Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of EAp on the Growth of Arabidopsis thaliana and Nicotiana benthamiana
2.2. EAp Treatment Affected the Growth and Nutritional Value of Brassica chinensis
2.3. EAp Varies According to Cultivation Strategy
2.4. Chemical Composition of EAp
3. Materials and Methods
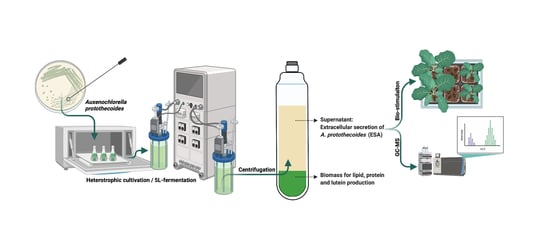
3.1. Microalgal Cultivation and EAp Preparation
3.1.1. Shake-Flask Culture
3.1.2. Fermentor Culture
3.2. Root Development in Petri Dish Culture
3.3. Plant Growth
3.4. EAp Composition Analysis
3.5. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2021, 38, 4. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, I.; Barone, V.; Fragalà, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of Microalgal Extracts from Chlorella vulgaris and Scenedesmus quadricauda on Germination of Beta vulgaris Seeds. Plants 2020, 9, 675. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Mieczyslaw, G.; Romanowska-Duda, Z. Improvements in Germination, Growth, and Metabolic Activity of Corn Seedlings by Grain Conditioning and Root Application with Cyanobacteria and Microalgae. Pol. J. Environ. Stud. 2014, 23, 1147–1153. [Google Scholar]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chla-mydomonas Green Microalgae on Medicago truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef]
- Jang, H.S.; Kang, N.S.; Kim, K.M.; Jeon, B.H.; Ji, W. Description and Application of a Marine Microalga Auxenochlorella protothecoides Isolated from Ulleung-do. Korean Soc. Life Sci. 2017, 27, 1152–1160. [Google Scholar]
- Xiao, Y.; He, X.; Ma, Q.; Lu, Y.; Bai, F.; Dai, J.; Wu, Q. Photosynthetic Accumulation of Lutein in Auxenochlorella protothe-coides after Heterotrophic Growth. Mar. Drugs 2018, 16, 283. [Google Scholar] [CrossRef]
- Higgins, B.; Nobles, D.; Ma, Y.; Wikoff, W.; Kind, T.; Fiehn, O.; Brand, J.; VanderGheynst, J.S. Informatics for improved algal taxonomic classification and research: A case study of UTEX 2341. Algal Res. 2015, 12, 545–549. [Google Scholar] [CrossRef] [Green Version]
- Seto, A.; Wang, H.L.; Hesseltine, C.W. Culture conditions affect eicosapentaenoic acid content of Chlorella minutissima. J. Am. Oil Chem. Soc. 1984, 61, 892–894. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S. Isolation and characterization of a novel lutein-producing marine microalga using high throughput screening. Food Res. Int. 2018, 116, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lu, Y.; Dai, J.; Wu, Q. Industrial Fermentation of Auxenochlorella protothecoides for Production of Biodiesel and Its Application in Vehicle Diesel Engines. Front. Bioeng. Biotechnol. 2015, 3, 164. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-density fermentation of microalga Chlorella protothecoides in bioreactor for micro-bio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, J.; Zhu, H.; Muhammad, A.; Deng, H.; Hu, Z.; Wu, Q. Inhibition of glucose assimilation in Auxenochlorella protothecoides by light. Biotechnol. Biofuels 2020, 13, 146. [Google Scholar] [CrossRef]
- Rui, X.; Yi, Z. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress—ScienceDirect. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Sun, P.-F.; Fang, W.-T.; Shin, L.-Y.; Wei, J.-Y.; Fu, S.-F.; Chou, J.-Y. Indole-3-Acetic Acid-Producing Yeasts in the Phyllosphere of the Carnivorous Plant Drosera indica L. PLoS ONE 2014, 9, e114196. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2015, 28, 1051–1061. [Google Scholar] [CrossRef]
- Kavipriya, R.; Dhanalakshmi, P.; Jayashree, S.; Nallamuthu, T. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotechnol. 2012, 3, 16–19. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Microalgae: New Source of Plant Biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- Lee, H.W.; Bi, X.; Henry, C.J. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022, 385, 132729. [Google Scholar] [CrossRef]
- Reis, A.R.; Favarin, J.L.; Malavolta, E.; Júnior, J.L.; Moraes, M.F. Photosynthesis, Chlorophylls, and SPAD Readings in Coffee Leaves in Relation to Nitrogen Supply. Commun. Soil Sci. Plant Anal. 2009, 40, 1512–1528. [Google Scholar] [CrossRef]
- Pastori, G.M.; Kiddle, G.; Antoniw, J.; Bernard, S.; Veljovic-Jovanovic, S.; Verrier, P.J.; Noctor, G.; Foyer, C.H. Leaf Vitamin C Contents Modulate Plant Defense Transcripts and Regulate Genes That Control Development through Hormone Signaling. Plant Cell 2003, 15, 939–951. [Google Scholar] [CrossRef]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.-C.; Gallie, D.R. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef]
- Abreu, A.P.; Rui, C.M.; Kazmerski, L. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Cook, J.; Zhang, J.; Norrie, J.; Blal, B.; Cheng, Z. Seaweed Extract (Stella Maris®) Activates Innate Immune Responses in Ar-abidopsis thaliana and Protects Host against Bacterial Pathogens. Mar. Drugs 2018, 16, 221. [Google Scholar] [CrossRef]
- Aitouguinane, M.; Bouissil, S.; Mouhoub, A.; Rchid, H.; Fendri, I.; Abdelkafi, S.; El-Hadj, M.D.O.; Boual, Z.; Dubessay, P.; Gardarin, C.; et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Mar. Drugs 2020, 18, 521. [Google Scholar] [CrossRef]
- Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-Phytopathogenic Activities of Macro-Algae Extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Akbari, P.; Ghalavand, A.; Sanavy, A.; Aghaalikhani, M.; Kalkhoran, S.S. Comparison of different nutritional levels and the effect of plant growth promoting rhizobacteria (PGPR) on the grain yield and quality of sunflower. Aust. J. Crop Sci. 2011, 5, 1570–1576. [Google Scholar]
- Gaume, A.; Machler, F.; De Leon, C.; Narro, L.; Frossard, E. Low-P tolerance by maize (Zea mays L.) genotypes: Significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 2001, 228, 253–264. [Google Scholar] [CrossRef]
- Petersen, W.; Böttger, M. Contribution of organic acids to the acidification of the rhizosphere of maize seedlings. Plant Soil 1991, 132, 159–163. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance. Plant Signal. Mol. 2019, 9, 157–168. [Google Scholar] [CrossRef]
- Mir, R.A.; Argal, S.; Ahanger, M.A.; Tomar, N.S.; Agarwal, R.M. Variation in Phenolic Compounds, Antioxidant Activity and Osmotica of Different Cultivars of Tagetes erecta L. at Different Growth Stages and Effect of its Leachates on Germination and Growth of Wheat (Triticum aestivum L.). J. Plant Growth Regul. 2021, 41, 907–921. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Bournonville, C.G.; Filippone, M.P.; Peto, P.; Trejo, M.F.; Couto, A.S.; de Marchese, A.M.; Ricci, J.C.D.; Welin, B.; Castagnaro, A.P. Strawberry fatty acyl glycosides enhance disease protection, have antibiotic activity and stimulate plant growth. Sci. Rep. 2020, 10, 8196. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, M.; Shim, C.; Bae, S.; Jang, S. Potential of Algae–Bacteria Synergistic Effects on Vegetable Production. Front. Plant Sci. 2021, 12, 656662. [Google Scholar] [CrossRef]
- El-Naggar, E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [Green Version]
- Hussain, H.; Mamadalieva, N.Z.; Ali, I.; Khan, E.; Xiao, J. Fungal glycosides: Structure and biological function. Trends Food Sci. Technol. 2021, 110, 611–651. [Google Scholar] [CrossRef]
- Lu, Y.; Zhai, Y.; Liu, M.; Wu, Q. Biodiesel production from algal oil using cassava (Manihot esculenta Crantz) as feedstock. J. Appl. Phycol. 2009, 22, 573–578. [Google Scholar] [CrossRef]
- Lorensia, A.; Budiono, R.; Suryadinata, R.V.; Tiarasari, N. Quantitative Determination of Epa and Dha in Fish Oil Capsules for Cardiovascular Disease Therapy in Indonesia by Gc-Ms. J. Public Health Res. 2021, 10, jphr-2021. [Google Scholar] [CrossRef]
- Nasaruddin, M.L.; Hölscher, C.; Kehoe, P.; Graham, S.F.; Green, B.D. Wide-ranging alterations in the brain fatty acid com-plement of subjects with late Alzheimer’s disease as detected by GC-MS. Am. J. Transl. Res. 2016, 8, 154–165. [Google Scholar]





| Sample | OM | N | P | K | Ca | Mg | Fe | Mn | Zn | AA |
|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | 0.12 | 0.42 | 0.015 | 0.006 | 0.003 | 0.001 | 0.001 | - | - | - |
| No. 2 | 1.18 | 0.31 | 0.05 | 0.044 | 0.037 | 0.016 | 0.0007 | 0.0001 | 0.0002 | 0.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Chen, X.; Ma, B.; Zhu, H.; Zheng, X.; Yu, J.; Wu, Q.; Li, R.; Wang, Z.; Xiao, Y. Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants. Mar. Drugs 2022, 20, 569. https://doi.org/10.3390/md20090569
Qu Y, Chen X, Ma B, Zhu H, Zheng X, Yu J, Wu Q, Li R, Wang Z, Xiao Y. Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants. Marine Drugs. 2022; 20(9):569. https://doi.org/10.3390/md20090569
Chicago/Turabian StyleQu, Yujiao, Xinxiang Chen, Beibei Ma, Huachang Zhu, Xuan Zheng, Jiazhen Yu, Qinghui Wu, Rong Li, Ziqiang Wang, and Yibo Xiao. 2022. "Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants" Marine Drugs 20, no. 9: 569. https://doi.org/10.3390/md20090569
APA StyleQu, Y., Chen, X., Ma, B., Zhu, H., Zheng, X., Yu, J., Wu, Q., Li, R., Wang, Z., & Xiao, Y. (2022). Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants. Marine Drugs, 20(9), 569. https://doi.org/10.3390/md20090569