An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
2.3. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
3.2. Risk of Bias Assessment of Selected Studies
3.3. Qualitative Thematic Analysis
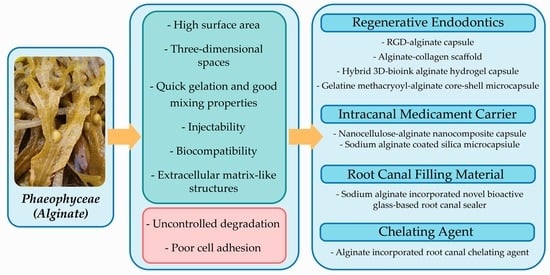
3.3.1. Role of Alginate in Regenerative Endodontics
3.3.2. Role of Alginate as Intracanal Medicament Carrier
3.3.3. Role of Alginate as Root Canal Filling Material
3.3.4. Role of Alginate as Reinforcement Material in a Chelating Agent
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tathe, A.; Ghodke, M.; Nikalje, A.P. A brief review: Biomaterials and their applications. Int. J. Pharm. Pharm. Sci. 2010, 2, 19–23. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The trends of dental biomaterials research and future directions: A mapping review. Saudi Dent. J. 2021, 33, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Bindal, P.; Kasim, N.H.A.; Ramasamy, T.S.; Dabbagh, A.; Moharamzadeh, K.; Chai, W.L. Dental pulp tissue engineering and regenerative endodontic therapy. In Biomaterials for Oral and Dental Tissue Engineering; Woodhead Publishing: Sawston, UK, 2017; pp. 297–318. [Google Scholar] [CrossRef]
- Bhoj, M.; Zhang, C.; Green, D.W. A first step in de novo synthesis of a living pulp tissue replacement using dental pulp MSCs and tissue growth factors, encapsulated within a bioinspired alginate hydrogel. J. Endod. 2015, 41, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, S.-Y.; Wu, J.-L.; Qiu, D.; Dong, Y.-M. A novel bioactive glass-based root canal sealer in endodontics. J. Dent. Sci. 2021, 17, 217–224. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Kiran, V.; Kurakula, M.; Rao, G.S.N.K.; Tabish, M.; Nayak, A.K. Use of alginates for drug delivery in dentistry. In Alginates in Drug Delivery; Academic Press: Cambridge, MA, USA, 2020; pp. 387–404. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate materials and dental impression technique: A current state of the art and application to dental practice. Mar. Drugs 2018, 17, 18. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.S.S.; Leong, J.Z.; Toh, E.Y.J.; Leow, Y.S. The scientific contribution of Malaysia’s researchers in the field of endodontics based on scopus database: A bibliometric analysis. Med. Health 2021, 16, 168–191. [Google Scholar] [CrossRef]
- Patel, E.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Oroactive dental biomaterials and their use in endodontic therapy. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 201–212. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.K.; Wu, B.; Ma, D. Effects of 3-dimensional bioprinting alginate/gelatin hydrogel scaffold extract on proliferation and differentiation of human dental pulp stem cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef]
- Devillard, R.; Remy, M.; Kalisky, J.; Bourget, J.M.; Kerouredan, O.; Siadous, R.; Bareille, R.; Amedee-Vilamitjana, J.; Chassande, O.; Fricain, J.C. In vitro assessment of a collagen/alginate composite scaffold for regenerative endodontics. Int. Endod. J. 2017, 50, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Garg, A. Textbook of Endodontics; Jaypee Brothers, Medical Publishers Pvt. Limited: New Delhi, India, 2010. [Google Scholar]
- Torabinejad, M.; Walton, R.E. Endodontics: Principles and Practice; Saunders/Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Jain, P. Current Therapy in Endodontics; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Duncan, H.F.; Cooper, P.R. Clinical Approaches in Endodontic Regeneration: Current and Emerging Therapeutic Perspectives; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Office of Health Assessment and Translation (OHAT) Risk of Bias Assessment Tool National Toxicology Programme (NTP). Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 20 July 2022).
- Wikoff, D.; Urban, J.D.; Harvey, S.; Haws, L.C. Role of risk of bias in systematic review for chemical risk assessment: A case study in understanding the relationship between congenital heart defects and exposures to trichloroethylene. Int. J. Toxicol. 2018, 37, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Evelyna, A.; Astifanni, T.K.; Ruth, I.; Asri, L.; Purwasasmita, B.S. Preparation of nanocellulose-alginate nanocomposites for chlorhexidine digluconate drug carrier. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012046. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, N.; Minakami, M.; Hatakeyama, J.; Haruna, C.; Morotomi, T.; Izumi, T.; Anan, H. Histologic evaluation of the effects of Emdogain gel on injured root apex in rats. J. Endod. 2014, 40, 1989–1994. [Google Scholar] [CrossRef]
- Girard, S.; Paque, F.; Badertscher, M.; Sener, B.; Zehnder, M. Assessment of a gel-type chelating preparation containing 1-hydroxyethylidene-1, 1-bisphosphonate. Int. Endod. J. 2005, 38, 810–816. [Google Scholar] [CrossRef]
- Lambricht, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Goldansaz, H.; Bouzin, C.; Preat, V.; Dupont-Gillain, C.; des Rieux, A. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent. Mater. 2014, 30, e349–e361. [Google Scholar] [CrossRef]
- Nurdin, D.; Purwasasmita, B.S. Synthesis and characterization of silica microcapsules with active compounds 2% chlorhexidine using sodium alginate and chitosan coating as medicament of root canal infection. Solids Struct. 2013, 2, 9–15. [Google Scholar]
- Liang, X.; Xie, L.; Zhang, Q.; Wang, G.; Zhang, S.; Jiang, M.; Zhang, R.; Yang, T.; Hu, X.; Yang, Z.; et al. Gelatin methacryloyl-alginate core-shell microcapsules as efficient delivery platforms for prevascularized microtissues in endodontic regeneration. Acta Biomater. 2022, 144, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Y.; Lee, T.H.; Chen, J.X.; Ng, H.Y.; Huang, T.H.; Shie, M.Y. Synergies of human umbilical vein endothelial cell-laden calcium silicate-activated gelatin methacrylate for accelerating 3D human dental pulp stem cell differentiation for endodontic regeneration. Polymers 2021, 13, 3301. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.; Noorani, T.; Ghani, N.N.A.; Ismail, N. Fracture resistance of the permanent restorations for endodontically treated premolars. Eur. J. Gen. Dent. 2018, 7, 56–60. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Nik Abdul Ghani, N.R.; Noorani, T.Y.; Kamarudin, A. Apical sealing ability of different endodontic sealers using glucose penetration test: A standardized methodological approach. Cumhur. Dent. J. 2020, 23, 79–87. [Google Scholar] [CrossRef]
- Galani, M.; Tewari, S.; Sangwan, P.; Mittal, S.; Kumar, V.; Duhan, J. Comparative evaluation of postoperative pain and success rate after pulpotomy and root canal treatment in cariously exposed mature permanent molars: A randomized controlled trial. J. Endod. 2017, 43, 1953–1962. [Google Scholar] [CrossRef]
- Qu, T.; Jing, J.; Ren, Y.; Ma, C.; Feng, J.Q.; Yu, Q.; Liu, X. Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix. Acta Biomater. 2015, 16, 60–70. [Google Scholar] [CrossRef]
- Gillette, B.M.; Jensen, J.A.; Wang, M.; Tchao, J.; Sia, S.K. Dynamic hydrogels: Switching of 3D microenvironments using two-component naturally derived extracellular matrices. Adv. Mater. 2010, 22, 686–691. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Hu, T.; Lo, A.C.Y. Collagen-alginate composite hydrogel: Application in tissue engineering and biomedical sciences. Polymers 2021, 13, 1852. [Google Scholar] [CrossRef]
- Waspe, J.; Bui, T.; Dishaw, L.; Kraft, A.; Luke, A.; Beronius, A. Evaluating reliability and risk of bias of in vivo animal data for risk assessment of chemicals—Exploring the use of the SciRAP tool in a systematic review context. Environ. Int. 2021, 146, 106103. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr. Guidelines for reporting pre-clinical in vitro studies on dental materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Country | Study Design | Theme | General Outcome |
|---|---|---|---|---|---|
| Zhang R et al. [22] | 2020 | China | In vitro and in vivo | Endodontic regeneration | hDPSCs and vascular endothelial growth factor (VEGF) were co-encapsulated in injectable hybrid RGD-alginate/laponite (RGD-Alg/Lap) hydrogel microspheres, which demonstrated adequate rheological properties, degradation rate, and cell viability. Additionally, it was found to promote the regeneration of pulp-like tissues and generate new microvessels. |
| Evelyna A et al. [23] | 2019 | Indonesia | In vitro | Intracanal medicament | When nanocellulose is combined with alginate and subsequently loaded with CHX digluconate 2% (w/v), the microcapsule appeared to be a viable option for intracanal drug delivery at pH 5.5 and pH 7.5. |
| Devillard R et al. [13] | 2016 | France | In vitro and ex vivo | Endodontic regeneration | When compared to synthetic materials, the collagen–alginate composite scaffold may offer significant advantage by allowing a favourable root canal healing environment amenable to regenerative endodontics. |
| Matsumoto N et al. [24] | 2014 | Japan | In vivo and in vitro | Endodontic regeneration | The findings showed that EMD does not irritate periapical tissue and may generate a favourable environment for periapical tissue recovery in comparison to PGA. |
| Girard S et al. [25] | 2005 | Switzerland | In vitro | Chemical preparation (Chelatingagent) | Aqueous gel containing 1-hydroxyethylidene-1, 1-bisphosphonate (HEBP) with 2% alginate appeared advantageous as a chelating agent over currently available product. |
| Lambricht L et al. [26] | 2014 | Belgium | In vitro and in vivo | Endodontic regeneration | Commercially available hyaluronic acid-based formulation can be a suitable delivery system for SCAP-based dental pulp regeneration strategies. |
| Nurdin D et al. [27] | 2013 | Indonesia | In vitro | Intracanal medicament | Silica microcapsules coated with sodium alginate and chitosan may be a promising carrier for releasing 2% CHX in the root canal at pH 6.5, as opposed to the normal pH of 7.4. |
| Athirasala A et al. [14] | 2018 | USA | In vitro | Endodontic regeneration | The suggested new bioink with alginate hydrogel demonstrated cytocompatibility and natural odontogenic potential, and it can be employed to manufacture scaffolds with sophisticated three-dimensional microarchitectures in the future for regenerative dentistry. |
| Bhoj M et al. [5] | 2015 | Hong Kong, China | In vitro | Endodontic regeneration | Simple templating allows RGD-alginate scaffolds to be constructed. When dual growth factors were added to cocultures of stem cells within RGD-alginate scaffolds, microenvironments were created that dramatically enhanced the proliferation of dental pulp stem cell/human umbilical vein endothelial cell combinations. |
| Huang G et al. [6] | 2021 | China | In vitro | Endodontic filling materials | The novel algin incorporated BG-based sealer exhibited acceptable flow, film thickness, setting time, solubility, and radiopacity with no cytotoxic effects on MG-63 cells. Dense hydroxyapatite crystals were found on the surface after 4 weeks of immersion in SBF. Furthermore, no difference in sealing performance was noted when compared to commercialised bioceramic sealer. |
| Yu H et al. [12] | 2019 | China | In vitro | Endodontic regeneration | The 3D-printed Alg-Gel scaffold is more suitable for the proliferation of hDPSCs than the Alg-Gel scaffold, and the scaffold extracts can better enhance cell proliferation and differentiation. |
| Liang X et al. [28] | 2022 | China | In vitro and in vivo | Endodontic regeneration | GelMA-alginate core-shell microcapsule system for co-cultivating and delivering hDPSC and HUVEC without microcapsule aggregation. The microcapsule system enhances cell proliferation, shows greater osteo- and odontogenic, and vasculogenic capacity. |
| Lai WY et al. [29] | 2021 | Taiwan, Republic of China | In vitro | Endodontic regeneration | hDPSC-based cell blocks with alginate–fish gelatine hydrogel core and Si ion-infused fish gelatine methacrylate hydrogel shell surrounding HUVEC were able to facilitate regeneration. The capacity to release Si ions improved numerous angiogenic signalling, increased the expression and secretion of angiogenesis-related and odontogenic-related biomarkers. |
| Studies | Domains | Overall RoB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Was the Administered dose/Exposure Level Adequately Randomized? | Was Allocation to Study Group Adequately Concealed? | Were Experimental Conditions Identical across Study Groups? | Were Research Personnel Blinded to the Study Group during the Study? | Were Outcome Data Complete without Attrition/Exclusion from Analysis? | Can We Be Confident in the Exposure Characterization? | Can We Be Confident with the Outcome Assessment (Including Blinding of Assessors)? | Were All Measured Outcomes Reported? | Were There No Other Potential Threats to Internal Validity? | ||
| Zhang R et al. [22] | PL | PH | DL | DH | PL | DL | DL | DL | DL | Tier 1 |
| Evelyna A et al. [23] | DH | DH | PL | DH | PL | DL | PL | DL | DL | Tier 1 |
| Devillard R et al. [13] | DH | PH | DL | PH | PL | DL | DL | DL | DL | Tier 1 |
| Matsumoto N et al. [24] | PL | PH | DL | PH | PL | DL | DL | DL | DL | Tier 1 |
| Girard S et al. [25] | PL | PH | DL | PH | PL | DL | DL | PL | DL | Tier 1 |
| Lambricht L et al. [26] | PH | PH | PL | DH | PL | PL | PL | PL | DL | Tier 1 |
| Nurdin D et al. [27] | PH | PH | PL | PH | DL | PL | PL | DL | DL | Tier 1 |
| Athirasala A et al. [14] | DH | PH | DL | PH | PL | DL | PL | PL | DL | Tier 1 |
| Bhoj M et al. [5] | DH | DH | DL | DH | PL | DL | DL | DL | DL | Tier 1 |
| Huang G et al. [6] | DH | DH | DL | DH | PL | PL | DL | DL | DL | Tier 1 |
| Yu H et al. [12] | DH | DH | DL | PH | PL | DL | PL | DL | DL | Tier 1 |
| Liang X et al. [28] | DH | DH | DL | DH | PL | DL | DL | DL | DL | Tier 1 |
| Lai WY et al. [29] | PH | PH | DL | PH | DL | DL | PL | DL | DL | Tier 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.S.S.; Cher, C.Y.; Goh, Y.H.; Chan, D.Z.K.; Karobari, M.I.; Lai, J.C.H.; Noorani, T.Y. An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review. Mar. Drugs 2022, 20, 539. https://doi.org/10.3390/md20080539
Lin GSS, Cher CY, Goh YH, Chan DZK, Karobari MI, Lai JCH, Noorani TY. An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review. Marine Drugs. 2022; 20(8):539. https://doi.org/10.3390/md20080539
Chicago/Turabian StyleLin, Galvin Sim Siang, Chia Yee Cher, Yong Hong Goh, Daryl Zhun Kit Chan, Mohmed Isaqali Karobari, Josephine Chang Hui Lai, and Tahir Yusuf Noorani. 2022. "An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review" Marine Drugs 20, no. 8: 539. https://doi.org/10.3390/md20080539
APA StyleLin, G. S. S., Cher, C. Y., Goh, Y. H., Chan, D. Z. K., Karobari, M. I., Lai, J. C. H., & Noorani, T. Y. (2022). An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review. Marine Drugs, 20(8), 539. https://doi.org/10.3390/md20080539