroptosis in Taxol-induced cell death and a Taxol-resistant phenotype in nasopharyngeal carcinoma regulated by autophagy. Cell Biol. Toxicol. 2020, 36, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008, 8, 740–754. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; de Torre-Minguela, C.; Gomez, A.I.; Águas, A.P.; Barbosa, M.A.; Pelegrín, P.; Barbosa, J.N. 3D chitosan scaffolds impair NLRP3 inflammasome response in macrophages. Acta Biomater. 2019, 91, 123–134. [Google Scholar] [CrossRef]
- Bueter, C.L.; Lee, C.K.; Rathinam, V.A.; Healy, G.J.; Taron, C.H.; Specht, C.A.; Levitz, S.M. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011, 286, 35447–35455. [Google Scholar] [CrossRef] [Green Version]
- Komi, D.E.A.; Sharma, L.; dela Cruz, C.S. Chitin and its effects on inflammatory and immune responses. Clin. Rev. Allergy Immunol. 2018, 54, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Bussink, A.P.; Speijer, D.; Aerts, J.M.F.G.; Boot, R.G. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics 2007, 177, 959–970. [Google Scholar] [CrossRef]
- Bouhenna, M.; Salah, R.; Bakour, R.; Drouiche, N.; Abdi, N.; Grib, H.; Lounici, H.; Mameri, N. Effects of chitin and its derivatives on human cancer cells lines. Environ. Sci. Pollut. Res. 2015, 22, 15579–15586. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Cruz, C.S.D.; Ahangari, F.; Ma, B.; Kang, M.J.; He, C.H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, C.A.; Chalouni, C.; Williams, A.; Hartl, D.; Lee, C.G.; Elias, J.A. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009, 182, 3573–3582. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills-Karp, M.; Nathan, A.; Page, K.; Karp, C.L. New insights into innate immune mechanisms underlying allergenicity. Mucosal Immunol. 2010, 3, 104–110. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Howard, B.A.; Liu, Y.; Neumann, A.K.; Li, L.; Menon, N.; Roach, T.; Kale, S.D.; Samuels, D.C.; Li, H.; et al. LYSMD3: A mammalian pattern recognition receptor for chitin. Cell Rep. 2021, 36, 109392. [Google Scholar] [CrossRef]
- Schlosser, A.; Thomsen, T.; Moeller, J.B.; Nielsen, O.; Tornøe, I.; Mollenhauer, J.; Moestrup, S.K.; Holmskov, U. Characterization of FIBCD1 as an acetyl group-binding receptor that binds chitin. J. Immunol. 2009, 183, 3800–3809. [Google Scholar] [CrossRef] [Green Version]
- Rozbeský, D.; Ivanova, L.; Hernychová, L.; Grobárová, V.; Novák, P.; Černý, J. Nkrp1 family, from lectins to protein interacting molecules. Molecules 2015, 20, 3463–3478. [Google Scholar] [CrossRef] [Green Version]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Seetharaman, J.; Kfanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-Å resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [Green Version]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Elieh-Ali-Komi, D.; Hamblin, M.R.; Daniel, E.-A.-K. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Koppolu, B.P.; Smith, S.G.; Zaharoff, D.A. Effect of chitosan properties on immunoreactivity. Mar. Drugs 2016, 14, 91. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Kapadnis, G.; Dey, A.; Dandekar, P.; Jain, R. Effect of degree of deacetylation on solubility of low-molecular-weight chitosan produced via enzymatic breakdown of chitosan. Polym. Int. 2019, 68, 1054–1063. [Google Scholar] [CrossRef]
- Jesus, S.; Marques, A.P.; Duarte, A.; Soares, E.; Costa, J.P.; Colaço, M.; Schmutz, M.; Som, C.; Borchard, G.; Wick, P.; et al. Chitosan nanoparticles: Shedding light on immunotoxicity and hemocompatibility. Front. Bioeng. Biotechnol. 2020, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Je, J.Y.; Kim, S.K. Chitooligosaccharides as potential nutraceuticals. production and bioactivities. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 65, pp. 321–336. [Google Scholar] [CrossRef]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Águas, A.P.; Barbosa, J.N. Macrophage polarization following chitosan implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef]
- Zou, P.; Yuan, S.; Yang, X.; Guo, Y.; Li, L.; Xu, C.; Zhai, X.; Wang, J. Structural characterization and antitumor effects of chitosan oligosaccharides against orthotopic liver tumor via NF-κB signaling pathway. J. Funct. Foods 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Shen, K.T.; Chen, M.H.; Chan, H.Y.; Jeng, J.H.; Wang, Y.J. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef]
- Nam, K.-S.; Kim, M.-K.; Shon, Y.-H. Chemopreventive Effect of Chitosan Oligosaccharide against Colon Carcinogenesis. J. Microbiol. Biotechnol. 2007, 17, 1546–1549. Available online: http://europepmc.org/abstract/MED/18062235 (accessed on 4 March 2022). [PubMed]
- Hu, H.; Xia, H.; Zou, X.; Li, X.; Zhang, Z.; Yao, X.; Yin, M.; Tian, D.; Liu, H. N-acetyl-chitooligosaccharide attenuates inflammatory responses by suppression of NF-κB signaling, MAPK and NLRP3 inflammasome in macrophages. J. Funct. Foods 2021, 78, 104364. [Google Scholar] [CrossRef]




| S.No | Extracts | Source | Effects on Inflammation | Effect on Cancer | Type of Cancer | Reference |
|---|---|---|---|---|---|---|
| 1 | Sesquiterpenoids | Soft coral (Sinularia leptoclados) | Inhibition and up-regulation of pro-inflammatory iNOS and cyclooxygenase-2 (COX-2) proteins | Not cytotoxic | HeLa | [51] |
| 2 | Diterpenoids | Dongsha Atoll soft coral (Sinularia flexibilis) | Pro-inflammatory iNOS and COX-2 (cyclooxygenase-2) proteins are inhibited and up-regulated | Moderate cytotoxicity | HeLa, SK-Hep1, and B16 cancer cells | [52] |
| 3 | Phenolic compound oleocanthal | Virgin olive oil | Reduced levels of cytokines, LTs, CRP, and PGs, as well as reduction of iNOS, COX, 5-LOX and NFB activity | Induces apoptosis | CRC cell line | [53] |
| 4 | Curcumin | Curcuma longa | Blocking of NF-κB activation | Selective cytotoxic effect | HeLa | [48,54] |
| 5 | Chito-oligosaccharides | Crustaceans, insects and fungi | Translational and transcriptional expression levels of iNOS, COX-2, TNF- and IL-6 are reduced. | Anticancer effect exhibited | HeLa and SW480 cell lines | [55] |
| 6 | Taxol | Pacific yew tree | Activates NLRP3 in macrophages causing inflammation | Strong anticancer effect | Breast, ovarian, pancreatic cancer | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhanjal, C.R.; Lingamsetty, R.; Pareddy, A.; Kim, S.-K.; Raval, R. Dual Role of Chitin as the Double Edged Sword in Controlling the NLRP3 Inflammasome Driven Gastrointestinal and Gynaecological Tumours. Mar. Drugs 2022, 20, 452. https://doi.org/10.3390/md20070452
Dhanjal CR, Lingamsetty R, Pareddy A, Kim S-K, Raval R. Dual Role of Chitin as the Double Edged Sword in Controlling the NLRP3 Inflammasome Driven Gastrointestinal and Gynaecological Tumours. Marine Drugs. 2022; 20(7):452. https://doi.org/10.3390/md20070452
Chicago/Turabian StyleDhanjal, Chetan Roger, Rathnamegha Lingamsetty, Anooshka Pareddy, Se-Kwon Kim, and Ritu Raval. 2022. "Dual Role of Chitin as the Double Edged Sword in Controlling the NLRP3 Inflammasome Driven Gastrointestinal and Gynaecological Tumours" Marine Drugs 20, no. 7: 452. https://doi.org/10.3390/md20070452
APA StyleDhanjal, C. R., Lingamsetty, R., Pareddy, A., Kim, S. -K., & Raval, R. (2022). Dual Role of Chitin as the Double Edged Sword in Controlling the NLRP3 Inflammasome Driven Gastrointestinal and Gynaecological Tumours. Marine Drugs, 20(7), 452. https://doi.org/10.3390/md20070452