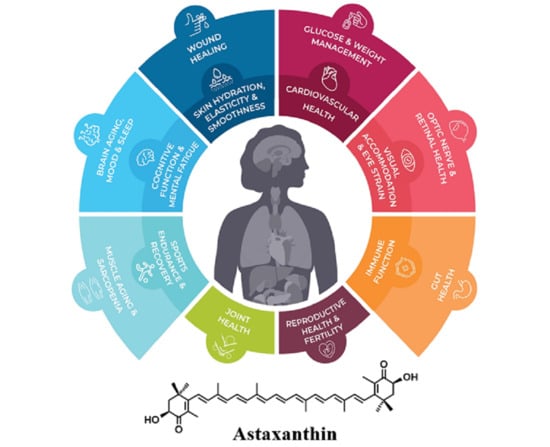
Astaxanthin: Past, Present, and Future
Abstract
:1. Introduction
2. Nature and Cultural Aspects of Astaxanthin
2.1. Astaxanthin; Chemistry, History of Discovery and Structural Investigation
2.1.1. Astaxanthin; Chemical Structure and its Properties
2.1.2. Astaxanthin; Discovery and History of Structural Investigation
2.1.3. The History of Astaxanthin Research in Japan
2.1.4. Astaxanthin; Optical Isomers
2.1.5. Astaxanthin; Geometric Isomers
2.1.6. Astaxanthin Fatty Acid Esters
2.1.7. Astaxanthin Aggregates
2.1.8. Carotenoproteins: Astaxanthin-Protein Complexes
2.1.9. Astaxanthin as a Powerful Antioxidant
Quenching Singlet Oxygen
| 1O2 Generator | EDN * | EDN * | NDPO2 * | EP * | |||
|---|---|---|---|---|---|---|---|
| Reference | [156] | [157] | [158] | [159] | |||
| Detection | Luminescence | Luminescence | Luminescence | Absorbance of DPBF | |||
| Solvent | CDCl3 | CDCl3/ CD3OD (2:1) | DMF/ CDCl3 (9:1) | CDCl3 | CDCl3/ CD3OD (2:1) | EtOH/CHCl3 /D2O (50:50:1) | EtOH/CHCl3/D2O (50:50:1) |
| 1. Carotenoids | |||||||
| Astaxanthin | 2.2 | 1.8 | 5.4 | 2.2 | 1.8 | 24.0 | 11.7 |
| Canthaxanthin | 2.2 | 1.3 | 2.0 | - | 1.2 | 21.0 | |
| Zeaxanthin | 2.0 | 0.73 | 3.4 | 1.9 | 0.12 | 10.0 | 11.2 |
| β-Cryptoxanthin | 2.0 | 0.27 | 1.7 | - | - | 6.0 | 7.0 |
| β-Carotene | 2.2 | 0.28 | 1.1 | 2.2 | 0.049 | 14.0 | 10.8 |
| Lycopene | 3.0 | 1.4 | 3.4 | - | - | 31.0 | 14.0 |
| Capsanthin | - | - | - | - | - | - | 12.1 |
| Lutein | 0.61 | 0.26 | 2.1 | 0.8 | - | 8.0 | 8.1 |
| α-Carotene | 0.66 | 0.23 | 0.93 | - | - | 19.0 | 10.0 |
| Fucoxanthin | 0.29 | 0.075 | 0.97 | - | 0.005 | - | - |
| Tunaxanthin | - | - | - | 0.15 | - | - | - |
| 2. Vitamin C | |||||||
| L-Ascorbic acid | - | - | 0.00089 | - | - | - | - |
| 3. Vitamin E | |||||||
| α-Tocopherol | 0.02 | 0.0039 | 0.049 | - | - | 0.28 | 0.13 |
| β-Tocopherol | - | - | - | - | - | 0.27 | 0.093 |
| γ-Tocopherol | - | - | - | - | - | 0.23 | 0.084 |
| δ-Tocopherol | - | - | - | - | - | 0.16 | 0.041 |
| Trolox | - | - | 0.010 | - | - | - | 0.042 |
| 4. Polyphenols/other phenolic antioxidants | |||||||
| α-Lipoic acid | 0.056 | 0.038 | 0.072 | - | - | 0.13 | 0.0019 |
| Ubiquinone-10 | 0.0019 | 0.0021 | 0.0068 | - | - | - | 0.062 |
| BHT | - | - | 0.004 | - | - | - | - |
| Caffeic acid | - | - | 0.0023 | - | - | - | 0.00069 |
| Ferulic acid | - | - | - | - | - | - | 0.00027 |
| CurcuminI | - | - | 0.0036 | - | - | - | - |
| (-)-EGCG | - | - | 0.0096 | - | - | - | 0.0051 |
| Gallic acid | - | - | 0.0023 | - | - | - | - |
| Pyrocatechol | - | - | 0.0055 | - | - | - | - |
| Pyrogallol | - | - | 0.0055 | - | - | - | - |
| Quercetin | - | - | 0.0018 | - | - | - | - |
| Resveratrol | - | - | 0.0018 | - | - | - | - |
| Sesamin | - | - | 0.0012 | - | - | - | - |
| Capsaicin | - | - | 0.0021 | - | - | - | - |
| Probucol | - | - | 0.00044 | - | - | - | - |
| Edaravon | - | - | 0.0067 | - | - | - | - |
Scavenging of Free Radicals and Inhibition of Lipid Peroxidation
Structures of Radical Cation and Dication of Astaxanthin as Predicted Based on DFT Calculations and Resonance Raman Spectroscopy
Biochemical Aspects of AX Properties against ROS
2.2. Astaxanthin; Distribution, Derivatives and Optical Structure in Nature
2.2.1. Bacteria and Archaea
2.2.2. Eukaryotes; Fungi and Protozoa
2.2.3. Eukaryotes; Algae and Higher Plants

2.2.4. Eukaryotes; Animals
In Case of Invertebrates
In Case of Vertebrates
2.2.5. Astaxanthin Content in Various Organisms
| Taxon | Scientific Name | Common Name | Astaxanthin | Reference | |||
|---|---|---|---|---|---|---|---|
| Form † | Stereoisomer (3R,3′R, meso, 3S,3′S) | Content (mg/100 g) | Origin | ||||
| Bacteria, Prtoteobacteria, Alphaproteobacteria | |||||||
| Paracoccus carotinifaciens (w/. mutation) | PanaFerd-AX | Free form | 3S,3′S | 2180 (50.2% of total Car) | De novo synthesis | [329,330] | |
| Paracoccus sp. strain N81106 (NBRC 101723) (Agrobacterium auranticum) (w/. mutation) | N/A | Free form and glycoside | 3S,3′S | ~800 (63.2% of total Car) | De novo synthesis | [331] | |
| Brevundimonas sp. M7 (w/. mutation) | N/A | Free form ** | 3S,3′S ** | 130 | De novo Synthesis ** | [186] | |
| Sphingomonas astaxanthinifaciens TDMA-17 | N/A | Free form | 3S,3′S ** | 96.0 (34.3% of total Car) | De novo Synthesis ** | [182] | |
| Paracoccus haeundaensis KCCM 10460 (Co-culture w/. Lactic Acid Bacteria) | N/A | Free form | 3S,3′S ** | 82.1 | De novo synthesis | [332] | |
| Paracoccus bogoriensis BOG6T (DSM16578, LMG2279) | N/A | Free form | 3S,3′S | 40 (10.8% of total Car) | De novo synthesis | [183] | |
| Brevundimonas spp. | N/A | Free form ** | 3S,3′S ** | 2.8~36.5 | De novo Synthesis ** | [186] | |
| Sphingomicrobium astaxanthinifaciens CC-AMO-30B | N/A | Free form | 3S,3′S ** | 4.0 | De novo Synthesis ** | [185] | |
| Brevundimonas sp. strain SD212 (NBRC 101024) | N/A | Free form | 3S,3′S | N/A (9.9% of total Car) | De novo synthesis | [181] | |
| Archaea | |||||||
| Halobacterium salinarium NRC-1 | N/A | Free form ‡ | N/D | 26.5 (c.a.73% of total Car) | De novo Synthesis * | [203] | |
| Haloarcula hispanica ATCC 33960 | N/A | Free form ‡ | N/D | 1.7 (c.a.1.3% of total Car) | De novo Synthesis* | [203] | |
| Eukaryota, Fungi | |||||||
| Xanthophyllomyces dendrorhous (ATCC SD 5340) | Phaffia Yeast | Free form | 3R,3′R | 723.5~1247.8 (c.a. 73% of total Car) | De novo synthesis | [327,333] | |
| Eukaryota, Plantae | |||||||
| Adonis amurensis (Reddish flower varieties) | Amur adonis, pheasant’s eye | Fatty acid esters | 3S,3′S ** | ~3310 (in upper red part of petal) (c.a. 70% of total Car) | De novo Synthesis ** | [334] | |
| Adonis annua | Autumn pheasant’s eye, blooddrops | Fatty acid esters | 3S,3′S | 120~1000 (in dry petal) (c.a. 75% of total Car) | De novo Synthesis ** | [17,50] | |
| Adonis aestivalis | Summer pheasant’s eye | Fatty acid esters | 3S,3′S | 166 (in wet petal) (87.4% of total Car) | De novo synthesis | [265] | |
| Eukaryota, Plantae, Chlorophyta | |||||||
| Haematococcus lacustris | Haematococcus pluvilialis, Haematococcus algae | Fatty acid esters | 3S,3′S | ~9800 (in red cyst) (>90% of total Car) | De novo synthesis | [64,328] | |
| Neochloris wimmeri CCAP-213/4 | N/A | Fatty acid esters | 3S,3′S ** | ~1920 (c.a 85% of total Car) | De novo Synthesis ** | [227,228,232] | |
| Asterarcys quadricellulare PUMCC 5.1.1 | N/A | N/A | 3S,3′S** | ~1550 (c.a 13% of total Car) | De novo Synthesis ** | [236] | |
| Protosiphon botryoides SAG-731/1a | N/A | Fatty acid esters | 3S,3′S** | ~1430 (c.a 80% of total Car) | De novo Synthesis ** | [227,228] | |
| Scotiellopsis oocystiformis SAG-277/1 | N/A | Fatty acid esters | 3S,3′S ** | ~1090 (c.a 70% of total Car) | De novo Synthesis ** | [227,228] | |
| Chlorococcum sp. | N/A | Fatty acid esters | 3S,3′S ** | ~c.a. 700 (c.a. 32% of total Car) | De novo Synthesis ** | [335,336,337] | |
| Chlorella zofingiensis SAG-211/14 | Chlorella | Fatty acid esters | 3S,3′S ** | ~680 (c.a. 75% of total Car) | De novo Synthesis ** | [227,228] | |
| Scenedesmus vacuolatus SAG-211/15 | N/A | Fatty acid esters | 3S,3′S ** | ~270 (40–50% of total Car) | De novo Synthesis ** | [227,228] | |
| Chlamydocapsa spp. Strain 101-99/R2 | N/A | N/A | 3S,3′S ** | ~44.4 (20.3% of total Car) | De novo Synthesis ** | [338] | |
| Neochloris oleoabundans UTEX#1185 | N/A | Fatty acid esters | 3S,3′S ** | N/A | De novo Synthesis ** | [232] | |
| Dysmorphococcus globosus-HI | N/A | Free form/ Fatty acid esters | 3S,3′S ** | ~517,090?? | De novo Synthesis ** | [243] | |
| Eukaryota, Chromista, Bigyra, Labyrinthulomycetes | |||||||
| Aurantiochytrium sp. RH-7A-7 (w/. mutation) | Labyrinthulomycetes | N/A | 3S,3′S ** | -470 (c.a. 85% of total Car) | De novo Synthesis * | [218] | |
| Thraustochytrium sp. CHN-3 (FERM P-18556) | Labyrinthulomycetes | Free form ** | 3S,3′S ** | ~280 (~60% of total Car) | De novo Synthesis * | [339] | |
| Aurantiochytrium sp. KH-10 | Labyrinthulomycetes | Fatty acid esters/ Free form | 3S,3′S ** | ~81 (28% of total Car) | De novo Synthesis * | [340] | |
| Thraustochytrium sp. CHN-1 | Labyrinthulomycetes | Free form | 3S,3′S | 50 (c.a. 50% of total Car) | De novo Synthesis * | [341,342] | |
| Eukaryota, Chromista, Gyrista, Eustigmatales | |||||||
| Nannochloropsis gaditana strain S4 (w/. mutation) | Nannochloropsis | Free form | 3S,3′S? | ~219 (14.4% of total Car) | De novo Synthesis | [219] | |
| Nannochloropsis oculata | Nannochloropsis | Free form | 3S,3′S? | 3.4 ng/106 cells | De novo Synthesis | [343] | |
| Nannochloropsissalina | Nannochloropsis | Free form | 3S,3′S? | 9.6 ng/106 cells | De novo Synthesis | [343] | |
| Eukaryota, Excavata, Euglenozoa | |||||||
| Euglena sanguinea | Euglena | Fatty acid esters/free | 3S,3′S | ~1.9 (80% of total Car) | De novo Synthesis | [256,344] | |
| Trachelomonas volvocina | Euglena | Fatty acid esters/ Free form | 3S,3′S * | N/A | De novo Synthesis | [215] | |
| Animals (Invertebrate), Coelenterata | |||||||
| Velella velella | By-the-wind sailor (Jerry fish) | Free form | Mixtures of stereoisomers | N/A | Accumulated from dietary Crustaceans | [98] | |
| Aurelia aurita | (Jerry fish) | Fee form/ Fatty acid esters (minor) | N/A | 12.2 (c.a.67% of total Car) | N/A | [279] | |
| Metridium senile var. fimbriatum | Frilled anemone (Sea anemone) | Fatty acid esters (in ovary) | Mixtures of stereoisomers | N/A | Oxidative metabolite of β-carotene | [345,346] | |
| Corynactis californica | Strawberry anemone (Sea anemone) | Fatty acid esters | N/A | N/A | Oxidative metabolite of β-carotene | [347] | |
| Animals (Invertebrate), Mollusca, Gastropoda | |||||||
| Clione limacina | Sea angel | Free form | 3S,3′S | 0.051 (1.1% of total Car) | Oxidaive metabolite of zeaxanthin | [348] | |
| Paedoclione doliiformis | Sea angel | Free form | 3S,3′S | 0.8 (5.5% of total Car) | Oxidaive metabolite of zeaxanthin | [348] | |
| Semisulcospira libertina | Terestorial Snail (Kawanina in Japanese) | Free form | 3S,3′S | 0.2 (6.5% of total Car) | Oxidaive metabolite of zeaxanthin | [349] | |
| Fushinus perplexu | Spindle shell | Free form | 3S,3′S | 0.2 (4.0% of total Car) | Oxidative metabolite of β-carotene | [350] | |
| Pomacea canaliculata | Apple snail | Free form | 3S,3′S | 5.0 in gonad, 2.31 in egg (~75% of total Car) | Oxidative metabolite of β-carotene | [351] | |
| Animals (Invertebrate), Mollusca, Cephalopoda | |||||||
| Octopus vulgaris | Common octopus | Fatty acid esters/ Free form | Mixtures of stereoisomers (46:22:32) | 3.2 in liver (c.a.80% of total Car) | Accumulated from dietary crustaceans | [288] | |
| Watasenia scintillans | Firefly squid | Fatty acid esters/ Free form | Mixtures of stereoisomers (40:6:54) | 5.0 in liver (>90% of total Car) | Accumulated from dietary crustaceans | [288] | |
| Animals (Invertebrate), Mollusca, Polyplacophora | |||||||
| Placiphorella japoonica | Chiton | Free form | Mixtures of stereoisomers (5:3:2) | 1.25 (~34% of total Car) | Oxidative metabolite of β-carotene | [352] | |
| Acanthochitona defilippii | Chiton | Free form | 3S,3′S | 1.55 in gonad (~4.0% of total Car) | Oxidative metabolite of β-carotene | [352] | |
| Liolophura japonica | Chiton | Free form | 3S,3′S | 0.8 in viscera (~10% of total Car) | Oxidative metabolite of β-carotene | [352] | |
| Animals (Invertebrate), Echinodermata | |||||||
| Peronella japonica | Sea urchin | Free form | Mixtures of stereoisomers (3:7:90) | ~3.0 in gonad (c.a.43% of total Car) | Oxidative metabolite of β-carotene | [301] | |
| Asteria pectinifera | Starfish | Free form | Mixtures of stereoisomers (50:25:25) | ~1.35 (% of total Car) | Oxidative metabolite of β-carotene | [305] | |
| Asterias amurensis | Starfish | Free form | Mixtures of stereoisomers (48:25:27) | ~4.64 (% of total Car) | Oxidative metabolite of β-carotene | [305] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Lobsters, rock lobsters and crawfishes) | |||||||
| Procambarus clarkii | Louisiana crawfish | Fatty acid ester/ Free form | Mixtures of stereoisomers | 7.9–19.8 in carapace | Oxidative metabolite of β-carotene | [353] | |
| Pontastacus leptodactylus (Astacus leptodactylus) | Turkish crayfish | Fatty acid esters /Free form | Mixtures of stereoisomers ** | 5.0 in carapace 0.13 in muscle 0.98 in intestine (82.5% of total Car) | Oxidative metabolite of β-carotene ** | [354] | |
| Panulirus japonicus | Japanese Spiny Lobster (Ise-ebi) | Free form/ Fatty acid esters | Mixtures of stereoisomers (20:20:56) | 3.3 in carapace (65% of total Car) | Oxidative metabolite of β-carotene | [355] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Copepoda | |||||||
| Tigriopus californicus | Red marine copepod | Free form (major) | 3S,3′S (major) | ~423 | Oxidative metabolite of β-carotene | [290,356] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Eucarida | |||||||
| Euphausia superba | Antarctic krill | Fatty acid esters/ Free form | 3R,3′R (Major, ~70%) | ~566 in eye | Oxidative metabolite of β-carotene ? | [291,357] | |
| Euphausiapacifica | Pacific krill (Isada) | Fatty acid esters/ Free form | 3R,3′R (Major) | ~ 252 in eye | Oxidative metabolite of β-carotene ? | [357,358] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Prawns and shrimps) *** | |||||||
| Pandalus borealis | Atlantic shrimp (Northern prawn) | Fatty acid esters/ Free form | Mixtures of stereoisomers (25:52:23) | ~28.48 in carapace | Oxidative metabolite of β-carotene | [62,359,360] | |
| Penaeus japonicus | Japanese tiger prawn (Kuruma-ebi) | Fatty acid esters/ Free form | Mixtures of stereoisomers (12:40:48) | ~13 in carapace | Oxidative metabolite of β-carotene | [361,362] | |
| Penaeus semisulcatus | Green tiger prawn | Fatty acid esters/ Free form | Mixtures of stereoisomers (19:44:57) | ~15.6 in carapace | Oxidative metabolite of β-carotene | [362] | |
| Penaeus monodon | Black tiger prawn | Fatty acid esters/ Free form | Mixtures of stereoisomers (16:43:41) | ~7.3 in carapace | Oxidative metabolite of β-carotene | [362] | |
| Litopenaeus vannamei | Whiteleg shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (23:44:32) | ~5.8 in carapace | Oxidative metabolite of β-carotene | [362] | |
| Metapenaeus joyneri | Shiba shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (14:46:40) | ~3.3 in carapace | Oxidative metabolite of β-carotene | [362] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda , Brachyura (Crabs) *** | |||||||
| Chionoecetes japonicus | Red snow crab (Beni-zuwai crab) | Fatty acid esters/ Free form ** | Mixtures of stereoisomers ** | ~23 in carapace (with demineralization treatment) | Oxidative metabolite of β-carotene? | [363] | |
| Chionoecetes opilio | Snow crab (Zuwai crab) | Fatty acid esters/ Free form | Mixtures of stereoisomers? | ~11.9 in carapace (~91.7% of total Car) | Oxidative metabolite of β-carotene? | [364] | |
| Callinectes sapidus | Blue crab | Fatty acid esters/ Free form | Mixtures of stereoisomers? | ~9.8 (with demineralization treatment) | Oxidative metabolite of β-carotene? | [365] | |
| Cancer pagurus | Brown crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (56:24:20) | 0.37 in carapace | Oxidative metabolite of β-carotene? | [366,367] | |
| Animals (Invertebrate), Arthropoda, Crustacea, Decapoda (Others) *** | |||||||
| Paralithodes brevipes | Hanasaki crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (26:9:6) | ~2.4 in carapace (~39.9% of total Car) | Oxidative metabolite of β-carotene | [368] | |
| Paralithodes camtschaticus | Red king crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (45–55:7–19:27–48) | ~0.35 in carapace (~97% of total Car) | Oxidative metabolite of β-carotene | [277,369] | |
| Cervimunida princeps | Squat lobster | Fatty acid esters/ Free form | Mixtures of stereoisomers (26:9:65) | ~ 0.45 in carapace (~100% of total Car) | Oxidative metabolite of β-carotene | [369] | |
| Upogebia major | Japanese mud shrimp | Fatty acid esters/ Free form | Mixtures of stereoisomers (72:21:7) | ~ 0.25 in carapace (~100% of total Car) | Oxidative metabolite of β-carotene | [369] | |
| Birgus latro | Coconut crab | Fatty acid esters/ Free form | Mixtures of stereoisomers (9:41:50) | ~ 0.3 in carapace (~96% of total Car) | Oxidative metabolite of β-carotene | [370] | |
| Asellus aquaticus | Isopoda | Free form/ Fatty acid esters? | N/A | ~0.52 (~37.5% of total Car) | Oxidative metabolite of β-carotene? | [371] | |
| Pleuroncodes planipes | Red crab langostilla | Fatty acid esters/ Free form | Mixtures of stereoisomers (3–4:1:3–4) | N/A | Oxidative metabolite of β-carotene? | [372] | |
| Animals (Invertebrate), Arthropoda, Arachnida, Acari | |||||||
| Balaustium murorum | Red velvet mite | Free form/ Fatty acid esters | 3S,3′S ** | ~61,530 mg/ 100 g protein (60% of total Car) | Oxidative metabolite of zeaxanthin (De novo Synthesis **) | [373] | |
| Panonychus citri | Citrus red mite | Fatty acid esters | 3S,3′S ** | ~263 mg/ 100 g protein (42.5% of total Car) | De novo Synthesis ** | [374] | |
| Tetranychus kanzawai | Kanzawa spider mite | Fatty acid esters | 3S,3′S ** | Undefined | De novo Synthesis | [375] | |
| Tetranychus urticae | Two-spotted spider mite | Fatty acid esters | 3S,3′S ** | Undefined | De novo Synthesis | [296,376] | |
| Eylais hamata | Hydracarina | Free form/ Fatty acid esters (minor) | N/A | 12.2 (c.a.67% of total Car) | N/A | [377] | |
| Eylais extendens | Hydracarina | N/A | N/A | Undefined (c.a.70% of total Car) | N/A | [378] | |
| Schizonobia sycophanta | Parasite mite | Fatty acid ester | 3S,3′S | Undefined (30% of total Car) | De novo Synthesis ** | [49,289] | |
| Animals (Invertebrate), Arthropoda, Arachnida, Araneae | |||||||
| Trichonephila clavata | Arachnida spider | ?? | Mixtures of stereoisomers (2:1:1) | 0.02 (1.9% of total Car) | Oxidative metabolite of β-carotene | [299] | |
| Animals (Invertebrate), Arthropoda, Insecta | |||||||
| Locusta migratoria | Migratory locust | Free form | Mixtures of stereoisomers (2:1:1) | 0.25 in brown form (12.5% of total Car) | Oxidative metabolite of β-carotene | [16,293] | |
| Aiolopus thalassinus tamulus | Grasshopper | Free form | Mixtures of stereoisomers (2:1:1) | 0.09 in brown form (3.0% of total Car) | Oxidative metabolite of β-carotene | [293] | |
| Schistocerca gregaria | Desert locust | Free form | Mixtures of stereoisomers * | N/A | Oxidative metabolite of β-carotene | [16] | |
| Animals (Vertebrate), Fish (Salmonidae) | |||||||
| Oncorhynchus nerka (Wild, anadromous form) | Sockeye salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 2.6–3.8 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | [308] | |
| Oncorhynchus nerka (Wild, non-anadromous form) | Kokanee salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | −0.8 in muscle 0.4–2.8 in skin −1.1 in golnad (−94% of total Car) | Accumulated from dietary crustaceans | [379,380] | |
| Oncorhynchus kisutch | Coho salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 1.0–2.1 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | [308] | |
| Salvelinus alpinus (Wild) | Arctic char | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 0.86 in muscle in egg (−30% of total Car) | Accumulated from dietary crustaceans | [308,381] | |
| Salmo salar (Wild) | Atlantic salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers (depending on AX source) | 0.6–0.8 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | [308,382] | |
| Oncorhynchus keta | Chum salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mainly 3S,3′S (in ovary) | 0.1–0.5 in muscle −0.7 in egg 0.1 in skin (male) (4.8–90% of total Car) | Accumulated from dietary crustaceans | [28,29,308,383] | |
| Oncorhynchus gorbuscha | Pink salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.4–0.7 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | [308] | |
| Oncorhynchus tshawytscha | Chinook salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.54 in muscle in egg (% of total Car) | Accumulated from dietary crustaceans | [308] | |
| Oncorhynchus masou (Wild, anadromous form) | Masu salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.3–0.8 in muscle 0.03–0.8 in skin 0.7–1.7 in egg (1.9–80% of total Car) | Accumulated from dietary crustaceans | [308,380,384] | |
| Oncorhynchus masou ishikawae (Wild, anadromous form) | Red-spotted masu salmon | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.2 in muscle trace in skin N/D in egg (1.9–68.5% of total Car) | Accumulated from dietary crustaceans | [380] | |
| Oncorhynchus masou rhodurus | Biwa trout | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | 0.2 in muscle −0.1 in skin (3.2–58.3% of total Car) | Accumulated from dietary crustaceans | [380] | |
| Oncorhynchus mykiss (Wild, pigmented phenotype) | Rainbow trout | Free form (muscle/egg)/ Fatty acid esters (skin) | Mixtures of stereoisomers | trace in muscle 0.8 in skin trace in egg (1.9–42.3% of total Car) | Accumulated from dietary crustaceans | [385] | |
| Animals (Vertebrate), Fish (Non-Salmonidae ) | |||||||
| Sebastolobus macrochir | Broadbanded thornyhead (Kichiji rockfish) | Fatty acid esters (skin) | N/A | 26 in skin (>90% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | [386] | |
| Plectropomus leopardus | Coral trout (Suziara) | Fatty acid esters/ Free form (skin) | Mixtures of stereoisomers (13:7:80) | 19.5 in skin (84.8% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | [387] | |
| Epinephelus fasciatus | Blacktip grouper (Akahata) | Fatty acid esters (skin) | N/A | 2.27 in skin (74% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | [386] | |
| Beryx splendens | Splendid alfonsino (Kinmedai) | Fatty acid esters (skin) | Mixtures of stereoisomers | 0.9 in skin (c.a. 100% of total Car) | Accumulated from dietary crustaceans or Oxidative metabolite of β-carotene/ zeaxanthin? | [386] | |
| Pagrus malor | Red sea bream (Madai) | Fatty acid esters (skin) | Mixtures of stereoisomers (38:0:62) | ~2 in skin (wild) 0.25 in skin (firmed w/o. AX) / 0.98 (firmed with. AX) (~c.a 45% of total Car) | Accumulated from dietary crustaceans and supplementary pigment | [277,386,388] | |
| Carassius auratus | Goldfish (Kingyo/Hibuna) | Free/ Fatty acid esters (skin) | 3S,3′S | 0.58 (whole body) (~47% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [307] | |
| Branchiostegus japonicus | Red tilefish (Red amadai) | Fatty acid esters (skin) | Mixtures of stereoisomers (24:24:52) | 0.39 in skin (35.8% of total Car) | Accumulated from dietary crustaceans | [389] | |
| Animals (Vertebrate), Amphibian | |||||||
| Cynops pyrrhogaster | Japanese newt | Free form/ Fatty acid esters | N/A | 4.55 in skin (c.a.21% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [309] | |
| Salamandra salamandra | Fire salamander | Free form/ Fatty acid esters | N/A | 0.23 (37.5% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [390] | |
| Lissotriton vulgaris (Triturus vulgaris) | Smooth newt/common newt | Free form | N/A | 0.1 (−23.5% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [390] | |
| Ranitomeya sirensis | Sira poison frog | Free form/ Fatty acid esters ? | N/A | N/A (-c.a. 40% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [294] | |
| Bufo bufo | Common toad | Free form/ Fatty acid esters | N/A | N/D in muscle and liver 0.02 in skin 0.35 in intestine (25.8–57.4% of total Car) 0.23 in gonads (95.8% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [390] | |
| Bufotes viridis (Bufo viridis) | European green toad | Free form/ Fatty acid esters | N/A | N/D in muscle and liver 0.02 in skin 0.35 in intestine (25.8–57.4% of total Car) 0.23 in gonads (95.8% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin | [391] | |
| Pelobates fuscus | European common spadefoot toad | Free form | N/A | 1.1 in liver (19.6% of total Car) | Oxidative metabolite of β-carotene/ zeaxanthin ** | [390] | |
| Melanophryniscus rubriventris | (Aposematic poison toad) | N/A (Free form/ ester form?) | N/A | Undefined | Oxidative metabolite of β-carotene ** | [392] | |
| Animals (Invertebrate), Reptile | |||||||
| Chlamydosaurus kingii. (the western red-frilled form) | Frillneck lizard | N/A (Free form?) | N/A | Undefined | Oxidative metabolite of β-carotene | [312] | |
| Chrysemys picta | Painted turtle | N/A | N/A | c.a 0.11 in leg skin | Oxidative metabolite of β-carotene | [393] | |
| Trachemys scripta | Red-eared slider | N/A | N/A | c.a 0.06 in skin around eye spot | Oxidative metabolite of β-carotene | [393] | |
| Animals (Invertebrate), Aves *** | |||||||
| Lagopus lagopus scoticus | Red grouse | Free form/ Fatty acid esters | N/A | 317.8 in combs N/D in plasma (-c.a.81.6% of total Car) | Oxidative metabolite of β-carotene | [394] | |
| Pygoscelis papua | Gentoo penguins | Free form | N/A | 2.19 in blood, breeding adults and chicks | Accumulated from dietary crustaceans, fishes | [395,396] | |
2.3. Biosynthesis and Metabolism of Astaxanthin
2.3.1. Overview of Carotenoid Biosynthetic Pathways in Bacteria, Fungi, and Higher Plants
2.3.2. Overview of Astaxanthin Biosynthetic Pathways in Bacteria, Algae and Plants
2.3.3. How Can Hematococcus Algae Achieve Ultra-High Concentrations of Astaxanthin Biosynthesis?
2.3.4. Metabolism of Astaxanthin in Animals
Overviews of Metabolism of Astaxanthin in Animals; General Remarks
Metabolic Conversion to Astaxanthin by Cytochrome P450
| Name P450 | Origin | Super-Family, Clan | Methodlogy of Functional Analysis | Reference | |
|---|---|---|---|---|---|
| CYP2J19 | Aves/Testudines | CYP2 | Genetics Heterologous expression Homology | [321,552,553,557] | |
| CYP2AE2 | Zebra fish; | Danio albolineatus | CYP2 | Genetics Heterologous expression | [556,557] |
| (Actinopterygii: Cypriniformes) | |||||
| CYP2J2 | Anole Lizards; | Anolis favillarum | CYP2 CYP2 | Genetics | [313] |
| CYP2J6 | (Reptilia: Iguania, (Lepidosauria)) | ||||
| CrtS (CYP5139Q1) | Phaffia Yeast; | Xanthophyllomyces dendrorhous | CYP3 | Heterologous expression | [212,213,558] |
| (Fungi: Basidiomycetes) | |||||
| CYP384A1 | Spider mites; | Tetranychus kanzawai | CYP3 | Genetics | [375] |
| CYP383A1 | (Arthropoda: Chelicerata) | CYP3 | Putative (Closest homologue of CYP384A) | ||
| CYP3A80 | Sira poison Frog; | Ranitomeya sirensis (Amphibia: Anura) | CYP3 | Genetics | [294] |
| CYP3-like | Anchialine Shrimp; | Halocaridina rubra | CYP3 | Putative | [559] |
| (Arthropoda: Crustacea: Decapoda) | |||||
| P450 like | Copeods; | Acartia fossae | N/D | Putative | [560] |
| (Arthropoda: Crustacea: Copepoda) | |||||
| P450 like | Pelagic tunicate; | Oikopleura dioica | N/D | Putative | |
| (Chordata: Tunicata: Appendicularia) | |||||
2.4. Chemical Synthesis and Analysis of Astaxanthin
2.4.1. Chemical Synthesis of Astaxanthin
2.4.2. Quantitative and Qualitative Analysis of Astaxanthin
2.5. Relationship between Human Culture and Astaxanthin
2.5.1. Historical Exposure of Human Societies to Astaxanthin Sources in Nature
2.5.2. Human Culture Shift towards Sustainability: Haematococcus algae as a Promising Source of Natural Astaxanthin
3. Industrial Use of Astaxanthin
3.1. Astaxanthin as a Pigment and Beyond: Astaxanthin in Aquaculture and Poultry Industries
3.1.1. Applications in Aquaculture
3.1.2. Applications in Poultry and Livestock Farming
3.2. Sustainable Commercial Production of Astaxanthin by Haematococcus algae
3.3. Human Uses of Astaxanthin
3.3.1. Ever-growing Interest in Human Applications of Astaxanthin
Market History and Evolution
Health Claims and Regulations
| (A) Oxidative Stress. | ||||||
|---|---|---|---|---|---|---|
| Author/Year/ Reference | Study Design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Oxidative stress markers in “metabolic disorder” | ||||||
| Rad NR. et al. 2022 [691] | Randomized, double-blind, placebo-controlled prospective study | 50 Type 2 Diabetes Mellitus (T2DM) patients receiving metformin | 0, 10 mg/day | 12 weeks |
| Investigation of additive synergistic effects on metformin (1000–2000 mg/day). Significantly increased blood total antioxidant capacity (TAC) levels at the end of the intervention only in the AX-treated group, while MDA remained unchanged. Similarly, increased SOD and catalase activity in blood and increased Nrf2 protein in PBMCs were observed at the end of the intervention only in the AX-treated group. No safety concerns were identified in this study. |
| Ishiwata S. et al. 2021 [692] | Open-labeled, prospective study | 17 patients with systolic heart failure | 12 mg/day * | 3 months |
| After 3 months of AX supplementation, significantly decreased serum d-ROM (Diacron-Reactive Oxygen Metabolites), p = 0.018, but no change in plasma biological antioxidant potential (BAP) or urinary ratio of 8-OHdG/Cr No safety concerns were identified in this study. |
| Shokri-Mashhadi, N. et al. 2021 [693] | Randomized, double-blind, placebo-controlled prospective study | 44 patients with type 2 diabetes | 0, 8 mg/day | 8 weeks |
| Decrease plasma levels of MDA (p < 0.05) No safety concerns were identified in this study. |
| Kato T. et al. 2020 [694] | Open-labeled, prospective study | 16 patients with systolic heart failure | 12 mg/day * | 3 months |
| Increased left ventricular ejection fraction (LVEF) from 34.1 ± 8.6% to 38.0 ± 10.0% (p = 0.031), and the 6-min walk distance increased from 393.4 ± 95.9 m to 432.8 ± 93.3 m (p = 0.023). Significant relationships were observed between percent changes in serum dROM level and those in LVEF. No safety concerns were identified in this study. |
| Coombes J.S et al. 2016 [695] Fassett, R.G. et al. 2008, [696] | Randomized, double-blind, placebo-controlled prospective study | 58 renal transplant recipients | 0, 12 mg/day | 12 months |
| There was no effect on oxidative stress in renal transplant recipients. (The XANTHIN trial) No safety concerns were identified in this study. |
| Takemoto M. et al. 2015 [697] | Case report | 1 Werner syndrome patient | 12 mg/day * | 6 months |
| There was no significant changes after AX intervention in MDA-modified LDL. No safety concerns were identified in this study. |
| Choi H.D. et al. 2011 [698] | Randomized, two-arm, prospective study | 23 obese and overweight subjects | 5 and 20 mg/day | 3 weeks |
| 5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, SOD increased by 193%, and TAC increased by 121% after 3 weeks compared to baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared to baseline (p < 0.01). No safety concerns were identified in this study. |
| Choi, H.D. et al. 2011 [699] | Randomized, double-blind, placebo-controlled, prospective study | 27 overweight subjects | 0, 20 mg/day | 12 weeks |
| MDA reduced by 17.3% and 29% after 8 and 12 weeks compared to placebo (p < 0.01), ISP reduced by 40.2% and 52.9% after 8 and 12 weeks compared to placebo (p < 0.01), SOD increased by 124.8% after 12 weeks compared to placebo (p < 0.01), and TAC increased by 130.1% after 12 weeks compared to placebo (p < 0.05). No safety concerns were identified in this study. |
| Iwabayashi M. et al. 2009 [700] | Open-labeled, prospective study | 35 healthy female subjects (with high oxidative stress, postmenopausal) | 12 mg/day | 8 weeks |
| Increased blood biological antioxidant potential (Biological Antioxidant Potential (BAP); +4.6%, p < 0.05). No safety concerns were identified in this study. |
| Kim Y.K. et al. 2004 [701] | Open-labeled, prospective study | 15 healthy postmenopausal females | 0, 2, 8 mg/day | 8 weeks |
| Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostane excretion did not decrease significantly. No safety concerns were identified in this study. |
| Oxidative stress markers in “skin” | ||||||
| Chalyk, N. et al. 2017 [702] | Open-label, prospective study | 31 subjects; 18 obese, 8 overweight, 5 healthy, over the age of 40 | 4 mg/day | 92 days |
| Plasma MDA decreased with AX by 11.2% on day 15 and by 21.7% on day 29 (N.S.). No safety concerns were identified in this study. |
| Yoon HS. et al. 2014 [703] | Randomized, double-blind, placebo-controlled prospective study | 44 healthy females with wrinkles grade ≥ 2 (≥40 yrs.) | 0, 2 mg/day * | 12 weeks |
| AX (2 mg/day) combined with collagen hydrolysate (3 g/day). Skin biopsy after UV irradiation: no difference in oxidative markers (Thymine dimers, 8-OHdG) between the two groups in histological evaluation. Regarding mRNA expression, significantly upregulated expression of procollagen type I tended to upregulate fibrillin-1, while significantly downregulated MMP1 and MMP12 in the AX group compared to the placebo. No safety concerns were identified in this study. |
| Satoh A. et al. 2009 [704] | Randomized, single-blind, placebo-controlled prospective study | 27 patients with atopic dermatitis | 0, 12 mg/day | 4 weeks. |
| The Th1/Th2 balance shifted significantly toward Th1, and urinary 8-OHdG concentrations decreased slightly but significantly. No safety concerns were identified in this study [Article in Japanese] |
| Oxidative stress markers in “ophthalmology; after cataract surgery “ | ||||||
| Hashimoto H. et al. 2021 [705] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| The antioxidant effect of AX was analyzed in relation to age. None of the parameters were correlated with age before AX intake; however, only total hydroperoxide values were significantly correlated after AX intake (r = 0.4, p < 0.05). Total hydroperoxide levels were similar in younger and older age groups (<70 vs. ≥70 years) before AX intake but significantly decreased in younger age groups after intake (−0.21 ± 0.18 vs. −0.05 0.31, p < 0.05), resulting in a significant difference (p < 0.05). Thus, the previously observed decrease in mean total hydroperoxide levels after AX intake was likely due to a greater response in the younger age group analysis associated with this study [706]. No safety concerns were identified in this study. |
| Hashimoto H. et al. 2019 [707] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| In this analysis, the effect of AX intake on the relationship between VEGF levels and ROS-related parameters before and after bilateral cataract surgery was analyzed by gender. VEGF, hydrogen peroxide, and total hydroperoxide levels in the aqueous humor, as well as O2•− scavenging activity, were measured. For women only, VEGF levels and O2•− scavenging activity before AX intake were negatively correlated (r = −0.6, p < 0.01) and positively correlated with total hydrogen peroxide levels before and after AX intake (r = 0.7, p < 0.01, respectively). Analysis associated with this study [706] No safety concerns were identified in this study. |
| Hashimoto H. et al. 2016 [708] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Superoxide anion scavenging activity (U/mL): 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared to baseline, p < 0.05. Total hydroperoxides (d-ROM) from 1.16 ± 0.18 at 0 weeks reduced to 1.04 ± 0.31 after 2 weeks were of supplementation compared to baseline, p < 0.05. Analysis associated with this study [706]. No safety concerns were identified in this study. |
| Hashimoto, H. et al. 2013 [709] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) increased superoxide scavenging activity (p< 0.05) Analysis associated with this study [706] No safety concerns were identified in this study. |
| Hashimoto H. et al. 2011 [706] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05). No safety concerns were identified in this study. [Article in Japanese] |
| Oxidative stress markers in “sports/musculoskeletal function “ | ||||||
| Kawamura A. et al. 2021 [710] | Randomized open-labeled, prospective study | 26 healthy male subject (22.3 ± 0.3 yrs.) | N/A (1mg AX/100g salmon) * | 10 weeks |
| Serum carbonylated protein level as an oxidative stress marker tended to be lower immediately after exercise than before exercise in the intervention group only (p = 0.056). No safety concerns were identified in this study. |
| McAllister M.J. et al. 2021 [711] | Randomized, double-blind, placebo-controlled, crossover study | 14 healthy young subjects, (23 ± 2 yrs.) | 0, 6 mg/day | 4 weeks |
| Glutathione was ∼7% higher following AX compared with placebo (p < 0.05). There was no effect on plasma hydrogen peroxide or MDA (p > 0.05). Advanced oxidation protein products (AOPP) were reduced by ∼28% (N.S.; p = 0.45). No safety concerns were identified in this study. |
| Baralic, I. et al. 2015 [712] | Randomized, double-blind, placebo-controlled, prospective study | 40 healthy subjects (young soccer players) | 0, 4 mg/day | 90 days |
| Improved prooxidant-antioxidant balance (PAB; p < 0.05) No safety concerns were identified in this study. |
| Baralic I. et al. 2013 [713] | Randomized, double-blind, placebo-controlled prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days |
| Protected thiol groups against oxidative modification (increase in SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) No safety concerns were identified in this study. |
| Djordjevic B. et al. 2013 [714] | Randomized, double-blind, placebo-controlled prospective study | 32 healthy subjects (soccer players) | 0, 4 mg/day | 90 days |
| Regular training significantly increased O2•¯ levels (main training effect, p < 0.01). TBARS and AOPP levels did not change throughout this study. Decreased post-exercise TAS levels only in the placebo group (p < 0.01). Increased total SH levels in both the AX and placebo groups (by 21% and 9%, respectively), and the effect of supplementation was marginally significant (p = 0.08). Decreased basal SOD activity in both the placebo and AX groups at the end of this study (main training effect, p < 0.01). No safety concerns were identified in this study. |
| Klinkenberg L.J. et al. 2013 [715] | Randomized, double-blind, placebo-controlled, prospective study | 32 well-trained male cyclists (25 ± 5 years, V˙O2peak = 60 ± 5 mL·kg−1·min−1, Wmax = 5.4 ± 0.5 W·kg−1) | 0, 20 mg/day * | 4 weeks |
| Not significant (N.S.); changes in markers of antioxidant capacity (trolox equivalent antioxidant capacity; TEAC, uric acid, and MDA). No safety concerns were identified in this study. |
| Res T. et al. 2013 [716] | Randomized, double-blind, placebo-controlled, prospective study | 32 trained male cyclists or triathletes (25 ± 1 years, V˙O2peak = 60 ± 1 mL·kg−1·min−1, Wmax = 395 ± 7 W) | 0, 20 mg/day | 4 weeks |
| N.S.; Plasma TAC (p = 0.90) or attenuated malondialdehyde levels (p = 0.63). Whole-body fat oxidation rates during submaximal exercise (from 0.71 +/− 0.04 to 0.68 ± 0.03 g.min and from 0.66 ± 0.04 to 0.61 ± 0.05 g.min in the Placebo and AX groups, respectively; p = 0.73), time trial performance (from 236 ± 9 to 239 ± 7 and from 238 ± 6 to 244 ± 6 W in the Placebo and AX groups, respectively; p = 0.63). No safety concerns were identified in this study. |
| Djordjevic B. et al. 2011 [714] | Randomized, double-blind, placebo-controlled, prospective study | 32 male elite soccer players | 0, 4 mg/day | 90 days |
| Changes in elevated O2•¯ concentrations after football exercise were statistically significant only in the placebo group (exercise × supplement effect, p < 0.05); TAS values decreased significantly after exercise only in the placebo group (p < 0.01). After the intervention, total SH content increased in the SH group (21% and 9%, respectively), and the effect of AX was marginally significant (p = 0.08). Basal SOD activity was significantly reduced in both the Placobo and AX groups at the end of this study (main effect of training, p < 0.01). .No safety concerns were identified in this study. |
| Bloomer, R.J. et al. 2005 [717] | Randomized, placebo-controlled, prospective study | 20 resistance trained male subjects (25.1 ± 1.6 years) | 0, 4 mg/day * | 3 months |
| N.S.; Muscle soreness, creatine kinase (CK), and muscle performance were measured before and through 96 h of eccentric exercise No safety concerns were identified in this study. |
| Oxidative stress markers in “geriatrics “ | ||||||
| Nakanishi R. et al. 2021 [718] | Randomized, double-blind, placebo-controlled prospective study | 29 nursing home residents healthy elderly subjects (80.9 ± 1.5 yrs.) | 0, 12 mg/twice a day * (0, 24 mg/day) | 16 weeks |
| Decrease in d-ROM values with the AX group (p < 0.01) but not with the placebo group; No safety concerns were identified in this study. |
| Petyaev I.M., et al. 2018 [719] | Randomized, blinded, four-arm, prospective study | 32 subjects with oxidative stress, 8 subjects taking AX only, (60–70 yrs) | 0, 7 mg/day * with DC | 4 weeks |
| Reduced serum oxidized LDL by 55.4% after 4 weeks (p < 0.05). Reduced MDA by 52.7% after 4 weeks (p < 0.05). Increase in serum nitric oxide (NO) levels (p = 0.054). No safety concerns were identified in this study. |
| Kiko T. et al. 2012 [720] | Randomized, double-blind, placebo-controlled prospective study | 30 healthy subjects (56.3 ± 1.0 yrs.) | 0, 6, 12 mg/day | 12 weeks |
| Amyloid β (Aβ) 40 and Aβ42 concentrations were much higher in erythrocytes (RBC) than in plasma. RBC Aβ levels increased with aging. After AX supplementation, RBC Aβ concentrations decreased. The RBC Aβ levels were positively correlated with RBC PLOOH and inversely correlated with AX concentration in RBC. A study related to the ref. [721] No safety concerns were identified in this study. |
| Oxidative stress markers in “fatigues” | ||||||
| Imai A. et al. 2018 [722] | Randomized, double-blind, placebo-controlled crossover study | 42 healthy subjects | 0, 6 mg/day * | 4 weeks |
| Elevated PCOOH levels during mental and physical tasks were attenuated by AX supplementation. No safety concerns were identified in this study. |
| Hongo N. et al. 2017 [723] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 12 mg/day * | 12 weeks |
| The rate of change in BAP values at week 12 was not significantly different between the AX group and the control group. No safety concerns were identified in this study. [Article in Japanese] |
| Oxidative stress markers in “other disorders and unhealthy condition” | ||||||
| Ledda A. et al. 2017 [724] | Open-labeled, two-arm prospective study | 59 patients with genitourinary cancers (prostate or bladder malignancies) who had undergone and completed cancer treatments (radiotherapy, chemotherapy or intravesical immunotherapy with increased oxidative stress and residual symptoms) | 0, 8 mg/day * | 6 weeks |
| Oncotris: containing 264 mg/day curcumin, 500 mg/day extract of cordyceps, and 8 mg/day AX (from EP217785227). Oncotris supplementation reduced plasma d-ROM levels. No safety concerns were identified in this study. |
| Yagi H. et al. 2013 [725] | Case reports | 34 OAB patients with anticholinergic agent-resistant (75.5 ± 8.0 years) | 0, 12 mg/day * | 8 weeks |
| Significantly improved international prostate symptom score (IPSS), QOL scores, benign prostatic hyperplasia impact index (BII) scores, and urinary 8-OHdG in patients AX could improve both urinary symptoms and QOL for anticholinergic agent-resistant OAB. No safety concerns were identified in this study. [Article in Japanese] |
| Kim, J.H. et al. 2011 [726] | Randomized, repeated measured, prospective study | 39 heavy smokers, 39 non-smokers | 0, 5, 20, or 40 mg/day | 3 weeks |
| 5 mg/day: MDA and ISP were significantly lower after 2 and 3 weeks compared to baseline in smokers (p < 0.05). SOD and TAC significantly increased after 1, 2, and 3 weeks compared to baseline in smokers (p < 0.05) 20 mg/day: MDA and ISP significantly were lower after 1, 2, and 3 weeks compared to baseline in smokers (p < 0.05). SOD and TAC significantly increased after 1, 2, and 3 weeks compared to baseline in smokers (p < 0.05). 40 mg/day: MDA and ISP were significantly lower after 1, 2, and 3 weeks compared to baseline in smokers (p < 0.05). SOD and TAC significantly increased after 2 and 3 weeks compared to baseline in smokers (p < 0.05). No safety concerns were identified in this study. |
| Yamada T. et al. 2010 [727] | Open-labeled, prospective study | 6 healthy subjects and 6 Sjoegren’s syndrome (SS) subjects | 12 mg/day | 2 weeks |
| Reduced protein oxidation (−10%, p < 0.05) No safety concerns were identified in this study. |
| Oxidative stress markers in “healthy subjects” | ||||||
| Chen JT, Kotani K. 2017 [728] | Randomized, double-blind, placebo-controlled, prospective study | 29 healthy females | 0, 12 mg/day | 3 months |
| N.S.: Serum d-ROM levels, urinary 8-OHdG, and BAP following AX treatment. A Significant increase in blood leukocytes was also found in the AX-treated group. No safety concerns were identified in this study. |
| Balcerczyk A. et al. 2014 [729] | Randomized, double-blind, placebo-controlled prospective study | 66 healthy females, (35–55 yrs.) | 0, 15 mg/day * | 12 weeks |
| Test supplement (NucleVital Q10): omega-3 acids (1350 mg/day), ubiquinone (300 mg/day), lycopene (45 mg/day), lutein palmitate (30 mg/day), zeaxanthin palmitate (6 mg/day), L-selenomethionine (330 mg/day), cholecalciferol (30 µg/day), α-tocopherol (45 mg/day), and AX (15 mg/day). Oxidative stress: significantly increased TAC of plasma and activity of erythrocyte SOD, with slight effects on oxidative stress biomarkers in erythrocytes: MDA and 4-hydroxyalkene levels. Antiaging effect: significant changes in mRNA expression of SIRT1 and 2 in PBMCs. No safety concerns were identified in this study. |
| Miyazawa T. et al. 2011 [730] | Randomized, double-blind, placebo-controlled, prospective study | 30 middle- aged & senior subjects (mean: 50.6 yrs.) | 0, 1, 3 mg/day | 12 weeks |
| Erythrocyte AX concentrations There was no significant changes in erythrocyte phospholipid hydroperoxide concentration after astaxanthin intake in either the 1 mg/day or 3 mg/day groups. No safety concerns were identified in this study. |
| Nakagawa K. et al. 2011 [721] | Randomized, double-blind, placebo-controlled prospective study | 30 healthy subjects | 0, 6, 12 mg/day | 12 weeks |
| 6 mg/day: Reduction in total phospholipid hydroperoxides (PLOOH) at 12 weeks compared to baseline (p < 0.01) and compared to placebo (p < 0.05). Reduced phosphatidyl ethanolamine hydroperoxide (PEOOH) at 12 weeks compared to baseline (p < 0.05) and compared to placebo (p < 0.05). Increased plasma AX concentration at 12 weeks (86 nM) compared to baseline (p < 0.01, 6 to 9 nM) and compared to placebo (p < 0.01, 8 nM). 12 mg/day: 48% reduction in total PLOOH at 12 weeks compared to baseline (p < 0.01) and 35% less total PLOOH at 12 weeks compared to control (p < 0.05). The 12 mg/day group had 46% less phosphatidylcholine hydroperoxide (PCOOH) at 12 weeks compared to baseline (p < 0.01). No safety concerns were identified in this study. |
| Peng L. et al., 2011 [731] | Randomized, placebo-controlled study | 115 healthy subjects | 0, 40 mg/day | 90 days |
| Comparing with the control group, MDA contents in the test group decreased significantly (p < 0.01), and SOD and GSH-Px activities increased significantly (p < 0.01). No safety concerns were identified in this study. |
| Park J.S. et al. 2010 [732] | Randomized, double-blind, placebo- controlled, prospective study | 42 healthy subjects | 0, 2, 8 mg/day | 8 weeks |
| 2 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared to placebo (p < 0.05). 8 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared to placebo (p < 0.05). No safety concerns were identified in this study. |
| Karppi, J. et al. 2007 [733] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 8 mg/day | 3 months |
| Decreased oxidation of fatty acids in healthy men (p < 0.05) No safety concerns were identified in this study. |
| Oxidative stress markers in “infertility” | ||||||
| Jabarpour M. et al. 2023 [652] | Randomized, placebo-controlled prospective study | 53 Patients with polycystic ovary syndrome (PCOS) | 0, 6 mg/twice a day (0,12 mg/day) | 60 days |
| Antioxidant markers: Increased levels of total antixoidant capacity (TAC) in follicular fluid. ART outcomes: higher rates of high-quality oocytes, high-quality embryo, and oocyte maturity in the AX group (the oocyte number, fertilization rate, and fertility rate; N.S.). No safety concerns were identified in this study. |
| Rostami S. et al. 2023 [653] | Randomized, triple-blind, placebo-controlled prospective study | 50 Patients of endometriosis (stage III/ IV) | 0, 6 mg/day | 12 weeks |
| Antioxidant markers: Increased serum levels of TAC (p = 0.004) and superoxide dismutase (SOD, 13.458 ± 7.276 vs. 9.040 ± 5.155; p = 0.010) were observed in the AX intervention group after therapy. In addition, serum Malondialdehyde (MDA, p = 0.031) decreased significantly after AX treatment. No safety concerns were identified in this study. |
| Ghantabpour T. et al. 2022 [734] | N/A | The first phase; 10 semen samples from healthy men, the second phase; 25 semen samples from healthy men | 0, 0.5, 1, 2 μM | N/A |
| Supplementation of sperm freezing medium with 1 µM AX was found to improve all parameters of sperm motility and viability (p ≤ 0.05). In addition, reduced levels of ROS parameters (intracellular hydrogen peroxide and superoxide) compared to the control group (p ≤ 0.05). AX also significantly reduced phosphatidylserine exogenous levels (p ≤ 0.05) and lipid peroxidation (p ≤ 0.05) after the freeze-thaw process. (in vitro study) |
| Gharaei R. et al. 2022 [651] | Randomized, double-blind, placebo-controlled prospective study | 40 Patients with polycystic ovary syndrome (PCOS) | 0, 8 mg/day | 40 days |
| AX supplementation resulted in significantly higher serum catalase and TAC levels in the AX group compared with the placebo group. However, there were no significant differences in serum MDA and SOD levels between groups. The expression of antioxidant genes such as Nrf2, HO-1, and NQ-1 was significantly increased in the granulosa cells (GC) of the AX group. No safety concerns were identified in this study. |
| Comhaire F.H. et al. 2005 [735] | Randomized, double-blind, placebo-controlled, prospective study | 30 males with infertility of ≥ 12 months | 0, 16 mg/day | 3 months |
| Significantly decreased ROS (chemiluminescence) in spermatozoa in the Astaxanthin group (n = 11), but not in the placebo group (n = 19). No safety concerns were identified in this study. |
| (B) Skin Health | ||||||
| Author/year/ reference | Study Design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of the effects on the skin under normal condition. | ||||||
| Sudo A et al. 2020 [736] | Placebo-controlled prospective study | 11 healthy subject (College floorball athletes for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil) studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in floorball athletes. Significant improvements in ‘firmness’ and ‘whiteness’, which are subjective measures of skin condition, were observed in the V7 supplementation group compared to pre-supplementation, while no significant changes were observed in the placebo group. No safety concerns were identified in this study. [Article in Japanese] |
| Sudo A et al. 2019 [737] | Placebo-controlled prospective study | 19 healthy subject (College softball player for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil) studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in college softball players. Subjective symptoms were evaluated with the VAS. The change of VAS in PRE and POST in the V7 group showed statistically significant improved skin blemishes; a comparison of V7 and placebo POST showed statistically significant improved skin elasticity and whitening. The percent change between PRE and POST in V7 was statistically significantly higher in dull skin and total score. No safety concerns were identified in this study. [Article in Japanese] |
| Chalyk, N. et al. 2017 [702] | Open-label, prospective study | 31 subjects; 18 obese, 8 overweight, 5 healthy, over the age of 40 | 4 mg/day | 92 days |
| Morphological analysis of the residual skin surface components (RSSC; age-related changes in corneocyte desquamation, microbial presence, and lipid droplet size): decreased levels of corneocyte desquamation (p = 0.0075) and microbial presence (p = 0.0367); increase in lipid droplet size among obese (body mass index >30 kg/m2) subjects (p = 0.0214). No safety concerns were identified in this study. |
| Tominaga K. et al. 2017 [738] | Randomized, double-blind, placebo-controlled prospective study | 65 healthy female (age, 35–60 years) with a wrinkle grade of 2.5 to 5.0 | 0, 6, 12 mg/day | 16 weeks |
| Water content (cheeks): no significant difference from the placebo group regarding improvement. However, it was significantly worse in the placebo group over the period, but unchanged in the low and high AX-treated groups. TEWL (cheek): no significant change during the study. Wrinkle depth (eye rims): no significant difference. However, it worsened during the study period in the placebo group but did not change in the low and high AX-treated groups. Elasticity (cheeks): significantly improved in the two AX groups IL-1α: increased during the study but not in the high AX dose group (p < 0.05). No safety concerns were identified in this study. |
| Tsukahara H. et al, 2016 [739] | Randomized, double-blind, placebo-controlled prospective study | 40 healthy subjects, those concerned about skin dullness or age-related skin deterioration. | 0, 3 mg/day | 8 weeks |
| TEWL (left cheek): significantly improved. Moisture content (left cheek): significant improvement. Melanin and color difference (left cheek): Melanin improved significantly. Elasticity (left cheek): Significant improvement in partial (R6). Facial image analysis (left part): “texture” significantly improved. No safety concerns were identified in this study. [Article in Japanese] |
| Phetcharat L. et al. 2015 [740] | Randomized, double-blind, placebo-controlled, prospective study | 34 healthy subjects with wrinkles on the face (crow’s-feet) (35–65 yrs.) | 4 mg/day | 8 weeks |
| The comparative control was rose hip powder. Therefore, the results of the evaluation before and after the intervention in the AX group are shown. Moisture content (forehead): improved from the pre-treatment level at week 8 (p < 0.001) in the AX group. Elasticity (cheeks): improved from the pre-treatment level in the AX group at week 8 (p < 0.05). Crow’s-feet wrinkle depth (buttocks of the eyes): improved from pre-treatment levels at Weeks 4 and 8 in the AX group (p < 0.05). No safety concerns were identified in this study. |
| Yoon HS. et al. 2014 [703] | Randomized, double-blind, placebo-controlled prospective study | 44 healthy females with wrinkles grade ≥ 2 (≥40 yrs.) | 0, 2 mg/day * | 12 weeks |
| AX (2 mg/day)is combined with collagen hydrolysate (3 g/day). Skin condition of non-UV- irradiated skin: significantly improved TEWL (cheek) at week 12 (p = 0.045), tended to improve water content (cheek), tended to improve elasticity (cheek) at week 4, and significantly improved at week 12. No safety concerns were identified in this study. |
| Suganuma K. et al. 2012 [741] | Randomized, double-blind, placebo-controlled, prospective study | 44 female subjects (Mean 37.26 yrs.) | 0, 6 mg/day * | 20 weeks |
| Water content (cheeks): increasing trend over the study period. The AX+VC+VE group showed an increase compared to the VC+VE (p < 0.10). Elasticity (upper cheekbones): no significant change. Fine wrinkles: significant improvement in the AX+VC+VE group (also improved compared to VC+VE group) No safety concerns were identified in this study. |
| Tominaga K. et al. 2012 [742] (Study 2) | Randomized, double-blind, placebo-controlled, prospective study | 36 male subjects (20 to 60 yrs.) | 0, 3 mg/twice a day * (0,6 mg/day) | 6 weeks |
| TEWL: significantly decreased in AX (p < 0.01). Water content: increasing trend in the left cheek in the AX group (p = 0.08). Elasticity: significantly improved in the AX group (p < 0.05). Fine wrinkles: the total area ratio and volume of wrinkles in the AX group decreased (p < 0.05). Sebum production: decreased in the AX group (p = 0.085). No safety concerns were identified in this study. |
| Evaluation of protective effect against UV irradiation | ||||||
| Ito N. et al. 2018 [743] | Randomized, double-blind, placebo-controlled, prospective study | 22 healthy subjects with skin phototype was type II or III (30–56 yrs.) | 0, 6 mg/day | 10 weeks |
| Subjective skin condition was assessed on a visual analog scale; the AX group showed an increase in minimum erythema dose (MED) compared to placebo. The AX group had a reduced loss of skin moisture in the irradiated area compared with the placebo. Subjective skin conditions for “improvement of rough skin” and “texture” in non-irradiated areas were significantly improved by AX. No safety concerns were identified in this study. |
| Carrascosa JM et al. 2017 [744] | Randomized, double-blind, placebo-controlled, prospective study | 31 healthy subjects with skin phototypes II and III | 4 mg/day * | 56 days |
| Intervention: Genosun oral a combination of AX (4 mg), β-carotene(4.8 mg), vitamin E (6 mg), vitamin C(40 mg), lutein (2.4 mg), and lycopene (2.4 mg). MED at Days 1, 29, and 57 was evaluated.The Intervention group showed a significant increase over placebo in the tolerance to an erythemal dose of UVR. Increased UVR tolerance was reflected in an increase in MED of 12.4% and 20.51% over baseline after 29 and 57 days, respectively, with a significant difference between treatment and control groups at the end of the study. No safety concerns were identified in this study. |
| Yoon HS. et al. 2014 [703] | Randomized, double-blind, placebo-controlled prospective study | 44 healthy females with wrinkles grade ≥ 2 (≥40 yrs.) | 0, 2 mg/day * | 12 weeks |
| AX (2 mg/day) combined with collagen hydrolysate (3 g/day). Skin biopsy after UV irradiation: no difference in oxidative markers (Thymine dimers, 8-OHdG) between the two groups in histological evaluation. Regarding mRNA expression, significantly upregulated expression of procollagen type I tended to upregulate fibrillin-1, while significantly downregulated MMP1 and MMP12 in the AX group compared to placebo. No safety concerns were identified in this study. |
| Satoh A. et al. 2011 [745] | Randomized, single-blind, placebo-controlled prospective study | 26 healthy subjects | 0, 3 mg/day | 4 + 4 weeks |
| After 4 weeks of administration, UV light was irradiated (2 MED), and the given test substance was administered for another 4 weeks. Skin color was evaluated with a colorimeter, a mexameter, and a skin color scale before administration, before UV irradiation, and 1, 7, 14, 21, and 28 days after UV irradiation. The results showed that the L value of the colorimeter and skin color scale scores were significantly higher in the Ax group than in the placebo group, and the amount of melanin after UV irradiation was significantly lower in the Ax group than in the placebo group. No safety concerns were identified in this study. [Article in Japanese] |
| Yamashita E. 2006 [746] | Randomized, single-blind, placebo-controlled, prospective study | 49 female subjects (Mean 47 yrs.) | 0, 2 mg/twice a day (0, 4 mg/day) * | 6 weeks |
| Water content (left cheek): significant improvement in the 6-week treatment group (compared to the start of treatment). Elasticity (left eye rim): significant improvement in the placebo group at weeks 3 and 6 (p < 0.05). Inspection/Palpation by a dermatologist: improvement in fine lines, wrinkles, and elasticity (week 6). Skin surface observation: improvement in fine lines, wrinkles, and elasticity (week 6). No safety concerns were identified in this study. |
| Yamashita E. 2002 [747] | Randomized, double-blind, placebo-controlled, prospective study | 16 healthy female subjects with dry skin | 0, 2 mg/day * | 4 weeks |
| Moisture content: trend of improvement in the AX group compared to placebo at week 2 on the left eye corner and at week 4 on the cheeks, with significant improvement at week 4 on the eye corner (p < 0.05). Wrinkle depth (eye corner): no significant difference. Subjective symptoms (questionnaire): subjective improvement in spots/freckles (week 2), acne/wipes (week 4). Inspection/Palpation by a dermatologist: improvement in smoothness, moistness, and firmness. No safety concerns were identified in this study. [Article in Japanese] |
| Evaluation of efficacy in skin diseases. | ||||||
| Satoh A. et al. 2009 [704] | Randomized, single-blind, placebo-controlled prospective study | 27 patients with atopic dermatitis | 0, 12 mg/day | 4 weeks. |
| Severity (SCORAD), pruritus (VAS), quality of life (Skindex-16, STAI), immune function (Th1/Th2, blood catecholamines), and antioxidant status (urinary 8-OHdG, isoprostanes) were assessed. There were significant differences in the degree of itching between the Ax and placebo groups. However, there was significant improvement in Skindex-16 symptoms and STAI status anxiety in the Ax group. In addition, the Th1/Th2 balance shifted significantly toward Th1. No safety concerns were identified in this study [Article in Japanese] |
| (C) Eye health | ||||||
| Author/year/ reference | Study design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of efficacy in “asthenopia (eyestrain)”. | ||||||
| Sekikawa T. et al. 2023 [748] | Randomized, double-blind, placebo-controlled prospective study | 59 healthy subjects with VDT operation (Mean 39 yrs.) | 0, 9 mg/day | 6 weeks |
| Visual acuity: In participants ≥40 yrs, AX had a higher protective effect of on corrected visual acuity of the dominant eye after visual display terminal (VDT) work at 6 weeks after intake in the AX group vs the control group (p < 0.05). In <40 yrs, no significant difference between the AX and control groups. Functional visual acuity and pupil constriction rate: No significant difference between the AX and control groups No safety concerns were identified in this study. |
| Kizawa Y. et al. 2021 [749] | Randomized double-blind, placebo-controlled, prospective study | 40 healthy subjects with VDT operation | 0, 6 mg/day * | 6 weeks |
| Intervention (active group): 72 mg anthocyanin from blueberry (bilberry) extract, 10 mg lutein and 6 mg AX. After 6 weeks, there was a significant improvement in the active group compared to the placebo group in the average percentage of pupillary response in both eyes and in the dominant eye before and after operating the visual display terminal. In addition, the scores for “A sensation of trouble in focusing the eyes” and “Difficulty in seeing objects in one’s hand and nearby, or fine print” were significantly improved in the active group compared to the placebo group before and after ingestion. No statistically significant improvements were observed in tear degradation time, visual acuity, Schirmer test value, macular pigment optical density level, or muscle hardness. No safety concerns were identified in this study. |
| Sudo A et al. 2019 [750] | Open-labeled, prospective study | 19 healthy females (Mean 47.3 yrs.) | 0, 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil) studied the efficacy of V7 on subjective fatigue and skin conditions in typical middle-aged females. Subjective symptoms were evaluated with the VAS. The change in VAS in PRE and POST in the V7 group showed no statistically significant improved in eye strain. No safety concerns were identified in this study. [Article in Japanese] |
| Sudo A et al. 2019 [737] | Placebo-controlled prospective study | 19 healthy subject (College softball player for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil) studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in college softball players. Subjective symptoms were evaluated with the VAS. A comparison of V7 and placebo POST showed statistically significant increases in eye strain. The percent change between PRE and POST in V7 was statistically significantly higher in eye strain. No safety concerns were identified in this study. [Article in Japanese] |
| Kono K. et al. 2014 [751] | Randomized, double-blind, placebo-controlled prospective study | 48 healthy subjects who complained of eye strain | 0, 4 mg/day * | 4 weeks |
| Test supplement (Enkin; lutein (10mg), 20 mg of bilberry extract, and 26.5 mg of black soybean hull extract (a total of 2.3 mg of cyanidin-3-glucoside in both extracts), DHA (50mg), and AX (4mg). The variation of the “near-point accommodation” of both eyes from baseline to 4 weeks after-intervention in the test supplement group (TS) was significantly higher than in the placebo (P) group (1.321 ± 0.394 diopter (D) in the TS group and 0.108 ± 0.336 D in the P group, p = 0.023, respectively). Regarding subjective symptoms, there was a significant improved on “stiff shoulders or neck” and “blurred vision” in the TS group compared to the P group (p < 0.05). No safety concerns were identified in this study. |
| Nagaki Y. et al. 2010 [46] | Randomized, single-blind, placebo-controlled prospective study | 82 healthy subjects with VDT operation (6 h or more per day for more than 1 year) and frequently experienced eyestrain | 0, 9 mg/day | 4 weeks |
| (1) The post-treatment accommodation ability of the AX group with respect to value and rate of change was significantly higher than that of the control group. (2) The distribution of rate of change also showed significant improvement in post-treatment accommodation ability of the AX group when compared to control group. (3) Subjective questionnaire regarding 4 conditions (“eyestrain,” “hazy vision,” “flickering images,” and “my shoulders/back feel stiff”) showed that the AX group significantly improved compared to the of control group. No safety concerns were identified in this study. [Article in Japanese] |
| Kajita M et al. 2009 [47] | Open-labeled, prospective study | 82 healthy males with presbyopia (Mean 53.9 yrs.) | 6 mg/day | 4 weeks |
| The pupillary constriction ratio before and after AX supplementation was measured by TriIRIS C9000. The change in subjective symptoms after supplementation was examined by a questionnaire. The results showed a significant increase in pupillary constriction ratio after supplementation with AX, therefore suggesting that astaxanthin may also improve the accommodation function of the eye and some subjective symptoms related to presbyopia in middle-aged and older people with complaints of eye strain. No safety concerns were identified in this study. [Article in Japanese] |
| Seya Y. et al. 2009 [752] | Open-labeled, prospective study | 10 healthy subjects with VDT operation (Mean Age 24.6 yrs., VDT 6.9 h/day) | 6 mg/day | 4 weeks. |
| The effects of visual fatigue on reaction times measured in a visual pursuit task. Regardless of the duration of the intake period for AX, the reaction times at the early trials/blocks of the reaction time task were shorter than those at the late trials/blocks during a long-lasting, 500-trial experimental session. In addition, the reaction times at the late stages (14th and 28th days) of the AX intake were shorter than those at the early stage (1st day). No safety concerns were identified in this study [Article in Japanese] |
| Tsukahara H. et al. 2008 [753] | Open-labeled, prospective study | 13 healthy subjects with shoulder Stiffness | 6 mg/days * | 4 weeks |
| 6mg of AX and 50mg of flaxseed lignin. All patients completed the efficacy evaluation, which confirmed significant improvement in physical symptoms such as shoulder stiffness, physical fatigue, mental irritability, cold hands and feet, eye fatigue, and redness of the eyes. At the end of the treatment, laser Doppler graphics also confirmed a significant increase in blood flow in the shoulders. No safety concerns were identified in this study. [Article in Japanese] |
| Iwasaki T. et al. 2006 [44] | Randomized, double-blind, placebo-controlled crossover study | 39 healthy females (Mea 20.5 yrs.) | 0, 6 mg/day | 2 weeks |
| Accommodative function and subjective symptoms relating to eyestrain were measured before and after the task and after the 10-min rest following the task. The data were then compared between the AX and Placebo groups by the double-blind cross-over method. After the task, accommodation contraction and relaxation times were extended in both the AX and Placebo groups. Comparison between the two groups showed that after the task, accommodation relaxation time was significantly extended in the Placebo group, in contrast to AX. Accommodative contraction and relaxation times were significantly prolonged after the 10-min rest in the P group as compared to AX. The symptoms of eye fatigue, eye heaviness, blurred vision, and eye dryness in the Placebo group increased; however, the AX group only showed increases in eye fatigue and eye heaviness. No safety concerns were identified in this study. [Article in Japanese] |
| Nagaki Y. et al. 2006 [45] | Randomized, double-blind, placebo-controlled, prospective study | 48 healthy subjects with VDT operation (6 h or more per day for more than 1 year) and frequently experienced eyestrain | 0, 6 mg/day | 4 weeks |
| 1. Significantly improved the magnitude of change in amplitude of accommodation before and after supplementation in the AX supplemented group compared with the control group. 2. Significantly better scores of the distribution of the percentage change in amplitude of accommodation after supplementation in the AX supplemented group compared with the control group. 3. In the subjective asthenopia evaluation, the AX supplemented significantly improved for the two items “dimness of sight” and “stiff shoulders and back” compared with the control group, and an improvement tendency was seen in “heavy head.” No safety concerns were identified in this study. [Article in Japanese] |
| Nitta T. et al. 2005 [41] | Randomized, placebo-controlled, prospective study | 30 health subjects (Mean time consumed for close work (e.g., VDT work) was approx. 7 h./day) | 0, 6, 12 mg/day | 4 weeks |
| l. Significantly increased the objective accommodation power of the AX 12 mg group compared to that of pre-dosing. 2. Significantly shortened was the positive accommodation time in the AX 6 mg and the 12 mg groups compared to those of pre-dosing, and the negative accommodation time was significantly shortened in the AX placebo and the 6 mg groups compared to those of pre-dosing. 3. VAS; many parameters of subjective symptoms were improved in the AX 6 mg group. No safety concerns were identified in this study. [Article in Japanese] |
| Shiratori K. et al. 2005 [42] | Randomized, placebo- controlled, prospective study | 39 healthy subjects who complained of eyestrain | 0, 6 mg/day | 4 weeks |
| 1. Significantly higher sub-objective accommodation power (changing rate) in the AX group than that of the control group. 2. Significantly higher rate of positive and negative accommodation times (rate of change) in the AX group compared to those of the control group. 3. In the AX group, subjective degree of asthenopia (eye strain) measured by VAS showed significant improvement in two parameters, i.e., “My eyes get bleary” and “I get irritated easily”, compared to the control group. No safety concerns were identified in this study. [Article in Japanese] |
| Takahashi N. et al. 2005 [43] | Open-labeled, prospective study | 10 healthy subjects | 6 mg/day | 2 weeks |
| Effects of astaxanthin on accommodative recovery derived from a rest after VDT work were studied. Evaluated (9 dominant eyes) by values for objective diopter, HFC (High Frequency Component in Accommodative micro-fluctuation), and accommodative reaction. Increased HFC after rest was significantly restrained by AX supplementation compared to the increase shortly after working. No safety concerns were identified in this study. [Article in Japanese] |
| Nakamura A. et al. 2004 [40] | Randomized, placebo- controlled, prospective study | 49 healthy subjects | 0, 2, 4, 12 mg/day | 4 weeks |
| For far visual acuity (5 m), there was no significant difference in the results for uncorrected visual acuity between the 0 mg group and the 2 mg group before and after the start of peroral administration; however, uncorrected visual acuity improved significantly for the 4 mg group and 12 mg group (p < 0.05). For corrected visual acuity, no significant difference was found for any of the groups. No significant changes were found in refraction or flicker fusion frequency. For the accommodation test, positive accommodation time was shortened significantly for the 4mg group and the 12 mg group (p < 0.05). No significant change was found in the other items. For pupillary reflex, no significant difference was found in the miosis ratio (%), T1 (ms), T2 (ms), or VC (mm2/s). No safety concerns were identified in this study. [Article in Japanese] |
| Nagaki Y. et al. 2002 [39] | Randomized, placebo- controlled, prospective study | 26 VDT subjects + 13 non-VDT subjects (control) | 0, 5 mg/day | 4 weeks |
| Group A: 13 non-VDT workers/no supplementation. Group B: 13 VDT workers with AX, 5 mg/day, for 4 weeks, Group C: 13 VDT workers with placebo, 5 mg/day, for 4 weeks. Accommodation amplitudes in Groups B and C before supplementation were significantly (p < 0.05) lower than in Group A. After AX supplementation, the accommodation amplitude in Group B was significantly (p < 0.01) larger than before supplementation, while the accommodation amplitude in Group C after placebo supplementation was unchanged. The CFFs in Groups B and C before supplementation were significantly (p < 0.05) lower than in Group A. The CCFs in Groups B and C did not change after supplementation. Amplitudes and latencies of P100 in PVEP in Groups B and C before supplementation were similar to those in Group A and did not change after supplementation. No safety concerns were identified in this study. |
| Evaluation of benefits in cataract surgery | ||||||
| Hashimoto H. et al. 2021 [705] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| We analyzed the antioxidant effect of AX in relationship to age. None of the parameters were correlated with age before AX intake, but only total hydroperoxide values were significantly correlated after AX intake (r = 0.4, p < 0.05). Total hydroperoxide levels were similar in younger and older age groups ( < 70 vs. ≥70 years) before AX intake, but significantly decreased in younger age groups after intake (−0.21 ± 0.18 vs. −0.05 ± 0.31, p < 0.05), resulting in a significant difference (p < 0.05). Thus, the previously observed decrease in mean total hydroperoxide levels after AX intake was likely due to a greater response in the younger age group. Analysis associated with the study [706]. No safety concerns were identified in this study. |
| Hashimoto H. et al. 2019 [707] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| In this analysis, the effect of AX intake on the relationship between VEGF levels and ROS-related parameters before and after bilateral cataract surgery was analyzed by gender. VEGF, hydrogen peroxide, and total hydroperoxide levels in the aqueous humor, as well as O2 scavenging activity, were measured. For women only, VEGF levels and O2 scavenging activity before AX intake were negatively correlated (r = −0.6, p < 0.01) and positively correlated with total hydrogen peroxide levels before and after AX intake (r = 0.7, 0.8, p < 0.01, respectively). Analysis associated with this study [706] No safety concerns were identified in this study. |
| Hashimoto H. et al. 2016 [708] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Superoxide anion scavenging activity (U/mL): 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared to baseline, p < 0.05. Total hydroperoxides (U CARR) from 1.16 ± 0.18 at 0 weeks were reduced to 1.04 ± 0.31 after 2 weeks of supplementation compared to baseline, p < 0.05. Analysis associated with this study [706]. No safety concerns were identified in this study. |
| Hashimoto, H. et al. 2013 [709] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) increased superoxide scavenging activity (p < 0.05). Analysis associated with this study [706] No safety concerns were identified in this study. |
| Hashimoto H. et al. 2011 [706] | Open-labeled, prospective study | 35 subjects who underwent bilateral cataract surgery (intraocular lens implantation) (Mean c.a 71 yrs.) | 6 mg/day | 2 weeks |
| Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) No safety concerns were identified in this study. [Article in Japanese] |
| Evaluation of efficacy in other ophthalmological categories. | ||||||
| Yoshida K. et al. 2023 [754] | Randomized, double-blind, placebo-controlled prospective study | 57 healthy subjects | 0, 6 mg/day | 8 weeks |
| Active group: 10 mg lutein, 2 mg zeaxanthin, and 6 mg of AX. Significantly improved eye–hand coordination after visual display terminal (VDT) operation at 8 weeks in the active group. No clear improvement in the effect of the supplementation on smooth-pursuit eye movements. The active group also showed a significant increase in macular pigment optical density (MPOD) levels. No safety concerns were identified in this study. |
| D’Aloisio R. et al., 2022 [755] | Retrospective study | 15 AMD patients treated with daily oral nutritional supplement with AX. 13 AMD patients treated w/o. daily oral nutritional supplement (control) | 0, 12 mg/day * | 6 months |
| Nutritional supplement: 100 mg lutein, 80 mg bromelain, 120 mg VC, 30 mg VE, 400 μg folic acid, Zn, Cu, 1000 IU D3 vitamin, and 12 mg AX (Astazin 10). There was a statistically significant difference in choriocapillary vessel density (CCVD) values between cases and controls at baseline (p < 0.001) and at follow-up (p < 0.001); choroidal thickness measurements were statistically significant between cases and controls (p = 0.002) and in cases at follow-up (p < 0.001). No safety concerns were identified in this study. |
| Tian L. et al. 2022 [756] | Open-labeled, prospective study | 60 middle-aged and elderly patients with mild-to-moderate dry eye disease (DED) | 6 mg/twice a day (12mg/day) | 30 days |
| Significantly improved (p < 0.05) to varying degrees after treatment compared to pre-treatment for the ocular surface disease index (OSDI) score, non-invasive tear break-up time (NIBUT), fluorescein break-up time (BUT), corneal fluorescein staining (CFS) score, eyelid margin signs, MG expressivity, mibum quality, and blink frequency, but no differences were found for tear meniscus height, Schirmer I test, conjunctival hyperemia, tear fluid lipid layer thickness, meibum quality, meibomian gland dropout (MGDR), incomplete blink rate, Visual acuity (VA), intraocular pressure (IOP). No safety concerns were identified in this study. |
| Huang J.Y. et al. 2016 [757] | Randomized, double-blind, placebo-controlled prospective study | 43 patients with dry eye disease (DED) | 0, 2 mg/day * | 16 weeks |
| Supplement: Commercially available antioxidant supplements contain anthocyanosides, vitamins A, C, and E, and crudely extracted additives from several Chinese herbal extracts and AX. Lower diastolic blood pressure in the treated group. There were no statistically significant differences in systolic blood pressure, dry eye symptoms, serum anti-SSA and anti-SSB, visual acuity, intraocular pressure, or fluorescein corneal staining between the two groups. Significantly improved tear film break time scores and Schirmer test without local anesthesia in the treatment group. Tear ROS levels differed between groups and decreased after treatment. The overall subjective impression was significantly improved by treatment compared to placebo. No safety concerns were identified in this study. |
| Piermarocchi S. et al. 2012 [758] | Randomized, two-arm, prospective study | 145 patients with nonexudative (dry) age-related macular degeneration (AMD) (72.5 ± 7 yrs.) | 0, 4 mg/days * | 24 months |
| Two-year results of the CARMIS study: Interventions were vitamin C (180 mg), vitamin E (30 mg), zinc (22.5 mg), copper (1 mg), lutein (10 mg), zeaxanthin (1 mg), and AX (4 mg). The treated group showed stabilization of visual acuity (VA), with significantly (p = 0.003) better VA scores compared to the non-treated group at 24-month follow-up. An improvement in contrast sensitivity (CS, p = 0.001) and final mean National Eye Institute visual function questionnaire (NEI VFQ-25) composite scores at 12 and 24 months were higher in the treated group compared to the non-treated group (p < 0.001). No safety concerns were identified in this study. |
| Saito M. et al. 2012 [685] | Randomized, double-blind, placebo-controlled, prospective study | 20 healthy subjects | 0, 12 mg/day | 4 weeks |
| Significant increase in the macular square blur rate (SBR) after 4 weeks after AX (p = 0.018). No statistical difference in the macular SBR was detected in the placebo group (p = 0.598). No safety concerns were identified in this study. |
| Parisi V.. et al. 2008 [759] | Randomized, two-arm, prospective study | 27 patients with nonadvanced AMD and visual acuity ≥0.2 logarithm of the minimum angle of resolution (69.4 ± 4.3 yrs.) | 0, 4 mg/days * | 12 months |
| one-year results of the CARMIS study; Interventions were vitamin C (180 mg), vitamin E (30 mg), zinc (22.5 mg), copper (1 mg), lutein (10 mg), zeaxanthin (1 mg), and AX (4 mg). In nonadvanced AMD eyes, selective dysfunction of the central retina (0°−5°) was ameliorated by carotenoid and antioxidant supplementation. There were no functional changes in the more peripheral (5°−20°) retina. No safety concerns were identified in this study. |
| Nagaki Y. et al. 2005 [684] | Randomized, placebo-controlled, prospective study | 36 health subjects (c.a. 41 yrs.) | 0, 6 mg/day | 4 weeks |
| After 4 weeks of supplementation, retinal capillary blood flow in the AX group was significantly (p < 0.01) higher than before supplementation in both eyes, while retinal capillary blood flow in the placebo group after placebo treatment was unchanged. Intraocular pressures in both groups remained unchanged during the supplementation period. No safety concerns were identified in this study. [Article in Japanese] |
| Sawaki et al. 2002 [760] | Randomized, double-blind, placebo-controlled, prospective study | 18 healthy male Subjects (College handball player) | 0, 6 mg/day | 4 weeks |
| No changes in static and dynamic visual acuity measurements were observed before and after administration of AX. Regarding the deep vision measurements after administration of AX, the AX group showed better values compared to the placebo group. Flicker values after AX administration showed that visual acuity was significantly more acute in the AX group than in the placebo group. No safety concerns were identified in this study. [Article in Japanese] |
| (D) Cardiovascular Health: Dyslipidemia, Glucose/Lipid Metabolisms, Chronic Inflammation and Type 2 Diabetes | ||||||
| Author/year/ reference | Study Design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of efficacy in obesity, dyslipidemia, hypertension, glucose intolerance and T2DM. | ||||||
| Ciaraldi T.P. et al. 2023 [761] | Randomized, double-blind, placebo-controlled prospective study | 34 Obese subjects with prediabetes and dyslipidaemia | 0, 12 mg/day | 24 weeks |
| After 24 weeks, there was a significant decrease in low-density lipoprotein (−0.33 ± 0.11 mM) and total cholesterol (Chol, −0.30 ± 0.14 mM) (both p < 0.05) in the AX group. Reduced levels of the cardiovascular disease (CVD) risk markers fibrinogen (−473 ± 210 ng/mL), L-selectin (−0.08 ± 0.03 ng/mL), and fetuin-A (−10.3 ± 3.6 ng/mL) (all p < 0.05) in the AX group. The trend of improvement in insulin action was also observed in insulin-stimulated whole-body glucose treatment (+0.52 ± 0.37 mg/m2/min, p = 0.078), as well as in fasting [insulin] (−5.6 ± 8.4 pM, p = 0.097) and HOMA2-IR (−0.31 ± 0.16, p = 0.060). No consistent, significant differences from baseline were observed in any of these results in the placebo group. No safety concerns were identified in this study. |
| Saeidi A. et al. 2023 [762] | Randomized placebo-controlled prospective study | Obese subjects; 15 control group (CG), 15 supplement group (SG), 15 training group (TG), 15 training plus supplement group (TSG). BMI: 33.6 ± 1.4 | 0, 20 mg/day | 12 weeks |
| Intervention: AX with/without high-intensity functional training. BW,% of Fat, BMI, Fat-Free Mass: After 12 weeks, there was significant improvement in SG, TG, and TSG, but not in CG (p < 0.05). VO2 peak: Increases after 12 weeks of exercise were significant in the TG (p = 0.0001) and TSG (p =0.0001) but not in the CG (p = 0.32) and SG (p = 0.21). Lipid profile (HDL, LDL, Total Cholesterol(Chol.), and Triglycerides(TG)): After 12 weeks, there was significant improvement in SG, TG, and TSG, but not in CG (p < 0.05). Metabolic Factors (Insulin, Glucose, HOMA-IR): Glucose and Insulin levels decreased significantly in the SG (p <0.001), TG (p < 0.001), and TSG (p <0.001), but not significantly in the CG (p > 0.05), and decreased HOMA-IR following 12 weeks of training in the SG (p = 0.0001), TG (p = 0.0001), and TSG (p = 0.0001), while the difference in HOMA-IR in the CG was not significant (p = 0.17). Adipokines and Growth Differentiation Factors: [Cq1/TNF-related protein 9 and 2 (CTRP9 and CTRP2) levels, and growth differentiation factors 8 and 15 (GDF8 and GDF15)] were measured. There were significant differences in all indicators between the groups (p < 0.05). No safety concerns were identified in this study. |
| Wika A.A. et al. 2023 [763] | Randomized, double-blind, placebo-controlled prospective study | 19 obese subjects (Mean, age: 27.5 yrs; BF%: 37.9; BMI: 33.4 kg/m2; VO2peak: 25.9 ml·kg−1·min−1) | 0, 12 mg/day | 4 weeks |
| Subjects performed a graded exercise test on a cycling ergometer and were measured for changes in glucose and lactate levels, fat and carbohydrate (CHO) oxidation rates, heart rate, and rating of perceived exertion (RPE). Although there were no changes in fat oxidation rate, blood lactate or glucose, or RPE (all p > 0.05), a significant decrease in CHO oxidation rate was observed in the AX group only, before and after supplementation. In addition, in the AX group, heart rate decreased by 7% during the graded exercise stress test. No safety concerns were identified in this study. |
| Rad NR. et al. 2022 [691] | Randomized, double-blind, placebo-controlled prospective study | 50 Type 2 Diabetes Mellitus (T2DM) patients receiving metformin | 0, 10 mg/day | 12 weeks |
| Investigation of additive synergistic effects on metformin (1000–2000 mg/day). T2DM: After the intervention, while FBS, HbA1c (probably maintained in the high-normal range by metformin even at baseline), and systolic blood pressure (in the normal range) tended to decrease in both groups, blood lipids (within normal range) remained unchanged. Significantly reduced FBS in the AX group rather than the placebo group. Oxidative stress: Significantly increased blood TAC levels at the end of the intervention only in the AX-treated group, while MDA remained unchanged. Similarly, increased SOD and catalase activity in blood and increased Nrf2 protein in PBMCs were observed at the end of the intervention only in the AX-treated group. No safety concerns were identified in this study. |
| Shokri-Mashhadi, N. et al. 2021 [693] | Randomized, double-blind, placebo-controlled prospective study | 44 patients with T2DM | 0, 8 mg/day | 8 weeks |
| Decrease plasma levels of MDA and IL-6 (p < 0.05) and decrease the expression level of miR-146a, associated with inflammatory markers (fold change: −1/388) (p < 0.05). No safety concerns were identified in this study. |
| Urakaze M et al. 2021 [764] | Randomized, double-blind, placebo-controlled prospective study | 44 subjects including prediabetes (Av. 46–48 yrs.) | 0, 12 mg/day | 12 weeks |
| Glucose levels at 120 min after the 75 g oral glucose tolerance test (OGTT) were significantly lower than before supplementation. HbA1c (p < 0.05), apo E (p < 0.05), and MDA-modified LDL (p < 0. 05) also decreased, while total cholesterol, triglycerides, and HDL-C levels were unchanged. Matuda index, a measure of insulin resistance, was improved in AX-treated subjects compared to pre-treatment. Plasma AX levels were undetectable at baseline and increased to 122.69 ng/mL (c.a. 205 nM) after 4 weeks in the intervention group, and this level was maintained until 12 weeks. No safety concerns were identified in this study. |
| Birudaraju D. et al. 2020 [765] | Randomized, double-blind, placebo-controlled prospective study | 22 healthy subjects (48.8 ± 16.0 yrs.) | 0, 6 mg/twice a day * (0,12 mg/day) | 4 weeks |
| Combination with Cavacurcumin, Eicosapentaenoic acid (Omega-3s), AX and γ-linoleic acid (Omega-6) (CEAG) The CEAG group had significantly lower mean systolic blood pressure at 4 weeks [4.7 ± 6.8 (p = 0.002)] compared to the placebo group. A significant decrease in high-sensitivity C-reactive protein (hsCRP) (−0.49 ± 1.9 vs. + 0.51 ± 2.5, p = 0.059) and a blunt increase in IL-6 (+0.2 vs. +0.4, placebo = 0.60) were observed compared to placebo. No safety concerns were identified in this study. |
| Chan K. et al. 2019 [766] | Randomized, double-blind, placebo-controlled prospective study | 54 patients With type 2 diabetes | 0, 6, 12 mg/day | 8 weeks |
| Increased plasma AX levels and decreased fasting plasma glucose and HbA1c levels. In the 12 mg AX group, there was a reduction in plasma triglyceride, total cholesterol, and LDL levels. Lowered changes in plasma IL-6 and TNF- levels and plasma vWF level and higher changes in AT-III level. In 12 mg AX group, there were decreased changes in plasma FVII and PAI-1levels. No safety concerns were identified in this study. |
| Landi F et al. 2019 [767] | Randomized open-labeled, prospective study | 47 subjects with higher level of serum Chol. (not needing statins or statin intolerant) (58.7± 8.7 yrs.) | 0.5 mg/day * in Nutraceutical B | 6 weeks |
| Nutraceutical B: policosanol (10 mg), red yeast rice (200 mg; 3 mg monacolin K), Berberine (500 mg), Astaxanthin (0.5 mg), folic acid (200 mcg), and Coenzyme Q10 (2 mg) Both nutraceutical combinations improved the lipid profile including total Chol., HDL-Chol., LDL-Chol., and TG. No safety concerns were identified in this study. |
| Mashhadi N.S. et al. 2018 [768] | Randomized, double-blind, placebo-controlled, prospective study | 44 participants with type 2 diabetes | 0, 8 mg/day | 8 weeks |
| Increased the serum adiponectin concentration, reduced visceral body fat mass (p < 0.01), serum triglyceride and very-low-density lipoprotein (VLDL) cholesterol concentrations, systolic blood pressure, fructosamine concentration (p < 0.05), and marginally reduced the plasma glucose concentration (p = 0.057). No safety concerns were identified in this study. |
| Sarkkinen ES., et al. 2018 [769] | Randomized, double-blind, placebo-controlled, prospective study | 35 overweight subjects with mildly or moderately elevated blood pressure | N/A (0, 4 g of kirill oil powder /day) | 56 days |
| Average values of hematological measurements were within the reference range for all subjects, and no significant changes were observed in blood pressure or lipid levels. No serious adverse events were reported. |
| Canas J. A. et al. 2017 [770] | Randomized, double-blind, placebo-controlled, prospective study | 20 children with simple obesity (BMI > 90%) | 500 μg/day * (MCS) | 6 months |
| Mixed-carotenoid supplementation (MCS) increased carotene, total adiponectin, and high-molecular-weight adiponectin in plasma compared to placebo; MCS decreased BMI z-score, waist-to-height ratio, and subcutaneous adipose tissue compared to placebo. AX was used as a part of MCS. No safety concerns were identified in this study. |
| Maki KC. et al. 2015 [771] | Randomized, double-blind, placebo-controlled, prospective study | 102 subjects with TAG 150–499 mg/dL and LDL cholesterol (LDL-C) ≥70 mg/dL | 0, 12 mg/day * | 8 weeks |
| Test food (PDL-0101): 1.8 g/day eicosapentaenoic acid, 100 mg/day tocopherol-freeγ/δ tocotrienols enriched with geranylgeraniol, extracted from annatto, and 12 mg/day AX. After 8 weeks of treatment, PDL-0101 significantly reduced median TAG compared to placebo (−9.5% vs. 10.6%, p < 0.001), but there was no significant change in mean LDL-C (−3.0% vs. −8.0% for PDL-0101 and placebo, respectively, p = 0.071), no significant change in mean high-density lipoprotein cholesterol (approximately 3% reduction in both groups, p = 0.732), or median oxidized LDL concentration (5% vs. −5% for PDL-0101 and placebo, respectively, p = 0.112). No safety concerns were identified in this study. |
| Takemoto M. et al. 2015 [697] | Case report | 1 Werner syndrome patient | 12 mg/day * | 6 months |
| Improved blood transaminase concentrations before AX intervention and 3 and 6 months after initiation were AST 40 IU/L, 41 IU/L, and 20 IU/L; ALT 69 IU/L, 62 IU/L, and 34 IU/L; GGT 38 IU/L, 41 IU/L, and 35 IU/L; and cholinesterase 360 IU/L, 366 IU/L, and 331 IU/L, respectively. Liver-to-spleen Hounsfield units on CT were 0.41 before AX initiation, 0.71 at 3 months, and 0.94 at 6 months. No significant changes after AX intervention in hyaluronic acid, a marker of liver fibrosis; high-sensitivity C-reactive protein, a marker of inflammation; or MDA-modified LDL. No safety concerns were identified in this study. |
| Ni Y. et al. 2015 [772] | Randomized, single-blind, placebo-controlled, prospective study | 12 NASH patients | 0, 12 mg/day | 24 weeks |
| Improved steatosis (p < 0.05), marginally improved lobular inflammation (p = 0.15), and NAFLD activity score (p = 0.08) No safety concerns were identified in this study. |
| Choi H.D. et al. 2011 [698] | Randomized, two-arm, prospective study | 23 obese and overweight subjects | 5 and 20 mg/day | 3 weeks |
| 5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, SOD increased by 193%, and TAC increased by 121% after 3 weeks compared to baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared to baseline (p < 0.01). Decreased LDL cholesterol and ApoB. No safety concerns were identified in this study. |
| Choi, H.D. et al. 2011 [699] | Randomized, double-blind, placebo-controlled, prospective study | 27 overweight subjects | 0, 20 mg/day | 12 weeks |
| MDA reduced by 17.3% and 29% after 8 and 12 weeks compared to placebo (p < 0.01), ISP reduced by 40.2% and 52.9% after 8 and 12 weeks compared to placebo (p < 0.01), SOD increased by 124.8% after 12 weeks compared to placebo (p < 0.01), and TAC increased by 130.1% after 12 weeks compared to placebo (p < 0.05). No safety concerns were identified in this study. |
| Yoshida H. et al. 2010 [773] | Randomized, double-blind, placebo- controlled, prospective study | 61 non-obese subjects with fasting serum triglyceride of 120–200mg/dl and without diabetes and hypertension | 0, 6, 12, 18 mg/day | 12 weeks |
| Multiple comparison: triglycerides were significantly decreased by 12 and 18 mg/day, and HDL-cholesterol was significantly increased by 6 and 12 mg. Serum adiponectin was increased by AX (12 and 18 mg/day), and changes in adiponectin were positively correlated with changes in HDL-cholesterol. No safety concerns were identified in this study. |
| Satoh A. et al. 2009 [774] | Open-labeled, prospective study | 20 subjects at risk for developing metabolic syndrome (from 127 healthy subjects) | 4, (8, 20) mg/day | 4 weeks |
| When subjects who met the diagnostic criteria for metabolic syndrome in Japan (SBP ≥ 130 mmHg, DBP ≥ 85 mmHg, TG ≥ 150 mg/dL, FG ≥ 100 mg/dL) at the start of the study were selected from the 4mg group, there was a significant decrease in SBP (p < 0.01). On the other hand, there was no significant decrease in DBP. Reduced TG after treatment (218 mg/dL) than the baseline value (292 mg/dL), marginally reduced fasting glucose after the intervention (p < 0.1). No safety concerns were identified in this study |
| Uchiyama A. et al. 2008 [775] | Open-labeled, prospective study | 17 subjects at risk for developing metabolic syndrome | 8 mg twice day | 3 months |
| Significant decreases in plasma HbAlc (p = 0.0433) and TNF-α levels (p = 0.0022) and an increase in adiponectin concentration (p = 0.0053). N.S: body weight, BMI, and waist circumference. The blood concentration reached the plateau after a month of treatment and was retained at that level until 3 months of treatment (0.2–0.25 μg/mL (0.34–0.42 μmol/L)). No safety concerns were identified in this study. |
| Evaluation of efficacy in cardiovascular diseases (CVDs) patients | ||||||
| Heidari M. et al. 2023 [776] | Randomized, double-blind, placebo-controlled prospective study | 44 CAD patients (40–65 yrs.), angiographic evidence of 50% stenosis in at least one of the major coronary arteries, 25 < BMI <35 | 0, 12 mg/day | 8 weeks |
| 12 mg of AX or placebo (microcrystalline cellulose) groups along with a low-calorie diet for a period of 8 weeks. Significant reductions in total cholesterol (−14.95 ± 33.57 mg/dL, p < 0.05) and LDL-C (−14.64 ± 28.27 mg/dL, p < 0.05) in the AX group with coronary artery disease (CAD). However, TG and HDL-C levels could not be affected by AX did not change “serum” levels of Sirtuin1 and TNF-α, Body composition, or glycemic indices. (Comments: BMI 25–27, mild obesity, non-insulin resistance with normoglycemia, normal TC, slightly elevated TG, and very low HDL-Chol. The effect on body composition seems to be more influenced by the low calorie diet. The clinical significance of the serum-free form of Sirt-1 remains unclear. No safety concerns were identified in this study. |
| Ishiwata S. et al. 2021 [692] | Open-labeled, prospective study | 17 patients with systolic heart failure | 12 mg/day * | 3 months |
| After 3 months of AX supplementation, the ”Specific Activity Scale” score increased from a median of 4.5 (interquartile range, 2.0) to 6.5 (interquartile range, 1.1) metabolic equivalents (p = 0.001), and the physical and mental component summary scores increased from 46.1 ± 9.2 to 50.8 ± 6.8 (p = 0.015) and 48.9 ± 9.1 to 53.8 ± 4.8 (p = 0.022), respectively. There was a linear relationship between baseline heart rate or mental component summary score and rate of change in the “Specific Activity Scale” score (r = 0.523, p = 0.031, r = −0.505, p = 0.039, respectively). Furthermore, there was a direct relationship between ischemic etiology and the rate of change in the physical component summary score (r = 0.483, p = 0.049, respectively). There was also a linear relationship between the rate of change in the “Specific Activity Scale” score and the rate of change in the mental component summary score (r = 0.595, p = 0.012). No safety concerns were identified in this study. |
| Kato T. et al. 2020 [694] | Open-labeled, prospective study | 16 patients with systolic heart failure | 12 mg/day * | 3 months |
| Increased left ventricular ejection fraction (LVEF) from 34.1 ± 8.6% to 38.0 ± 10.0% (p = 0.031), and the 6-min walk distance increased from 393.4 ± 95.9 m to 432.8 ± 93.3 m (p = 0.023). Significant relationships were observed between percent changes in dROM level and those in LVEF. No safety concerns were identified in this study. |
| Marazzi G et al. 2017 [777] | Randomized, single-blind, placebo-controlled, prospective study | 100 patients with CVD, percutaneous coronary intervention in the past 12 months, high-dose statin intolerance, and LDS treatment alone did not reduce LDL-C by more than 50%. | 0, 0.5 mg/day * (Armolipid Plus) | 12 months |
| The aim was to compare the efficacy and tolerability of low-dose statin (LDS) therapy versus combined therapy of LDS plus a nutraceutical combination (Armolipid Plus: red yeast rice (200 mg), policosanol (10 mg), berberine (500 mg), folic acid (0.2 mg), AX (0.5 mg), and coenzyme Q10 (2 mg) After 3 months, LDL-C and total cholesterol were significantly lowered in the LDS + Almolypid Plus (n = 50) group (p < 0.0001), and 70% of this group achieved the treatment goal (LDL-C <70 mg/dL), while patients in the LDS group did not. No safety concerns were identified in this study. |
| Evaluation of efficacy in postmenopausal females | ||||||
| Iwabayashi M. et al. 2009 [700] | Open-labeled, prospective study | 35 healthy female subjects (with high oxidative stress, postmenopausal) | 12 mg/day | 8 weeks |
| Increased blood biological antioxidant potential (Biological Antioxidant Potential (BAP); +4.6%, p < 0.05). After eight-week treatment with astaxanthin, significant improvement was observed in 5 of the 34 physical symptoms listed in the common questionnaire, including “tired eyes”, “stiff shoulders”, “constipation”, “gray hair”, and “cold skin”, and in 3 of the 21 mental symptoms, including “daily life is not enjoyable”, “difficulty falling asleep”, and “a sense of tension”. In addition, systolic (p = 0.021) and diastolic blood pressure (p < 0.001) significantly decreased. No safety concerns were identified in this study. |
| Kim Y.K. et al. 2004 [701] | Open-labeled, prospective study | 15 healthy postmenopausal females | 0, 2, 8 mg/day | 8 weeks |
| Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostane excretion did not decrease significantly. Increase HDL-cholesterol levels in 2 mg and 8mg group increased significantly after 8 weeks from 50.6 ± 5.8 to 60.4 ± 7.1 mg/dL, 44.4 ± 10.7 to 49.4 ± 2.7 mg/dL respectively (p < 0.05). In the 2mg group, triglyceride decreased significantly from 171.6 ± 67.4 mg/dL to 145.8 ± 5.1 mg/dL (p < 0.05). No safety concerns were identified in this study. |
| Evaluation of efficacy in healthy subjects | ||||||
| Takami M. et al. 2019 [778] | Open-labeled, prospective study | 20 healthy young male subjects | c.a, 4.5 mg/day * from salmon | 4 weeks |
| Increased maximum work load by training in both groups (p = 0.009), while increased oxygen consumption during exercise in the antioxidant group only (p = 0.014). There were positive correlations between maximum work load and fat (r = 0.575, p = 0.042) and carbohydrate oxidations (r =0.520, p = 0.059) in the antioxidant group. Higher carbohydrate oxidation during rest in the post-training than that in the pre-training only in the antioxidant group. More decreased levels of serum insulin and HOMA-IR after training were observed in the antioxidant group than in the control group. No safety concerns were identified in this study. |
| Fukamauchi M. et al. 2007 [779] | Randomized, double-blind, placebo- controlled, prospective study | 32 healthy subjects | 0, 6 mg/day | 6 weeks |
| The synergistic effects of AX intake (12 mg/day, 6 weeks) and aerobic exercise (walking) were studied. AX contributed to the reduction of body fat and suppressed the increase in blood lactate levels after exercise. No safety concerns were identified in this study. [Article in Japanese] |
| Others | ||||||
| Miyawaki H. et al. 2008 [686] | Single-blind, placebo-controlled prospective study | 20 healthy subjects | 0, 6 mg/days | 10 days |
| The time for blood to pass through the microchannel array flow analyzer decreased from 52.8 ± 4.9 s to 47.6 ± 4.2 s in the AX group (p < 0.01), and a significant difference was found when comparing values in the AX group (47.6 ± 4.2 s) and placebo group (54.2 ± 6.7 s) (p < 0.05). No safety concerns were identified in this study. |
| (E) Exercise/Sports performance, skeletal | ||||||
| Author/year/ reference | Study Design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of efficacy in exercise/sports performance in healthy subjects/athletes (muscular damages) | ||||||
| Barker G.A. et al. 2023 [780] | Double-blind, placebo-controlled prospective study | 19 Resistance-trained males | 0, 12 mg/day | 4 weeks |
| Significantly decreased in delayed onset muscle soreness (SORE score (p = 0.02)) for the AX group post-supplementation compared to pre-supplementation test. There is no effect on Placebo. Significantly decreased in VAS (visual analog scale; p = 0.01) for the AX group post-supplementation compared to the pre-supplementation test, whereas these had no effect in the Placebo group. No effect on performance. No safety concerns were identified in this study. |
| Nieman, D.C. et al. 2023 [781] | Randomized, double-blind, placebo-controlled crossover study | 18 healthy subjects (Capable of running 2.25 h on laboratory treadmills at 70% maximal oxygen consumption rate (VO2max)) | 0, 8 mg/day | 4 weeks |
| The running bout for 2.25 h induced significant muscle soreness, muscle damage, and inflammation; AX supplementation had no effect on exercise-induced muscle soreness, muscle damage, or increases in 6 plasma cytokines and 42 oxylipins. However, AX supplementation inhibited exercise-induced decreases in 82 plasma proteins (at 24 h post-recovery). Most of these proteins were involved in immune-related functions. Twenty plasma immunoglobulins were identified that differed significantly between the AX and placebo groups; plasma levels of IgM were significantly reduced after exercise but recovered in the AX trial but not in the placebo trial after a 24-h recovery period after exercise. No safety concerns were identified in this study. |
| Sudo A et al. 2019 [737] | Placebo-controlled prospective study | 19 healthy subject (College softball player for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil). Studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in college softball players. Subjective symptoms were evaluated with the VAS. The change of VAS in PRE and POST in the V7 group showed statistically significant improvement in 50 m running performance; a comparison of V7 and placebo POST showed statistically significant increases in leg fatigue, knee, and hip pain. The percent change between PRE and POST in V7 was statistically significantly higher in leg fatigue, hip and back pain, and total score. No safety concerns were identified in this study. [Article in Japanese] |
| Baralic, I. et al. 2015 [712] | Randomized, double-blind, placebo-controlled, prospective study | 40 healthy subjects (young soccer players) | 0, 4 mg/day | 90 days |
| The increase in neutrophil count and hs-CRP level was found only in the placebo group, indicating a significant blunting of the systemic inflammatory response in the subjects taking AX. Improved prooxidant-antioxidant balance (PAB; p < 0.05) AX supplementation improves the sIgA response and attenuates muscle damage. No safety concerns were identified in this study. |
| Baralic I. et al. 2013 [713] | Randomized, double-blind, placebo-controlled prospective study | 40 healthy subjects (soccer players) | 0, 4 mg/day | 90 days |
| Protected thiol groups against oxidative modification (increase in SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) No safety concerns were identified in this study. |
| Djordjevic B. et al. 2013 [714] | Randomized, double-blind, placebo-controlled prospective study | 32 healthy subjects (soccer players) | 0, 4 mg/day | 90 days |
| Regular training significantly increased O2•¯ levels (main training effect, p < 0.01). O2•¯ concentrations. Not change TBARS and AOPP levels throughout the study. Decreased TAS levels post- exercise only in the Placebo group (p < 0.01). The increased total SH group content both in the AX and in the placebo groups (by 21% and 9%, respectively) and supplementation effect were marginally significant (p = 0.08). Decreased basal SOD activity both in the Placebo and in the AX group by the end of the study (main training effect, p < 0.01). Significant decrease in basal CK and AST activities after 90 days (main training effect, p < 0.01 and p < 0.001, respectively). Post-exercise CK and AST levels were significantly lower in the AX group compared to the Placebo group (p < 0.05) No safety concerns were identified in this study. |
| Klinkenberg L.J. et al. 2013 [715] | Randomized, double-blind, placebo-controlled, prospective study | 32 well-trained male cyclists (25 ± 5 years, V˙O2peak = 60 ± 5 mL·kg−1·min−1, Wmax = 5.4 ± 0.5 W·kg−1) | 0, 20 mg/day * | 4 weeks |
| N.S; effect on exercise-induced cardiac troponin T release (p = 0.24), changes in antioxidant capacity markers (trolox equivalent antioxidant capacity, uric acid, and malondialdehyde). Markers of inflammation (high-sensitivity C-reactive protein) and exercise-induced skeletal muscle damage (creatine kinase). No safety concerns were identified in this study. |
| Djordjevic B. et al. 2011 [714] | Randomized, double-blind, placebo-controlled, prospective study | 32 male elite soccer players | 0, 4 mg/day | 90 days |
| Changes in elevated O2-¯ concentrations after soccer exercise were statistically significant only in the Placebo group (exercise × supplementation effect, p < 0.05); TAS values decreased significantly only in the Placebo group after exercise (p < 0.01). After intervention, total SH group content increased (21% and 9%, respectively), and the effect of AX was marginally significant (p = 0.08). Basal SOD activity was significantly reduced in both the Placobo and AX groups at the end of this study (main training effect, p < 0.01).Post-exercise CK and AST levels were significantly lower in the AX group than in the Placebo group (p < 0.05) No safety concerns were identified in this study. |
| Bloomer, R.J. et al. 2005 [717] | Randomized, placebo-controlled, prospective study | 20 resistance trained male subjects (25.1 ± 1.6 years) | 0, 4 mg/day * | 3 months |
| N.S; Muscle soreness, creatine kinase (CK), and muscle performance were measured before and through 96 h of eccentric exercise No safety concerns were identified in this study. |
| Sawaki et al. 2002 [760] | Randomized, double-blind, placebo-controlled, prospective study | 18 healthy male Subjects (College handball player) | 0, 6 mg/day | 4 weeks |
| Although there was no difference between the two groups in post-exercise CK levels or heart rate, the AX group had significantly lower blood lactate levels 2 min after exercise. No safety concerns were identified in this study. [Article in Japanese] |
| Evaluation of efficacy in exercise/sports performance in healthy subjects/athletes (performance/energy metabolisms) | ||||||
| Waldman H.S. et al. 2023 [782] | Randomized, double-blind, placebo-controlled crossover study | Resistance-trained males (Mean 23.4 yrs.) | 0, 12 mg/day | 4 weeks |
| AX supplementation had no statistical effect on markers of substrate metabolism, Wingate variables, or markers of muscle damage, inflammation, or delayed-onset muscle soreness during the graded exercise test (GXT) compared to placebo (p > 0.05). However, 4 weeks of AX supplementation significantly reduced oxygen consumption during the final phase of the GXT compared to placebo (12%, p = 0.02), reduced systolic blood pressure (approximately 7%, p = 0.04), and significantly reduced baseline insulin levels (c.a. 24%, p = 0.05). No safety concerns were identified in this study. |
| McAllister MJ. et al. 2022 [711] | Randomized, double-blind, placebo-controlled crossover study | 14 healthy subjects (23 ± 2 yrs) | 0, 6 mg/day | 4 weeks |
| A graded exercise test was performed after each treatment to measure substrate utilization during exercise at increased intensity. Glutathione was approximately 7% higher after AX treatment compared to placebo (p = 0.02, d = 0.48). Plasma hydrogen peroxide and malondialdehyde (MDA) did not differ between treatments (p > 0.05). Although not statistically significant (p = 0.45), highly oxidized protein products were reduced by approximately 28%. In the graded exercise stress test, mean fat oxidation rates did not differ between treatments (p > 0.05); however, fat oxidation was reduced from 50 to 120 W (p < 0.001) and from 85 to 120 W (p = 0.004) in both conditions. No safety concerns were identified in this study. |
| Brown, R.D. et al. 2021 [783] | Randomized, double-blind, placebo-controlled crossover study | 12 recreationally trained male cyclists (27.5 ± 5.7 years, VO2peak: 56.5 ± 5.5 mL⋅kg−1⋅min−1, Wmax: 346.8 ± 38.4 W) | 0, 12 mg/day | 7 days |
| Completion time of the 40-km cycling time trial improved by 1.2 ± 1.7% with AX supplementation, from 70.76 ± 3.93 min in the placebo condition to 69.90 ± 3.78 min in the AX condition (mean improvement time = 51 ± 71 s, p = 0.029, g = 0.21). Whole body fat oxidation rate was also greater in the AX group between 39 and 40 km (+0.09 ± 0.13 g ⋅ min−1, p = 0.044, g = 0.52) and respiratory exchange ratio was lower (−0.03 ± 0.04, p = 0.024, g = 0.60). No safety concerns were identified in this study. |
| Kawamura A. et al. 2021 [710] | Randomized open-labeled, prospective study | 26 healthy male subject (22.3 ± 0.3 yrs.) | N/A (1 mg AX/100g salmon) * | 10 weeks |
| The skeletal muscle mass was higher after training than before training in both the control and intervention groups (p < 0.05). Increased maximal voluntary contraction after training in the intervention group (p < 0.05), but not significantly increased in the control group. Higher resting oxygen consumption after training in the intervention group only (p < 0.05). Serum carbonylated protein level as an oxidative stress marker tended to be lower immediately after exercise than before exercise in the intervention group only (p = 0.056). No safety concerns were identified in this study. |
| McAllister M.J. et al. 2021 [711] | Randomized, double-blind, placebo-controlled, crossover study | 14 healthy young subjects, (23 ± 2 yrs.) | 0, 6 mg/day | 4 weeks |
| Glutathione was ~7% higher following AX compared with placebo (p < 0.05). There was no effect on plasma hydrogen peroxide or MDA (p > 0.05). Advanced oxidation protein products (AOPP) were reduced by ~28% (N.S.; p = 0.45) not affect substrate utilization during exercise. No safety concerns were identified in this study. |
| Fleischmann C. et al. 2019 [784] | Randomized, double-blind, placebo-controlled prospective study | 22 healthy subjects (23.1 ± 3.5 yrs.) | 0, 12 mg/day | 30 days |
| Decreased raised blood lactate caused by the VO2 Max test in the AX group (9.4 ± 3.1 and 13.0 ± 3.1 mM in the AX and Placebo groups, respectively, p < 0.02). Change in oxygen uptake during recovery (−2.02 ± 0.64 and 0.83 ± 0.79% of VO2 Max in the AX and Placebo groups, respectively, p = 0.001). N.S; anaerobic threshold or VO2 Max. physiological or biochemical differences in the heat tolerance test (HTT) (2 h walk at 40 °C, 40% relative humidity. No safety concerns were identified in this study. |
| Sudo A et al. 2020 [736] | Placebo-controlled prospective study | 11 healthy subjects (College floorball athletes for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil). Studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in floorball athletes. Subjective symptoms were evaluated with the VAS. The change in VAS in PRE and POST in the V7 group showed that fatigue was significantly alleviated overall and in the torso. Significantly improved on a seated toe touch, increasing from 48.6 cm pre-intake to 51.8 cm post-intake. No safety concerns were identified in this study. [Article in Japanese] |
| Takami M. et al. 2019 [778] | Open-labeled, prospective study | 20 healthy young male subjects | c.a, 4.5 mg/day * from salmon | 4 weeks |
| Increased maximum work load by training in both groups (p = 0.009), while increased oxygen consumption during exercise in the antioxidant group only (p = 0.014). There were positive correlations between maximum work load and fat (r = 0.575, p = 0.042) and carbohydrate oxidations (r =0.520, p = 0.059) in the antioxidant group. Higher carbohydrate oxidation during rest in the post-training than that in the pre-training only in the antioxidant group. No safety concerns were identified in this study. |
| Talbott I. et al. 2016 [785] | Randomized, double-blind, placebo-controlled prospective study | 28 recreational runners (42 ± 8 yerars) | 0, 12 mg/day | 8 weeks |
| Reduced average heart rate at submaximal endurance intensities (aerobic threshold, AeT, and anaerobic threshold, AT), but not at higher “peak” intensities. No safety concerns were identified in this study. |
| Res T. et al. 2013 [716] | Randomized, double-blind, placebo-controlled, prospective study | 32 trained male cyclists or triathletes (25 ± 1 years, V˙O2peak = 60 ± 1 mL·kg−1·min−1, Wmax = 395 ± 7 W) | 0, 20 mg/day | 4 weeks |
| N.S; total plasma antioxidant capacity (p = 0.90) or attenuated malondialdehyde levels (p = 0.63). Whole-body fat oxidation rates during submaximal exercise (from 0.71 +/− 0.04 to 0.68 ± 0.03 g.min and from 0.66 ± 0.04 to 0.61 ± 0.05 g.min in the Placebo and AX groups, respectively; p = 0.73), time trial performance (from 236 ± 9 to 239 ± 7 and from 238 ± 6 to 244 ± 6 W in the Placebo and AX groups, respectively; p = 0.63). No safety concerns were identified in this study. |
| Earnest C.P. et al. 2011 [786] | Randomized, double-blind, placebo-controlled, prospective study | 14 amateur endurance-trained subjects (18–39 years, V˙O2peak = 52.84 ± 3.5 mL·kg−1·min−1, Wmax = 330 ± 26 W | 0, 4 mg/day | 28 days |
| Improved performance in the 20-km cycling time trial in the AX group (n = 7, −121 s; 95% CI, −185, −53), but not in the Placebo group (n = 7, −19 s; 95% CI, −84, 45). The AX group significantly increased power output (20 W; 95% CI, 1, 38), while the Placebo group did not (1.6 W; 95% CI, −17, 20). N.S: carbohydrate, fat oxidation, and blood indices indicative of fuel mobilization. No safety concerns were identified in this study. |
| Malmstena C.L.L. et al. 2008 [787] | Randomized, double-blind, placebo-controlled prospective study | 40 young healthy subjects (17–19 years) | 0, 4 mg/day | 3 months |
| The increased average number of knee bending (squats) increased by 27.05 (from 49.32 to 76.37, AX group) vs. 9.0 (from 46.06 to 55.06, placebo subjects), p = 0.047. No safety concerns were identified in this study. |
| Tajima T. et al. 2004 [788] | Randomized, double-blind, placebo-controlled, crossover study | 18 healthy subjects (35.7 ± 4 years) | 0, 5 mg/day | 2 weeks |
| Increases in CVRR and HF/TF (Heart rate variability) were significant during exercise at 70% maximum heart rate (HRmax) intensity (p < 0.05). Moreover, after the AX supplementation there was, decreased minute ventilation (VE) during exercise at 70% HRmax (p < 0.05). Decreased LDL cholesterol (p < 0.05) and respiratory quotient after exercise. No safety concerns were identified in this study. [Article in Japanese] |
| Evaluation of efficacy in exercise performance in elderly subjects (sarcopenia) | ||||||
| Liu S.Z. et al. 2021 [789] | Randomized, double-blind, placebo-controlled, prospective study | 42 elderly subjects (65–82 yrs.) | 0, 12 mg/day * | 12 weeks |
| Intervention: 6 mg Zn, 10 mg tocotrienol and 12 mg AX. In endurance training (ET), specific muscular endurance was improved only in the AX group (Pre 353 ± 26 vs. Post 472 ± 41), and submaximal graded exercise test duration was improved in both groups (Placebo 40.8 ± 9.1% vs. AX 41.1 ± 6.3%). The increase in fat oxidation at low intensity after ET was greater in AX (Placebo 0.23 ± 0.15g vs AX 0.76 ± 0.18g) and was associated with reduced carbohydrate oxidation and improved exercise efficiency in men but not in women. Analysis associated with the study [790]. No safety concerns were identified in this study. |
| Nakanishi R. et al. 2021 [718] | Randomized, double-blind, placebo-controlled prospective study | 29 nursing home resident’s healthy elderly subjects (80.9 ± 1.5 yrs.) | 0, 12 mg/twice a day * (0, 24 mg/day) | 16 weeks |
| There was a decrease in d-ROM values with the AX group (p < 0.01) but not with the placebo group; the AX group had a therapeutic effect on 6-min walking distance compared with the placebo group (p < 0.05). The AX group had an increase in distance and number of steps in the 6-min walking test compared to the placebo group. Furthermore, the rate of increase in blood lactate levels after walking was lower in the AX group than in the placebo group (p < 0.01). No safety concerns were identified in this study. |
| Liu S.Z. et al. 2018 [790] | Randomized, double-blind, placebo-controlled, prospective study | 42 elderly subjects (65–82 yrs.) | 0, 12 mg/day * | 12 weeks |
| Administration of AX increased maximal voluntary force (MVC) by 14.4% (± 6.2%, p < 0.02), tibialis anterior muscle size (cross-sectional area, CSA) by 2.7% (±1.0%, p < 0.01), and specific impulse increased by 11.6% (MVC/CSA, ±6.0%, p = 0.05), respectively, whereas placebo treatment did not alter these characteristics (MVC, 2.9% ± 5.6%; CSA, 0.6% ± 1.2%; MVC/CSA, 2.4 ± 5.7%; all p > 0.6). No safety concerns were identified in this study. |
| Evaluation of efficacy in joint health (osteoarthritis (OA), carpal tunnel syndrome) | ||||||
| Stonehouse W et al. 2022 [791] | Randomized, double-blind, placebo-controlled prospective study | 235 Healthy adults (40–65 yrs, BMI >18.5 to <35 ) w/.clinically diagnosed with mild to moderate knee OA | 0, 0.35 mg/day (in krill oil) | 6 months |
| Knee pain scores (Western Ontario and McMaster Universities Osteoarthritis Index: WOMAC, numeric scale) improved in both groups, with greater improvement with krill oil than placebo. Knee stiffness and physical function also showed greater improvement with krill oil than placebo. NSAID usage, serum lipid profile, inflammatory markers, and safety markers did not differ between groups. No safety concerns were identified in this study. |
| Macdermid J.C. et al. 2012 [792] | Randomized, triple-blind, placebo-controlled prospective study | 63 patients with carpal tunnel syndrome | 0, 4 mg/days with splinting | 9 weeks |
| The Symptom Severity Scale (SSS) over the course of treatment in both AX treated and placebo groups (p = 0.002) showed no differences between the groups (p = 0.18). The Disability of Arm, Shoulder, and Hand Questionnaire and the Short Form 36-item Health Survey showed no effects over time or between treatment groups. No safety concerns were identified in this study. |
| (F) Brain Health | ||||||
| Author/year/ reference | Study design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of efficacy in cognitive functions | ||||||
| Hayashi M. et al. 2018 [793] | Randomized, double-blind, placebo-controlled prospective study | 54 healthy subjects (45–64 yrs.) | 0, 8 mg/day from Paracoccus | 8 weeks |
| (After 8 weeks of AX administration, the serum AX concentration increased to 0.173 ± 0.058 μg/mL (0.29 μM).( Not detected in the placebo group.) Evaluation: word memory test, verbal fluency test, and Stroop test. AX group significantly larger increase in blood AX level. No significant intergroup differences in the results of the evaluations. Subgroup analysis (<55 yrs. old and ≥55 yrs. old): “words recalled after 5 min” in word memory test in <55 year old subjects showed significant improvement in the AX group than in the placebo group, which was not found in ≥55 year old subjects. No safety concerns were identified in this study. |
| Hongo N. et al. 2018 [794] | Randomized, double-blind, placebo-controlled prospective study | 40 Healthy subjects aged 60–79 years reporting awareness of cognitive and/or physical decline (Mean 65.8 yrs.) | 0, 12 mg/day * | 12 weeks |
| AX group (12 mg AX, 10 mg tocotrienols, 6 mg zinc, and 600 IU vitamin D) and control group (same formulation w/o. AX). Cognitive functions/Fatigues: AX intake shortened the reaction time in working-memory tasks (particularly improved the speed of processing newly-provided visual information by comparing it with previously-provided information while appropriately retaining the previous information in the memory for several seconds). In addition, AX promoted efficacy in the improvement of self-assessed memory ability. Furthermore, in the subjects with improved endurance, AX enhanced the recognition memory function of nonverbal information, particularly the accuracy of memorizing faces and distinguishing them from newly presented faces. Moods/Stress: Improved the scores of “Anger-Hostility,” “Confusion-Bewilderment,” “Fatigue-Inertia,” and TMD in the POMS survey and the decrease in the score of “irritated” in the VAS survey in the AX group No safety concerns were identified in this study. [Article in Japanese] |
| Ito N. et al. 2018 [795] | Randomized, double-blind, placebo-controlled, prospective study | 14 patients diagnosed with MCI (57–78 yrs.; MMSE scores 24–27). | 0, 6 mg/day * | 12 weeks |
| Evaluation of cognitive improvement in patients with mild cognitive impairment (MCI). The Central Nervous System Vital Signs (CNSVS, also known as ‘Cognitrax’) test significantly improved “psychomotor speed” and “processing speed” in the AX group compared to the placebo group. No safety concerns were identified in this study. |
| Zanotta D. et al. 2014 [796] | Open-labeled, prospective study | 104 subjects diagnosed with mild cognitive impairment (Mean 71.2 yrs.) | c.a. 4.2 mg/day * | 60 days |
| Test supplement (Illumina); 20mg Bacosides from Bacopa monnieri, 30 mg Phosphatidylserine, 30 mg V.E and 2 mg AX. Significantly improved in the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) total score from 13.7 ± 5.8 at baseline to 9.7 ± 4.9 at 60 days and in the clock drawing test from 8.5 ± 2.3 to 9.1 ± 1.9. The greatest improvement in each component of the ADAS-cog was in the memory task. The largest improvement in each component of the ADAS-cog was in the memory tasks. In multivariate analysis, larger improvements in ADAS-cog scores were associated with less deterioration in baseline Mini-Mental State Examination scores. Efficacy was rated “excellent” or “good” by 62% of subjects. The study compounds were well tolerated, with one non-serious adverse event (gastric disturbances in a subject who was taking concomitant oral corticosteroids) reported in the entire study population and tolerability rated as “excellent” or “good” by 99% of subjects. |
| Katagiri M. et al. 2012 [797] | Randomized, double-blind, placebo-controlled, prospective study | 89 healthy middle-aged and elderly subjects who complained of age-related forgetfulness. | 0, 6, 12 mg/day | 12 weeks |
| After 12 weeks, CogHealth battery scores improved in the high-dose group (12 mg AX/day). Improved Groton Maze Learning Test scores were seen earlier in the low-dose group (6 mg AX/day) and in the high-dose group than in the placebo group. However, the sample size was too small to show significant differences in cognitive function between the AX and placebo groups. No safety concerns were identified in this study. |
| Satoh A. et al. 2009 [774] | Open-labeled, prospective study | 10 healthy male subjects (50–69 yrs.) | 12 mg/day | 12 weeks |
| In the CogHealth tasks (simple response, choice response, working memory, delayed memory, and divided attention), significant reductions in reaction time were observed in the “divided attention” task after 6 weeks and in all tasks after 12 weeks. Accuracy on the “working memory” task was significantly improved after 12 weeks of treatment; however, no such effect was observed on the “delayed recall” task. No safety concerns were identified in this study |
| Evaluation of efficacy in fatigue | ||||||
| Yamazaki I. et al, 2022 [798] | Randomized, double-blind, placebo-controlled prospective study | 257 subjects feeling fatigue with aging and on daily (Av. 44–45 yrs.) | 0, 6 mg/day * | 8 weeks |
| Combination with sesamins (sesame lignans). Visual Analogue Scale (VAS) scores of the fatigue feeling at 4 weeks in the test food groups compared to the placebo food groups (p < 0.05), and these scores tended to be lower in the test food groups than the placebo food groups at 8 weeks (p < 0.1). Subgroup analysis: (A) There was a trend toward greater improvement in the group with a greater VAS value at screening than in the group with a smaller VAS value for fatigue at screening. (B)The frequency of exercise at screening was divided into two groups: “most/several times a month” and “at least once a week/every day.” The “at least once a week/every day” group, which exercised more frequently, showed more improvement in the VAS. No safety concerns were identified in this study. [Article in Japanese] |
| Sudo A et al. 2020 [736] | Placebo-controlled prospective study | 11 healthy subjects (College floorball athletes for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil). Studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in floorball athletes. Subjective symptoms were evaluated with the VAS. The change in VAS in PRE and POST in the V7 group showed that fatigue was significantly alleviated overall and in the torso significantly improved on a seated toe touch, increasing from 48.6 cm pre-intake to 51.8 cm post-intake. No safety concerns were identified in this study. [Article in Japanese] |
| Sudo A et al. 2019 [750] | Open-labeled, prospective study | 19 healthy females (Mean 47.3 yrs.) | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil). Studied the efficacy of V7 on subjective fatigue and skin conditions in typical middle-aged females. Subjective symptoms were evaluated with the VAS. The change of VAS in PRE and POST in the V7 group showed statistically significantly improved general fatigue, leg fatigue, and the state of the lower back (p < 0.05, respectively); significantly improved dark spots, blotches, and the elasticity and appearance of the skin; no significant change in eye strain or memory loss. No safety concerns were identified in this study. [Article in Japanese] |
| Sudo A et al. 2019 [737] | Placebo-controlled prospective study | 19 healthy subject (College softball player for women in physical education | 0.3 mg/day * in V7 | 30 days |
| Test supplement (V7; astaxanthin, reduced coenzyme Q10, leucine, arginine, citrulline, DHA, Krill oil). Studied the efficacy of V7 on subjective fatigue, sports performance, and skin conditions in college softball players. Subjective symptoms were evaluated with the VAS. The change of VAS in PRE and POST in the V7 group showed statistically significant improvement in fatigue, shoulder pain, skin blemishes, and 50 m running performance; a comparison of V7 and placebo POST showed statistically significant increases in leg fatigue, knee and hip pain, skin elasticity and whitening, memory loss, and eye strain. The percent change between PRE and POST in V7 was statistically significantly higher in leg fatigue, hip and back pain, dull skin, eye strain, and total score. No safety concerns were identified in this study. [Article in Japanese] |
| Imai A. et al. 2018 [722] | Randomized, double-blind, placebo-controlled crossover study | 42 healthy subjects | 0, 6 mg/day * | 4 weeks |
| Elevated PCOOH levels during mental and physical tasks were attenuated by AX supplementation. Improved recovery from mental fatigue compared to placebo. No differences were found between AX and placebo in other secondary outcomes such as subjective feelings, work efficiency, and autonomic activity. The treatment group showed a significant reduction (p < 0.05) in fatigue during recovery after the mental task compared to the placebo group. (Evaluated by VAS) No safety concerns were identified in this study. |
| Hongo N. et al. 2017 [723] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 12 mg/day * | 12 weeks |
| Intent-to-treat (ITT) analysis; fatigue after physical and mental stress was significantly lower in the AX group than in the placebo at week 8; the change in POMS friendliness was significantly higher in the AX group than in the control group at week 8; the rate of change in BAP values at week 12 was not significantly different between the AX and control groups. The rate of change in BAP values at week 12 was not significantly different between the AX group and the control group. No safety concerns were identified in this study. [Article in Japanese] |
| Kono K. et al. 2014 [751] | Randomized, double-blind, placebo-controlled prospective study | 48 healthy subjects who complained of eye strain | 0, 4 mg/day * | 4 weeks |
| See Kono K. et al. (2014) in Table 4 (C). [Evaluation of efficacy in “asthenopia (eyestrain)”] |
| Tsukahara H. et al. 2008 [753] | Open-labeled, prospective study | 13 healthy subjects with shoulder Stiffness | 6 mg/days * | 4 weeks |
| See Tsukahara H.et al. (2008) in Table 4 (C). Evaluation of efficacy in “asthenopia (eyestrain)”. |
| Evaluation of efficacy in stress/mood/sleep quality | ||||||
| Hayashi M. et al. 2020 [799] | Randomized, double-blind, placebo-controlled prospective study | 54 healthy subjects (Av. 45–46 yrs.) | 0, 12 mg/day from Paracoccus | 8 weeks |
| After 8 weeks of AX administration, the serum AX concentration increased to 0.171 ± 0.082 μg/mL (0.29 μM) not detected in the placebo group. Evaluated with the Profile of Mood States 2nd Edition for stress (POMS2) and the Oguri-Shirakawa-Azumi Sleep Inventory. I did not observe any significant intergroup differences in stress or sleep. Subgroup analysis (>65 and ≤65 in the ”Depression-Dejection”in POMS): sleep of subjects who scored >65 (”Depression-Dejection”) showed significant improvement in the AX group compared with the placebo group; however, no significant improvement was observed in stress or the other subjects. No safety concerns were identified in this study. |
| Hongo N. et al. 2018 [794] | Randomized, double-blind, placebo-controlled prospective study | 40 Healthy subjects aged 60–79 years reporting awareness of cognitive and/or physical decline (Mean 65.8 yrs.) | 0, 12 mg/day * | 12 weeks |
| See Hongo N.et al. (2018) in Table 4 (G). [Evaluation of efficacy in cognitive functions] |
| Imai A. et al. 2018 [722] | Randomized, double-blind, placebo-controlled crossover study | 42 healthy subjects | 0, 6 mg/day * | 4 weeks |
| See Imai A. et al. (2018) in Table 4 (G). [Evaluation of efficacy in cognitive functions] |
| Hongo N. et al. 2017 [723] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 12 mg/day * | 12 weeks |
| See Hongo N.et al. 2017 in Table 4 (G). [Evaluation of efficacy in fatigue] |
| Saito H et al. 2017 [800] | Randomized, double-blind, placebo-controlled prospective study | 120 healthy subjects | 0, 1.5, 3 mg/day * (1.5 mg from krill, 3.0 mg from Haematococcus algae) | 12 weeks |
| Subjects were divided into four groups: (A) placebo, (B) zinc-rich food, (C) zinc- and AX-rich food (krill), and (D) placebo supplemented with zinc-enriched yeast and AX oil (from Haematococcus algae). Pittsburgh sleep quality index (PSQI): improved significantly within all groups at the endpoint. Total sleep time (TST): No significant difference was noted in any groups in comparison to group A. Sleep onset latency (SOL): significantly improved in group B and group D in comparison to the placebo group. Body positional changes: Although the number increased in the group A (3.74 ± 4.38), this increase was suppressed in group B ( 0.71 ± 5.01), group C (0.58 ± 5.75) and group D ( 0.95 ± 4.33) (N.S.). No safety concerns were identified in this study. |
| Iwabayashi M. et al. 2009 [700] | Open-labeled, prospective study | 35 healthy female subjects (with high oxidative stress, postmenopausal) | 12 mg/day | 8 weeks |
| Increased blood biological antioxidant potential (Biological Antioxidant Potential (BAP); +4.6%, p < 0.05). After eight-week treatment with astaxanthin, significant improvement was observed in five of the 34 physical symptoms listed in the common questionnaire, including “tired eyes”, “stiff shoulders”, “constipation”, “gray hair”, and “cold skin”, and in 3 of 21 mental symptoms, including “daily life is not enjoyable”, “difficulty in falling asleep”, and “a sense of tension”. In addition, systolic (p = 0.021) and diastolic blood pressure (p < 0.001) significantly decreased. No safety concerns were identified in this study. |
| (G) Infertility | ||||||
| Author/year/ reference | Study design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Evaluation of efficacy in gynecological disorders and assisted reproductive technology (ART) | ||||||
| Jabarpour M. et al. 2023 [652] | Randomized, placebo-controlled prospective study | 53 Patients with polycystic ovary syndrome (PCOS) | 0, 6 mg/twice a day (0,12 mg/day) | 60 days |
| ER stress (Granulosa cells): After the intervention, AX treatment reduced the mRNA expression levels of 78-kDa glucose-regulated protein (GRP78), CCAAT/enhancer-binding protein homologous protein (CHOP), and X-box-binding protein 1 compared to the placebo group, but activated transcription factor 6 (ATF6) was not statistically significant. However, AX significantly increased the ATF4 expression level. GRP78 and CHOP protein levels represented a considerable decrease in the treatment group after the intervention. Antioxidant markers: Increased levels of TAC in follicular fluid. ART outcomes: higher rates of high-quality oocytes, high-quality embryos, and oocyte maturity in the AX group (the oocyte number, fertilization rate, and fertility rate; N.S.) No safety concerns were identified in this study. |
| Rostami S. et al. 2023 [653] | Randomized, triple-blind, placebo-controlled prospective study | 50 Patients of endometriosis (stage III/ IV) | 0, 6 mg/day | 12 weeks |
| Antioxidant markers: Increased serum levels of total antioxidant capacity (TAC, p = 0.004) and superoxide dismutase (SOD, 13.458 ± 7.276 vs. 9.040 ± 5.155; p = 0.010) were observed in the AX intervention group after therapy. In addition, serum Malondialdehyde (MDA, p = 0.031) decreased significantly after AX treatment. Inflammation markers: Serum IL-1β (p = 0.000), IL-6 (p = 0.024), and TNF-α (p = 0.038) showed significantly lower levels after AX treatment. ART outcomes: AX supplementation resulted in a significantly improved number of oocytes retrieved (p = 0.043), mature (MII) oocytes (p = 0.041), and improved quality embryos (p = 0.024). No safety concerns were identified in this study. |
| Gharaei R. et al. 2022 [651] | Randomized, double-blind, placebo-controlled prospective study | 40 Patients with polycystic ovary syndrome (PCOS) | 0, 8 mg/day | 40 days |
| AX supplementation resulted in significantly higher serum Catalase and TAC levels in the AX group compared to the placebo group. However, there were no significant differences in serum MDA and SOD levels between groups. The expression of antioxidant genes such as Nrf2, HO-1, and NQ-1 was significantly increased in the granulosa cells (GC) of the AX group.ART: MII oocyte and high quality embryo rates were significantly increased in the AX group compared to the placebo group. There were no significant differences in chemical and clinical pregnancy rates between groups. No safety concerns were identified in this study. |
| Nakayama T. et al. 2015 [801] | Open-labeled, prospective study | 18 subjects with normal menstrual cycles who have severe menstrual cramps or hypermenorrhea | 12 mg/day | 12 weeks |
| VAS values for menstrual cramps: After 12 weeks of AX supplementation it, decreased significantly with duration of supplementation (p < 0.05 vs. pre; 60.2 ± 16.3, 4 weeks; 45.3 ± 17.0, 12 weeks; 38.5 ± 19.0). After12 weeks washout, it increased to 55.0 ± 18.3 (p < 0.05 vs. 12 weeks). VAS values for menstrual flow: Significantly decreased at 12 weeks (p < 0.05, pre; 67.0 ± 15.7 vs. 12 weeks; 45.1 ± 20.3), but increased again after 12 weeks of washout (55.6 ± 16.6, p < 0.05 vs. 12 weeks). Day-dysmenorrhea: Significantly decreased at 12 weeks (p < 0.05, pre; 2.5 ± 1.8 vs. 12 weeks; 1.3 ± 0.8), but increased again after 12 of weeks washout (2.5 ± 2.0, p < 0.05 vs. 12 weeks). Others: No significant changes in the frequency of NSAID dosing or the blood levels of CA125 and Hb over the study period. No safety concerns were identified in this study. [Article in Japanese] |
| Evaluation of efficacy in improving sperm quality and assisted reproductive technology (ART) | ||||||
| Dede G et al. 2022 [802] | N/A | 30 Semen samples from normozoospermic individuals | 0, 50, 100, 500 μM | N/A |
| Purpose: the protective efficacy of AX against the damage that occurs during sperm cryopreservation. The loss of motility due to cryopreservation of sperm was highest in the control group, and the least loss of motility was seen in the 100 μM AX group. Chromatin condensation of spermatozoa showed that the number of condensed spermatozoa was higher in the 100 μM AX group than in the other groups. (in vitro study) |
| Ghantabpour T. et al. 2022 [734] | N/A | The first phase; 10 semen samples from healthy men, the second phase; 25 semen samples from healthy men | 0, 0.5, 1, 2 μM | N/A |
| Supplementation of sperm freezing medium with 1 µM AX was found to improve all parameters of sperm motility and viability (p ≤ 0.05). In addition, there were reduced levels of ROS parameters (intracellular hydrogen peroxide and superoxide) compared to the control group (p ≤ 0.05). AX also significantly reduced phosphatidylserine exogenous levels (p ≤ 0.05) and lipid peroxidation (p ≤ 0.05) after the freeze-thaw process. (in vitro study) |
| Kumalic SI. et al. 2020 [803] | Randomized, double-blind, placebo-controlled prospective study | 72 patients with oligo-astheno-teratozoospermia (Av. 35–36 yrs) | 0, 16 mg/day | 3 months |
| In theAX group, no improvements in the total number of spermatozoa, concentration of spermatozoa, total motility of spermatozoa, morphology of spermatozoa, DNA fragmentation, and mitochondrial membrane potential of spermatozoa, or serum follicle-stimulating hormone were determined in patients with oligo-astheno-teratozoospermia. No safety concerns were identified in this study. |
| Terai K. et al. 2019 [649] | Randomized, two-arm, open-labeled, prospective study | 31 males with oligozoospermia and/or asthenozoospermia | 0 (HE), 16mg/day * | 12 weeks |
| Intervention; Combination of antioxidant supplements (L-carnitine, Zn, CoQ10, vitamin C, vitamin B12, vitamin E, and AX) and a Chinese herbal medicine, hochu-ekki-to (HE). There were no significant improvements in endocrinological findings in the supplement group. Although no statistically significant improvement was observed in semen volume, sperm concentration, or sperm motility, total motile sperm counts were significantly improved. On the other hand, the HE group tended to increase semen concentration, semen motility, and total motile sperm count, but not significantly improve any endocrinological factors or semen findings. No safety concerns were identified in this study. |
| Andrisani A. et al., 2015 [804] | N/A | 24 Semen samples from healthy male donors of proven fertility, 27 Semen samples from patients who had failed to conceive after at least one year of regular unprotected intercourse | 0, 2 μM | N/A |
| Evaluation of sperm capacitation, which involves a series of modifications, including regulation of reactive oxygen species, depletion of cholesterol in the sperm outer membrane, and protein tyrosine phosphorylation (Tyr-P) processes in the head region, that acquire sperm for the essential functions for fertilization of oocytes. AX successfully triggered Lyn translocation in patient-derived sperm(PG), bypassing the impaired ROS-related mechanisms for raft and Lyn translocation. In this study, ROS generation, lipid raft, and Lyn relocation are interdependent, leading to a continuous cellular acrosome reaction (AR). AX could be potentially used to ameliorate male idiopathic infertility by improving PG sperm function. A study related to the ref. [805]. (in vitro study) |
| Dona G. et al. 2013 [805] | N/A | 24 Semen samples from healthy male donors | 0 to 2 μM | N/A |
| The AX improved Tyr-P and acrosome reaction cells (ARC) values in sperm heads without affecting the ROS generation curve, while diamide successfully increased Tyr-P levels in flagella but did not increase ARC values. This suggests that AX, when inserted into the membrane, causes membrane changes, such as capsulation, that allow for Tyr-P conversion of the head. In other words, an acrosome reaction can occur, and more cells can be involved. A study related to the ref. [804]. (in vitro study) |
| Comhaire F.H. et al. 2005 [735] | Randomized, double-blind, placebo-controlled, prospective study | 30 males with infertility of ≥12 months | 0, 16 mg/day | 3 months |
| Significantly decreased ROS and Inhibin B and sperm linear velocity increased in the Astaxanthin group (n = 11), but not in the placebo group (n = 19). The total and per cycle pregnancy rates among the Astaxanthin group (54.5% and 23.1%) were higher compared with 10.5% and 3.6%, respectively, in the placebo cases (p = 0.028; p = 0.036). No safety concerns were identified in this study. |
| (H) Absorption Distribution Metabolism and Excretion (ADME) | ||||||
| Author/year/ reference | Study design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Continuous dosing pharmacokinetic evaluation | ||||||
| Urakaze M et al. 2021 [764] | Randomized, double-blind, placebo-controlled prospective study | 44 subjects Including Prediabetes (Av. 46–48 yrs.) | 0, 12 mg/day | 12 weeks |
| Plasma AX levels were undetectable at baseline and increased to 122.69 ng/mL (c.a. 205 nM) after 4 weeks in the intervention group, and this level was maintained until 12 weeks. No safety concerns were identified in this study. |
| Hayashi M. et al. 2020 [799] | Randomized, double-blind, placebo-controlled prospective study | 54 healthy subjects (Av. 45–46 yrs.) | 0, 12 mg/day from Paracoccus | 8 weeks |
| After 8 weeks of AX administration, the serum AX concentration increased to 0.171 ± 0.082 μg/mL (0.29 μM). Not detected in the placebo group. No safety concerns were identified in this study. |
| Hayashi M. et al. 2018 [793] | Randomized, double-blind, placebo-controlled prospective study | 54 healthy subjects (45–64 yrs.) | 0, 8 mg/day from Paracoccus | 8 weeks |
| After 8 weeks of AX administration, the serum AX concentration increased to 0.173 ± 0.058 μg/mL (0.29 μM). Not detected in the placebo group. No safety concerns were identified in this study. |
| Petyaev I.M., et al. 2018 [719] | Randomized, blinded, four-arm, prospective study | 32 subjects with oxidative stress, 8 subjects taking AX only, (60–70 yrs) | 0, 7 mg/day * with DC | 4 weeks |
| Plasma AX concertation was seen in the volunteers supplemented with a lycosomal formulation of dark chocolate (DC) containing 7 mg of co-crystalized AX (L-DC-ASTX). Increase in plasma AX concertation. Plasma AX concentrations after 4 weeks were higher in the L-DC-ASTX formulation than other groups (5.22–7.31 nmol/L vs. 17.34 nmol/L ). No safety concerns were identified in this study. |
| Coombes J.S et al. 2016 [695] Fassett, R.G. et al. 2008, [696] | Randomized, double-blind, placebo-controlled prospective study | 58 renal transplant recipients | 0, 12 mg/day | 12 months |
| Plasma AX concentrations were 0.29 ± 0.18 μmol/L at 6 months and 0.28 ± 0.17 μmol/L at 12 months (Mean ± SD). (The XANTHIN trial) No safety concerns were identified in this study. |
| Miyazawa T. et al. 2011 [730] | Randomized, double-blind, placebo-controlled, prospective study | 30 middle- aged & senior subjects (Mean: 50.6 yrs.) | 0, 1, 3 mg/day | 12 weeks |
| Erythrocyte AX concentrations after 4 or 12 weeks of supplementation (3 mg/day administration, 2.5 nM AX in packed cells for 4 weeks, and 2.9 nM for 4 weeks respectively) were significantly higher than after placebo or 1 mg AX supplementation. No safety concerns were identified in this study. |
| Miyazawa T. et al. 2011 [568] | Randomized, double-blind, placebo-controlled, prospective study | 20 middle- aged & senior subjects (Mean: 50.6 yrs.) | 1, 3 mg/day | 12 weeks |
| Plasma AX concentrations were significantly higher in a dose-dependent manner after AX supplementation than before in both the 1 mg/day (0.4, 12.3, and 18.9 nM for 0, 4, and 12 weeks after administration, respectively) and 3 mg/day (0.7, 14.4, and 62.4 nM for 0, 4, and 12 weeks after administration, respectively) groups. No safety concerns were identified in this study. |
| Nakagawa K. et al. 2011 [721] | Randomized, double-blind, placebo-controlled prospective study | 30 healthy subjects | 0, 6, 12 mg/day | 12 weeks |
| 6mg/day: Increased AX concentration in plasma after 12 weeks (86 nM) compared to baseline (p < 0.01, 6 to 9 nM) and compared to placebo (p < 0.01, 8 nM). 12mg/day: Increased AX concentration in plasma after 12 weeks (109 nM) compared to baseline (p < 0.01, 8 to 9 nM) and compared to placebo (p < 0.01, 8 nM) No safety concerns were identified in this study. |
| Park J.S. et al. 2010 [732] | Randomized, double-blind, placebo- controlled, prospective study | 42 healthy subjects | 0, 2, 8 mg/day | 8 weeks |
| Plasma AX concentration From 4 weeks after administration, c.a. 0.1 µM (2 mg AX/day), c.a. 0.12–0.14 µM (8 mg AX/day) No safety concerns were identified in this study. |
| Uchiyama A. et al. 2008 [775] | Open-labeled, prospective study | 17 subjects at risk for developing metabolic syndrome | 8 mg twice day | 3 months |
| The blood concentration reached the plateau after a month of treatment and was retained at that level unti1 3 months of treatment (0.2–0.25 μg/mL (0.34–0.42 μmol/L)). No safety concerns were identified in this study. |
| Karppi, J. et al. 2007 [733] | Randomized, double-blind, placebo-controlled, prospective study | 39 healthy subjects | 0, 8 mg/day | 3 months |
| AX supplementation elevated plasma AX levels to 0.032 nM (c.a. 0.02μg/mL, p < 0.001 for the change compared with the placebo group). No safety concerns were identified in this study. |
| Nagaki Y. et al. 2005 [684] | Randomized, placebo-controlled, prospective study | 36 health subjects (c.a. 41 yrs.) | 0, 6 mg/day | 4 weeks |
| The fasting plasma AX level in the AX group was significantly (p < 0.001, 35.6 ng/mL at 4 weeks) higher than before supplementation. The fasting plasma AX level in the placebo group after placebo treatment remained unchanged. No safety concerns were identified in this study. [Article in Japanese] |
| Single dosing pharmacokinetic evaluation | ||||||
| Madhavi D. et al. 2018 [806] | Open-label, crossover study | 6 healthy subjects | 60 mg | Single dosing |
| Test articles: AX-SR; 2.5% AX in a sustained-release matrix: AX (control); 10% AX (unformulated AX oil). Dissolution study: AX-SR formulation formed a stable dispersion in simulated gastric and intestinal fluids. Single-dose pharmacokinetic study (clinical study); AX-SR formulation (AUC0–24h: 4393 ± 869 ng/mL-h) showed 3.6 times higher bioavailability than AX oil (AUC0–24h: 1227 ± 1328 ng/mL-h) (p < 0.0005). AX oil showed very low absorption in 3 of 6 subjects, whereas the AX-SR formulation appeared in the blood of all subjects. No safety concerns were identified in this study. |
| Okada Y. et al. 2009 [807] | Open-labeled, prospective study | Healthy subjects; before the meal to nonsmokers (n = 7), after the meal to nonsmokers (n = 6), and after the meal to smokers (n = 7) | 48 mg | Single dosing |
| Dosing timing significantly affected AX bioavailability, including area under the curve (AUC0–168h, 2968 ± 959 μg·h/L in the pre-meal group and 7219 ± 3118 μg·h/L in the after-meal group), suggesting a higher availability in the after-meal group. Smoking also affected pharmacokinetic parameters, significantly reducing the elimination half-life (t1⁄2) of AX. No safety concerns were identified in this study. |
| Coral-Hinostroza G.N. et al. 2004 [808] | Open-labeled, prospective study | 3 male subjects (41–50 yrs, BMI 27.7–27.8) | 10, 100 mg | Single dose |
| Test meal: Dissolved the oily AX diesters in warm olive oil (30% of meal weight) and served as a dressing to a pasta salad. 100 mg (Cmax: 0.28 ± 0.1 mg/L, Tmax: 11.5 ± 1.2 hr, T1/2: 52.2 ± 39.5 hr. AUC(0–∞) 11.0 ± 2.8 mg h/L) Cmax at the low dose (10 mg): 0.08 mg/L (non-linear dose response). AX esters were not detected in plasma. No safety concerns were identified in this study |
| Mercke Odeberg J. et al. 2003 [809] | Open-labeled, prospective study | 32 healthy male Subjects (nonsmoker/no medications) | 40 mg | Single dose |
| Test meal: Haematococcus algal meal and dextrin in hard gelatin capsules (reference) and with long-chain triglyceride (palm oil) and polysorbate 80 (formulation A), glycerol mono- and dioleate and polysorbate 80, (formulation B), and glycerol mono- and dioleate, polysorbate 80 and sorbitan monooleate (formulation C). The highest bioavailability was observed with formulation B (3.7 times higher UC(0–∞) compared to the reference control). No safety concerns were identified in this study. |
| Østerlie M. et al. 2000 [810] | Open-labeled, prospective study | 3 male subjects (37–43 years, BMI 27.5–31.7) | 100 mg | Single dose |
| Test meal: prepared by dispersing the AX beadlets in warm water (40 °C) and mixing with olive oil (50% of meal weight) and cereals. Cmax: 1.3 ± 0.1 mg/L, Tmax: 6.7 ± 1.2 hr, T1/2: 21 ± 11 hr, AUC(0–∞) 42 ± 3 mg h/L. Accumulated 13Z-AX selectively, whereas the steroisomer distribution was similar to that of the experimental meal. AX was present mainly in VLDL containing chylomicrons (36–64% of total AX), whereas LDL and HDL contained 29% and 24% of total AX, respectively. The AX isomer distribution in plasma, VLDL/CM, LDL, and HDL were not affected by time. No safety concerns were identified in this study. |
| (I) Others | ||||||
| Author/Year/ Reference | Study Design | Subjects | Dose #,## | Duration | Major Outcome † | Description |
| Continuous dosing pharmacokinetic evaluation | ||||||
| Ledda A. et al. 2017 [724] | Open-labeled, two-arm prospective study | 59 patients with genitourinary cancers (prostate or bladder malignancies) who had undergone and completed cancer treatments (radiotherapy, chemotherapy or intravesical immunotherapy with increased oxidative stress and residual symptoms) | 0, 8 mg/day * | 6 weeks |
| Oncotris: containing 264 mg/day curcumin, 500 mg/day extract of cordyceps, and 8 mg/day AX (from EP217785227). Signs and symptoms (treatment-related) and the intensity of residual side effects were significantly reduced after 6 weeks in the supplemented group: minimal changes were seen in the control group. Oncotris supplementation was associated with significant improvements in blood cell counts and reductions in levels of plasma PSA and oxidative stress. No safety concerns were identified in this study. |
| Kaneko M et al. 2017 [811] | Open-label, prospective study | 10 male subjects | 24 mg/day | 28 days |
| Aerodynamic assessment, acoustic analysis, and the GRBAS scale (grade, roughness, breathiness, asthenia, and strain) were significantly worse in the no AX status (day 0) immediately after vocal loading but improved by 30 min after loading. On the other hand, in AST(+) (day 35), no statistical worsening of any of the phonatory parameters was observed when measured immediately after the vocal load. No safety concerns were identified in this study. |
| Yagi H. et al. 2013 [725] | Case reports | 34 OAB patients with anticholinergic agent-resistant (75.5 ± 8.0 years) | 0, 12 mg/day * | 8 weeks |
| Significantly improved international prostate symptom score (IPSS), QOL scores, benign prostatic hyperplasia impact index (BII) scores, and urinary 8-OHdG in patients AX could improve both urinary symptoms and QOL for anticholinergic agent-resistant OAB. No safety concerns were identified in this study. [Article in Japanese] |
| Park J.S. et al. 2010 [732] | Randomized, double-blind, placebo- controlled, prospective study | 42 healthy subjects | 0, 2, 8 mg/day | 8 weeks |
| Immunity Increased natural killer (NK) cell cytotoxic activity and increased total T and B cell subpopulations; however, did not influence populations of Th, Tcytotoxic or NK cells. No difference in TNF-α and IL-2 concentrations, but plasma IFN-γ and IL-6 increased after 8 weeks in subjects given 8 mg AX. No safety concerns were identified in this study. |
| Yamada T. et al. 2010 [727] | Open-labeled, prospective study | 6 healthy subjects and 6 Sjoegren’s syndrome (SS) subjects | 12 mg/day | 2 weeks |
| Although the increased amount of salivary secretion after the intake of AX for 2 weeks was faint in the SS group (1.02 g/2 min to 1.04 g/2 min, p = 0.69), a significant increase was also observed in the normal group (6.23 g/2 min to 7.02 g/2 min, p = 0.03). reduced protein oxidation (−10%, p < 0.05) No safety concerns were identified in this study. |
| Kupcinskas L. et al. 2008 [812] | Randomized, single-blind, placebo-controlled prospective study | 131 patients with functional dyspepsia with or without Helicobacter pylori infection | 0, 8, 20 mg/twice a day (0,16, 40 mg/day) | 4 weeks |
| No statistically significant differences were observed between the three treatment groups at the end of treatment (week 4) for mean scores on the Gastrointestinal Symptom Rating Scale (GSRS) for abdominal pain, indigestion, and reflux syndrome, and similar results were observed at the end of follow-up (week 8). The reflux syndrome tended to improve at the higher dose (40 mg) compared to the other treatment groups (16 mg and placebo), and this response was more pronounced (p = 0.04) in H.pylori-infected patients. No safety concerns were identified in this study. |
| Andersen LP. et al. 2007 [813] | Randomized, double-blind, placebo-controlled, prospective study | 44 patients with functional dyspepsia with or without Helicobacter pylori infection | 0, 20 mg/twice a day (0, 40 mg/day) | 4 weeks |
| There were no significant changes in either H. pylori density or interleukins during or after treatment; significant upregulation of CD4 (p < 0.05) and downregulation of CD8 (p < 0.001) were observed in H. pylori patients treated with AX. No safety concerns were identified in this study. |
4. Advantages of Astaxanthin from Haematococcus Algae
4.1. Absorption and Bioavailability
4.1.1. General Aspects of AX Absorption Dynamics
4.1.2. Molecular Mechanism of Intestinal Absorption of AX
4.1.3. Challenges in Improving Low Bioavailability
4.2. Safety of Haematococcus Astaxanthin
4.2.1. Genotoxicity
4.2.2. Acute and Sub-Chronic Toxicity
4.2.3. Reproductive and Developmental Toxicity
4.2.4. Toxicokinetics and Liver Toxicity
4.2.5. Human Safety
5. Prospective: The Future of Astaxanthin
5.1. Perspective: Health Promotion Potentials of Natural Astaxanthin
5.2. Prospective: Global Demand and Social Requirements for Astaxanthin
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grand View Research Inc. Astaxanthin Market Size, Share & Trends Analysis Report By Product (Oil, Softgel, Liquid), By Source (Natural, Synthetic), By Application (Aquaculture & Animal Feed, Nutraceuticals), By Region, And Segment Forecasts, 2021. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/global-astaxanthin-market (accessed on 1 November 2022).
- MarketsandMarkets Research Pvt., L. Astaxanthin Market Global Forecast To. Available online: https://www.marketsandmarkets.com/Market-Reports/astaxanthin-market-162119410.html (accessed on 1 November 2022).
- Research, E.M. Global Astaxanthin Market: By Product: Dried Algae Meal or Biomass, Oil, Softgel, Liquid, Others; By Source: Natural, Synthetic; By Application: Aquaculture and Animal Feed, Nutraceuticals, Others; Regional Analysis; Historical Market and Forecast (2018–2028); Market Dynamics; Competitive Landscape; Industry Events and Developments. Available online: https://www.expertmarketresearch.com/reports/astaxanthin-market (accessed on 1 November 2022).
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Handbook; Springer: New York City, NY, USA, 2004. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Britton, G. Carotenoids. In Natural Food Colorants; Hendry, G.A.F., Houghton, J.D., Eds.; Springer US: Boston, MA, USA, 1996; pp. 197–243. [Google Scholar]
- Newbigin, M.I. The Pigments of the Decapod Crustacea. J. Physiol. 1897, 21, 237–257. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize. Richard Kuhn and the Chemical Institute: Double Bonds and Biological Mechanisms. Available online: https://www.nobelprize.org/prizes/chemistry/1938/kuhn/facts/ (accessed on 1 January 2023).
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Lederer, E. Über die Farbstoffe des Hummers (Astacus gammarus L.) und ihre Stammsubstanz, das Astacin. Berichte Der Dtsch. Chem. Ges. 1933, 66, 488–495. [Google Scholar] [CrossRef]
- Kuhn, R.; Sörensen, N.A. Über Astaxanthin und Ovoverdin. Berichte Dtsch. Chem. Ges. (A B Ser.) 1938, 71, 1879–1888. [Google Scholar] [CrossRef]
- Stern, K.G.; Salomon, K. On Ovoverdin, the Carotenoid-Protein Pigment of the Egg of the Lobster. J. Biol. Chem. 1938, 122, 461–475. [Google Scholar] [CrossRef]
- Euler, H.; Hellstrom, H.; Malmberg, M. Salmic acid, a carotenoid of the salmon. Sven. Kem. Tidskr. 1933, 45, 151–152. [Google Scholar]
- Khare, A.; Moss, G.P.; Weedon, B.C.; Matthews, A.D. Identification of astaxanthin in Scottish salmon. Comp. Biochem. Physiol. B 1973, 45, 971–973. [Google Scholar] [CrossRef]
- Fox, D.L. Astaxanthin in the American flamingo. Nature 1955, 175, 942–943. [Google Scholar] [CrossRef]
- Manunta, C. Astaxanthin in insects and other terrestrial arthropods. Nature 1948, 162, 298. [Google Scholar] [CrossRef]
- Seybold, A.; Goodwin, T.W. Occurrence of Astaxanthin in the Flower Petals of Adonis annua L. Nature 1959, 184, 1714–1715. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Yoda, T.; Kobayashi, T.; Young, A.J. Molecular structures of carotenoids as predicted by MNDO-AM1 molecular orbital calculations. J. Mol. Struct. 2002, 604, 125–146. [Google Scholar] [CrossRef]
- Bartalucci, G.; Coppin, J.; Fisher, S.; Hall, G.; Helliwell, J.R.; Helliwell, M.; Liaaen-Jensen, S. Unravelling the chemical basis of the bathochromic shift in the lobster carapace; new crystal structures of unbound astaxanthin, canthaxanthin and zeaxanthin. Acta Crystallogr. B 2007, 63, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Carotenoids in Marine Organisms: Metabolism and Biological Activities "Kaiyo Seibutsu no Karotenoido: Taisya to Seibutsu Kassei" in Japanese; Kouseisha Kouseikaku Co., Ltd.: Tokyo, Japan, 1993. [Google Scholar]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoid Metabolism in Aquatic Animals. Adv. Exp. Med. Biol. 2021, 1261, 29–49. [Google Scholar] [CrossRef]
- Hata, M.; Hata, M. Carotenoid pigments i goldfish (Carassius auratus). Int. J. Biochem. 1971, 2, 11–19. [Google Scholar] [CrossRef]
- Hata, M.; Hata, M. Carotenoid metabolism in fancy red carp, Cyprinus carpio. I. Administration of carotenoids. Nippon. Suisan Gakkaishi 1975, 41, 653–655. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matsuguchi, H.; Katayama, T.; Simpson, K.L.; Chichester, C.O. The biosynthesis of astaxanthin. XVIII. The metabolism of the carotenoids in the prawn, Penaeus japonicus bate. Nippon. Suisan Gakkaishi 1976, 42, 197–202. [Google Scholar] [CrossRef]
- Kitahara, T. Carotenoids in the pacific salmon during the marine period. Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 78, 859–862. [Google Scholar] [CrossRef]
- Nakamura, K.; Hata, M.; Hata, M. A study on astaxanthin in salmon Oncorhyncus keta serum. Nippon. Suisan Gakkaishi 1985, 51, 979–983. [Google Scholar] [CrossRef]
- Ando, S. Stereochemical Investigation of Astaxanthin in the Ovaries of Chum Salmon Oncorhynchus keta during Spawning Migration. Bull. Fac. Fish. Hokkaido Univ. 1986, 37, 309–313. [Google Scholar]
- Ando, S.; Hatano, M. Metabolic pathways of carotenoids in chum salmon Oncorhynchus keta during spawning migration. Comp. Biochem. Physiol. B 1987, 87, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Miki, W.; Yamaguchi, K.; Konosu, S.; Takane, T.; Satake, M.; Fujita, T.; Kuwabara, H.; Shimeno, S.; Takeda, M. Origin of tunaxanthin in the integument of yellowtail (Seriola quinqueradiata). Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 195–201. [Google Scholar] [CrossRef]
- Matsuno, T.; Katsuyama, M.; Maoka, T.; Hirono, T.; Komori, T. Reductive metabolic pathways of carotenoids in fish (3S,3′S)-astaxanthin to tunaxanthin a, b and c. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 779–789. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef]
- Nishino, H. Cancer prevention by carotenoids. Mutat Res. 1998, 402, 159–163. [Google Scholar] [CrossRef]
- Yokoyama, A.; Izumida, H.; Miki, W. Production of Astaxanthin and 4-Ketozeaxanthin by the Marine Bacterium, Agrobacterium Aurantiacum. Biosci. Biotechnol. Biochem. 1994, 58, 1842–1844. [Google Scholar] [CrossRef]
- Misawa, N.; Kajiwara, S.; Kondo, K.; Yokoyama, A.; Satomi, Y.; Saito, T.; Miki, W.; Ohtani, T. Canthaxanthin biosynthesis by the conversion of methylene to keto groups in a hydrocarbon beta-carotene by a single gene. Biochem. Biophys Res. Commun. 1995, 209, 867–876. [Google Scholar] [CrossRef]
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995, 177, 6575–6584. [Google Scholar] [CrossRef]
- Kajiwara, S.; Kakizono, T.; Saito, T.; Kondo, K.; Ohtani, T.; Nishio, N.; Nagai, S.; Misawa, N. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol. Biol. 1995, 29, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Nagaki, Y.; Hayasaka, S.; Yamada, T.; Hayasaka, Y.; Sanada, M.; Uonomi, T. Effects of astaxanthin on accommodation, critical flicker fusion, and pattern visual evoked potential in visual display terminal workers. J. Tradit. Med. 2002, 19, 170–173. (In Japanse) [Google Scholar]
- Nakamura, A.; Isobe, A.; Ootaka, Y.; Abematsu, Y.; Nakata, D.; Honma, K.; Sakurai, S.; Shimada, Y.; Horiguchi, M. Changes in visual function following peroral astaxanthin. RinGan "Rinsho Ganka" 2004, 58, 1051–1054. (In Japanse) [Google Scholar] [CrossRef]
- Nitta, T.; Ohgami, K.; Shiratori, K.; Chin, S.; Yoshida, K.; Tsukahara, H.; Ohno, S. Effects of Astaxanthin on Accommodation and Asthenopia-Dose Finding Study in Healthy Volunteers-. Rinsho Iyaku J. Clin. Ther. Med. 2005, 21, 543–556. (In Japanse) [Google Scholar]
- Shiratori, K.; Ohgami, K.; Nitta, T.; Shinmei, Y.; Chin, S.; Yoshida, K.; Tsukahara, H.; Takehara, I.; Ohno, S. Effect of Astaxanthin on Accommodation and Asthenopia-Efficacy-Identification Study in Healthy Volunteers-. Rinsho Iyaku J. Clin. Ther. Med. 2005, 21, 637–650. (In Japanse) [Google Scholar]
- Takahashi, N.; Kajita, M. Effects of astaxanthin on accommodative recovery. Rinsho Iyaku J. Clin. Ther. Med. 2005, 21, 431–436. (In Japanse) [Google Scholar]
- Iwasaki, T.; Tawara, A. Effects of Astaxanthin on Eyestrain Induced by Accommodative Dysfunction. Atarashii Ganka J. 2006, 23, 829–834. (In Japanse) [Google Scholar]
- Nagaki, Y.; Mihara, M.; Tsukuhara, H.; Ohno, S. The supplementation effect of astaxanthin on accommodation and asthenopia. Rinsho Iyaku (J. Clin. Ther. Med.) 2006, 22. Available online: https://www.researchgate.net/publication/298644250_Effect_of_astaxanthin_on_accommodation_and_asthenopia (accessed on 1 August 2023). (In Japanse).
- Nagaki, Y.; Tsukahara, H.; Yoshimoto, T.; Masuda, K. Effect of Astaxanthin on Accommodation and Asthenopia. Japanese Review of Clinical Ophthalmology. Folia Opththalmol. Jpn. 2010, 3, 461–468. (In Japanse) [Google Scholar]
- Kajita, M.; Tsukahara, H.; Mio, K. The Effects of a Dietary Supplement Containing Astaxanthin on the Accommodation Function of the Eye in Middle-aged and Older People. Med. Consult. New Remedies 2009, 46, 325–329. (In Japanse) [Google Scholar]
- Ota, S. Frontiers in Phycology: Phylogenetic Studies on the Genus Haematococcus and Related Genera. Jpn. J. Phycol. 2014, 62, 11–14. (In Japanese) [Google Scholar]
- Veerman, A.; Borch, G.; Pedersen, R.; Liaaen-Jensen, S.; Schroll, G.; Altona, C. Animal Carotenoids. 10. Chirality of Astaxanthin of Different Biosynthetic Origin. Acta Chem. Scand. 1975, 29, 525. [Google Scholar] [CrossRef] [PubMed]
- Renstrøm, B.; Borch, G.; Skulberg, O.M.; Liaaen-Jensen, S. Optical purity of (3S,3′S)-astaxanthin from Haematococcus pluvialis. Phytochemistry 1981, 20, 2561–2564. [Google Scholar] [CrossRef]
- Andrewes, A.G.; Starr, M.P. (3R,3′R)-astaxanthin from the yeast Phaffia rhodozyma. Phytochemistry 1976, 15, 1009–1011. [Google Scholar] [CrossRef]
- Vecchi, M.; Müller, R.K. Separation of (3S, 3′S)-, (3R 3′R)- and (3S, 3′R)-astaxanthin via (−)-camphanic acid esters. J. High Resolut. Chromatogr. 1979, 2, 195–196. [Google Scholar] [CrossRef]
- Maoka, T.; Komori, T.; Matsuno, T. Direct diastereomeric resolution of carotenoids. J. Chromatogr. A 1985, 318, 122–124. [Google Scholar] [CrossRef]
- Euglert, G.; Vecchi, M. trans/cis Isomerization of Astaxanthin Diacetate/Isolation by HPLC. and Identification by1H-NMR. Spectroscopy of Three Mono-cis- and Six Di-cis-Isomers. Helv. Chim. Acta 1980, 63, 1711–1718. [Google Scholar] [CrossRef]
- Yao, G.; Muhammad, M.; Zhao, J.; Liu, J.; Huang, Q. DFT-based Raman spectral study of astaxanthin geometrical isomers. Food Chem. 2022, 4, 100103. [Google Scholar] [CrossRef]
- Honda, M.; Sowa, T.; Kawashima, Y. Thermal- and Photo-Induced Isomerization of All-E- and Z-Isomer-Rich Xanthophylls: Astaxanthin and Its Structurally-Related Xanthophylls, Adonirubin, and Adonixanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900462. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Zhang, Y.; Goto, M. Continuous production of Z-isomer-rich astaxanthin nanodispersions with stabilized Z-structures using selected ester-based nonionic emulsifiers. Food Chem. Adv. 2022, 1, 100028. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Liu, H.; Cao, Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J. Funct. Foods 2018, 44, 127–136. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Yang, C.; Hassan, Y.I.; Liu, R.; Zhang, H.; Chen, Y.; Zhang, L.; Tsao, R. Anti-Inflammatory Effects of Different Astaxanthin Isomers and the Roles of Lipid Transporters in the Cellular Transport of Astaxanthin Isomers in Caco-2 Cell Monolayers. J. Agric. Food Chem. 2019, 67, 6222–6231. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Z-Isomers of Astaxanthin Exhibit Greater Bioavailability and Tissue Accumulation Efficiency than the All-E-Isomer. J. Agric. Food Chem. 2021, 69, 3489–3495. [Google Scholar] [CrossRef]
- Breithaupt, D.E. Identification and Quantification of Astaxanthin Esters in Shrimp (Pandalus borealis) and in a Microalga (Haematococcus pluvialis ) by Liquid Chromatography−Mass Spectrometry Using Negative Ion Atmospheric Pressure Chemical Ionization. J. Agric. Food Chem. 2004, 52, 3870–3875. [Google Scholar] [CrossRef]
- Miao, F.; Lu, D.; Li, Y.; Zeng, M. Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. Anal. Biochem. 2006, 352, 176–181. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Matsui, K.; Nakamura, M.; Muramatsu, M.; Hanada, S. Fatty acids of astaxanthin esters in krill determined by mild mass spectrometry. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 136, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Grynbaum, M.D.; Hentschel, P.; Putzbach, K.; Rehbein, J.; Krucker, M.; Nicholson, G.; Albert, K. Unambiguous detection of astaxanthin and astaxanthin fatty acid esters in krill (Euphausia superba Dana). J. Sep. Sci. 2005, 28, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids, Volume 1A: Isolation and Analysis. Birkhäuser: Basel, Switzerland, 1995. [Google Scholar]
- USP. Astaxanthin Esters. In USP–NF; Ckville, M.D., Ed.; USP: Rockville, MD, USA, 2019. [Google Scholar] [CrossRef]
- Mori, Y.; Yamano, K.; Hashimoto, H. Bistable aggregate of all-trans-astaxanthin in an aqueous solution. Chem. Phys. Lett. 1996, 254, 84–88. [Google Scholar] [CrossRef]
- Köpsel, C.; Möltgen, H.; Schuch, H.; Auweter, H.; Kleinermanns, K.; Martin, H.-D.; Bettermann, H. Structure investigations on assembled astaxanthin molecules. J. Mol. Struct. 2005, 750, 109–115. [Google Scholar] [CrossRef]
- Giovannetti, R.; Alibabaei, L.; Pucciarelli, F. Kinetic model for astaxanthin aggregation in water–methanol mixtures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Fuciman, M.; Durchan, M.; Šlouf, V.; Keşan, G.; Polívka, T. Excited-state dynamics of astaxanthin aggregates. Chem. Phys. Lett. 2013, 568–569, 21–25. [Google Scholar] [CrossRef]
- Lu, L.; Hu, T.; Xu, Z. Structural characterization of astaxanthin aggregates as revealed by analysis and simulation of optical spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zajac, G.; Machalska, E.; Kaczor, A.; Kessler, J.; Bouř, P.; Baranska, M. Structure of supramolecular astaxanthin aggregates revealed by molecular dynamics and electronic circular dichroism spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 18038–18046. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and Optical Properties of Water-Soluble Xanthophyll Antioxidants: Aggregation vs Complexation. J. Phys. Chem. B 2013, 117, 10173–10182. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef]
- Dai, M.; Li, C.; Yang, Z.; Sui, Z.; Li, J.; Dong, P.; Liang, X. The Astaxanthin Aggregation Pattern Greatly Influences Its Antioxidant Activity: A Comparative Study in Caco-2 Cells. Antioxidants 2020, 9, 126. [Google Scholar] [CrossRef]
- Ding, L.; Yang, J.; Dai, M.; Li, S.; Yin, K.; Li, J. Effect of environmental factors on the aggregation behavior of astaxanthin in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 12150. [Google Scholar] [CrossRef]
- Musser, A.J.; Maiuri, M.; Brida, D.; Cerullo, G.; Friend, R.H.; Clark, J. The Nature of Singlet Exciton Fission in Carotenoid Aggregates. J. Am. Chem. Soc. 2015, 137, 5130–5139. [Google Scholar] [CrossRef]
- Bardeen, C.J. The Structure and Dynamics of Molecular Excitons. Annu. Rev. Phys. Chem. 2014, 65, 127–148. [Google Scholar] [CrossRef]
- Britton, G.; Helliwell, J.R. Carotenoid-Protein Interactions. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 99–118. [Google Scholar]
- Zagalsky, P.F. β-Crustacyanin, the blue–purple carotenoprotein of lobster carapace: Consideration of the bathochromic shift of the protein-bound astaxanthin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 1529–1531. [Google Scholar] [CrossRef]
- Britton, G.; Weesie, R.J.; Askin, D.; Warburton, J.D.; Gallardo-Guerrero, L.; Jansen, F.J.; de Groot, H.J.M.; Lugtenburg, J.; Cornard, J.-P.; Merlin, J.-C. Carotenoid blues: Structural studies on carotenoproteins. Pure Appl. Chem. 1997, 69, 2075–2084. [Google Scholar] [CrossRef]
- Cianci, M.; Rizkallah, P.J.; Olczak, A.; Raftery, J.; Chayen, N.E.; Zagalsky, P.F.; Helliwell, J.R. The molecular basis of the coloration mechanism in lobster shell: β-Crustacyanin at 3.2-Å resolution. Proc. Natl. Acad. Sci. USA 2002, 99, 9795–9800. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E.; Cianci, M.; Grossmann, J.G.; Habash, J.; Helliwell, J.R.; Nneji, G.A.; Raftery, J.; Rizkallah, P.J.; Zagalsky, P.F. Unravelling the structural chemistry of the colouration mechanism in lobster shell. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.M.; Tollenaere, A.; Hall, M.R.; Degnan, B.M. Evolution of a Novel Carotenoid-Binding Protein Responsible for Crustacean Shell Color. Mol. Biol. Evol. 2009, 26, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Folli, C.; Pincolini, E.; McClintock, T.S.; Rossle, M.; Berni, R.; Cianci, M. Structural characterization of recombinant crustacyanin subunits from the lobster Homarus americanus. Acta Crystallogr Sect. F Struct Biol. Cryst. Commun. 2012, 68, 846–853. [Google Scholar] [CrossRef]
- Buchwald, M.; Jencks, W.P. Properties of the crustacyanins and the yellow lobster shell pigment. Biochemistry 1968, 7, 844–859. [Google Scholar] [CrossRef]
- Salares, V.R.; Young, N.M.; Bernstein, H.J.; Carey, P.R. Resonance Raman spectra of lobster shell carotenoproteins and a model astaxanthin aggregate. A possible photobiological function for the yellow protein. Biochemistry 1977, 16, 4751–4756. [Google Scholar] [CrossRef]
- Tlusty, M.; Hyland, C. Astaxanthin deposition in the cuticle of juvenile American lobster (Homarus americanus): Implications for phenotypic and genotypic coloration. Mar. Biol. 2005, 147, 113–119. [Google Scholar] [CrossRef]
- Drozdova, P.; Saranchina, A.; Morgunova, M.; Kizenko, A.; Lubyaga, Y.; Baduev, B.; Timofeyev, M. The level of putative carotenoid-binding proteins determines the body color in two species of endemic Lake Baikal amphipods. PeerJ 2020, 8, e9387. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.N.; Freeman, J.A. The Crustacean Integument: Morphology and Biochemistry; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bliss, D.E.; Mantel, L.H. ; Integument, Pigments, and Hormonal Processes: Volume 9: Integument, Pigments and Hormonal Processes; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Stern, K.G.; Salomon, K. Ovoverdin, a Pigment Chemically Related to Visual Purple. Science 1937, 86, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Young, N.M.; Williams, R.E. The circular dichroism of ovoverdin and other carotenoproteins from the lobster Homarus americanus. Can J. Biochem. Cell. Biol. 1983, 61, 1018–1024. [Google Scholar] [CrossRef]
- Mantiri, D.M.H.; Nègre-Sadargues, G.; Castillo, R.; Milicua, J.-C.G. The Carotenoproteins During Embryogenesis and Larval Development of the European Lobster Homarus gammarus. J. Crustac. Biol. 2004, 24, 592–602. [Google Scholar] [CrossRef]
- Zagalsky, P. A study of the astaxanthin-lipovitellin, ovoverdin, isolated from the ovaries of the lobster, Homarus gammarus (L.). Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 589–597. [Google Scholar] [CrossRef]
- Zagalsky, P. Crystallisation of astaxanthin-proteins of Velella velella (Coelenterata: Chondrophora). Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 71, 235–236. [Google Scholar] [CrossRef]
- Zagalsky, P. Comparison of the apoprotein subunits of the lobster carapace astaxanthin-protein, α-crustacyanin, and of the apoproteins of Velella mantle pigment, λmax 600 nm, by peptide mapping. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 73, 997–1000. [Google Scholar] [CrossRef]
- Zagalsky, P.; Jones, R. Quaternary structures of the astaxanthin-proteins of Velella velella, and of α-crustacyanin of lobster carapace, as revealed in electron microscopy. Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 71, 237–242. [Google Scholar] [CrossRef]
- Chayen, N.E.; Boggon, T.J.; Raftery, J.; Helliwell, J.R.; Zagalsky, P.F. Purification, crystallization and initial X-ray analysis of the C1subunit of the astaxanthin protein, V600, of the chondrophore Velella velella. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 266–268. [Google Scholar] [CrossRef]
- Zagalsky, P.F.; Mummery, R.S.; Winger, L.A. Cross-reactivities of polyclonal antibodies to subunits, CRTA and CRTC, of the lobster carapace carotenoprotein, α-crustacyanin, and of monoclonal antibodies to human serum retinol-binding protein against carotenoproteins of different types and from separate invertebrate species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 385–391. [Google Scholar] [CrossRef]
- Shone, C.C.; Britton, G.; Goodwin, T.W. The violet carotenoprotein of the starfish, Asterias rubens. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 62, 507–513. [Google Scholar] [CrossRef]
- Liaaen-Jensen, S.; Kildahl-Andersen, G. Blue carotenoids. Arkivoc 2008, 6, 5–25. [Google Scholar] [CrossRef]
- Zagalsky, P.; Haxo, F.; Hertzberg, S.; Liaaen-Jensen, S. Studies on a blue carotenoprotein, linckiacyanin, isolated from the starfish Linckia laevigata (Echinodermata: Asteroidea). Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 339–353. [Google Scholar] [CrossRef]
- Cheesman, D.F. Ovorubin, a chromoprotein from the eggs of the gastropod mollusc Pomacea canaliculata. Proc. R. Soc. London. Ser. B: Boil. Sci. 1958, 149, 571–587. [Google Scholar] [CrossRef]
- Dreon, M.S.; Schinella, G.; Heras, H.; Pollero, R.J. Antioxidant defense system in the apple snail eggs, the role of ovorubin. Arch. Biochem. Biophys. 2003, 422, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dreon, M.S.; Ceolín, M.; Heras, H. Astaxanthin binding and structural stability of the apple snail carotenoprotein ovorubin. Arch. Biochem. Biophys. 2007, 460, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ronneberg, H.; Borch, G.; Fox, D.L.; Liaaen-Jensen, S. Animal carotenoids 19. Alloporin, a new carotenoprotein. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 62, 309–312. [Google Scholar] [CrossRef]
- Rønneberg, H.; Fox, D.L.; Liaaen-Jensen, S. Animal carotenoids—Carotenoproteins from hydrocorals. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 64, 407–408. [Google Scholar] [CrossRef]
- Fujii, R.; Yamano, N.; Hashimoto, H.; Misawa, N.; Ifuku, K. Photoprotection vs. Photoinhibition of Photosystem II in Transplastomic Lettuce (Lactuca sativa) Dominantly Accumulating Astaxanthin. Plant Cell Physiol. 2015, 57, 187–1529. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef]
- Kay Holt, T.; Krogmann, D.W. A carotenoid-protein from cyanobacteria. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1981, 637, 408–414. [Google Scholar] [CrossRef]
- Kawasaki, S.; Mizuguchi, K.; Sato, M.; Kono, T.; Shimizu, H. A Novel Astaxanthin-Binding Photooxidative Stress-Inducible Aqueous Carotenoprotein from a Eukaryotic Microalga Isolated from Asphalt in Midsummer. Plant Cell Physiol. 2013, 54, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Yamazaki, K.; Nishikata, T.; Ishige, T.; Toyoshima, H.; Miyata, A. Photooxidative stress-inducible orange and pink water-soluble astaxanthin-binding proteins in eukaryotic microalga. Commun. Biol. 2020, 3, 490. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Miyata, A.; Yoshida, R.; Ishige, T.; Takaichi, S.; Kawasaki, S. Distribution of the Water-Soluble Astaxanthin Binding Carotenoprotein (AstaP) in Scenedesmaceae. Mar. Drugs 2021, 19, 349. [Google Scholar] [CrossRef]
- Slonimskiy, Y.B.; Egorkin, N.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Microalgal protein AstaP is a potent carotenoid solubilizer and delivery module with a broad carotenoid binding repertoire. FEBS J. 2022, 289, 999–1022. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Miyazawa, S.; Yoshimura, S.; Shinzaki, Y.; Tomizawa, K.; Shindo, K.; Choi, S.K.; Misawa, N.; Miyake, C. Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J. 2008, 55, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Roding, A.; Dietzel, L.; Schlicke, H.; Grimm, B.; Sandmann, G.; Buchel, C. Production of ketocarotenoids in tobacco alters the photosynthetic efficiency by reducing photosystem II supercomplex and LHCII trimer stability. Photosynth. Res. 2015, 123, 157–165. [Google Scholar] [CrossRef]
- Harada, H.; Maoka, T.; Osawa, A.; Hattan, J.; Kanamoto, H.; Shindo, K.; Otomatsu, T.; Misawa, N. Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 2014, 23, 303–315. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Tabunoki, H.; Sugiyama, H.; Tanaka, Y.; Fujii, H.; Banno, Y.; Jouni, Z.E.; Kobayashi, M.; Sato, R.; Maekawa, H.; Tsuchida, K. Isolation, Characterization, and cDNA Sequence of a Carotenoid Binding Protein from the Silk Gland of Bombyx mori Larvae. J. Biol. Chem. 2002, 277, 32133–32140. [Google Scholar] [CrossRef]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef]
- Yuasa, M.; Kitamura, A.; Maoka, T.; Sakudoh, T.; Shimada, T.; Tsuchida, K. Astaxanthin and lutein compete for accumulation into the middle silk gland via Yellow cocoon gene (C′)-dependent control and produce a red cocoon of Bombyx mori. J. Insect Biotechnol. Sericology 2014, 83, 1–11. [Google Scholar] [CrossRef]
- Slonimskiy, Y.B.; Egorkin, N.A.; Ashikhmin, A.A.; Friedrich, T.; Maksimov, E.G.; Sluchanko, N.N. Reconstitution of the functional Carotenoid-Binding Protein from silkworm in E. coli. Int. J. Biol. Macromol. 2022, 214, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Egorkin, N.A.; Varfolomeeva, L.A.; Kleymenov, S.Y.; Minyaev, M.E.; Faletrov, Y.V.; Moysenovich, A.M.; Parshina, E.Y.; Friedrich, T.; et al. Structural basis for the carotenoid binding and transport function of a START domain. Structure 2022, 30, 1647–1659.e1644. [Google Scholar] [CrossRef]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Egorkin, N.A.; Varfolomeeva, L.A.; Faletrov, Y.V.; Moysenovich, A.M.; Parshina, E.Y.; Friedrich, T.; Maksimov, E.G.; Boyko, K.M.; et al. Silkworm carotenoprotein as an efficient carotenoid extractor, solubilizer and transporter. Int. J. Biol. Macromol. 2022, 223, 1381–1393. [Google Scholar] [CrossRef]
- Henmi, H.; Hata, M.; Hata, M. Studies on the carotenoids in the muscle of salmon-II. Astaxanthin and/or canthaxanthin-actomyosin complex in salmon muscle. Nippon. Suisan Gakkaishi 1989, 55, 1583–1589. [Google Scholar] [CrossRef]
- Henmi, H.; Hata, M.; Takeuchi, M. Studies on the carotenoids in the muscle of salmon. IV. Resonance Raman and circular dichroism studies of astaxanthin and/or canthaxanthin in salmon muscle. Nippon. Suisan Gakkaishi 1990, 56, 1825–1828. [Google Scholar] [CrossRef]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2021, 14, 107. [Google Scholar] [CrossRef]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 12365–12367. [Google Scholar] [CrossRef]
- Rutherford, A.; Krieger-Liszkay, A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 2001, 26, 648–653. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef]
- Ledford, H.K.; Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 2005, 28, 1037–1045. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet oxygen production in photosystem II and related protection mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef]
- Mano, C.M.; Prado, F.M.; Massari, J.; Ronsein, G.E.; Martinez, G.R.; Miyamoto, S.; Cadet, J.; Sies, H.; Medeiros, M.H.G.; Bechara, E.J.H.; et al. Excited singlet molecular O2 (1Δg) is generated enzymatically from excited carbonyls in the dark. Sci. Rep. 2014, 4, 5938. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P. Singlet molecular oxygen generated by biological hydroperoxides. J. Photochem. Photobiol. B Biol. 2014, 139, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P.; Prasad, A. Formation of singlet oxygen and protection against its oxidative damage in Photosystem II under abiotic stress. J. Photochem. Photobiol. B Biol. 2014, 137, 39–48. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Shichiri, M.; Tanito, M.; Yoshida, Y. Significance of Singlet Oxygen Molecule in Pathologies. Int. J. Mol. Sci. 2023, 24, 2739. [Google Scholar] [CrossRef]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Tamura, H.; Ishikita, H. Quenching of Singlet Oxygen by Carotenoids via Ultrafast Superexchange Dynamics. J. Phys. Chem. A 2020, 124, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.F.; Schalch, W.; Truscott, T. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Edge, R.; McGarvey, D.; Truscott, T. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, J.; Hashimoto, H.; Matsudaira, S.; Koyama, Y. Resonance Raman Spectra of Excited Triplet-States of Β-Carotene Isomers. Chem. Lett. 1985, 14, 311–314. [Google Scholar] [CrossRef]
- Hashimoto, H.; Koyama, Y. Time-resolved resonance Raman spectroscopy of triplet beta.-carotene produced from all-trans, 7-cis, 9-cis, 13-cis, and 15-cis isomers and high-pressure liquid chromatography analyses of photoisomerization via the triplet state. J. Phys. Chem. 2002, 92, 2101–2108. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B Biol. 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Oshima, S.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Terao, J. Inhibitory Effect of BETA.-Carotene and Astaxanthin on Photosensitized Oxidation of Phospholipid Bilayers. J. Nutr. Sci. Vitaminol. 1993, 39, 607–615. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M. Protective effects of astaxanthin against singlet oxygen induced damage in human dermal fibroblasts in vitro. Food Style 2009, 13, 84–86. [Google Scholar]
- Murotomi, K.; Umeno, A.; Sugino, S.; Yoshida, Y. Quantitative kinetics of intracellular singlet oxygen generation using a fluorescence probe. Sci. Rep. 2020, 10, 10616. [Google Scholar] [CrossRef] [PubMed]
- Nishino, A.; Maoka, T.; Yasui, H. Analysis of reaction products of astaxanthin and its acetate with reactive oxygen species using LC/PDA ESI-MS and ESR spectrometry. Tetrahedron Lett. 2016, 57, 1967–1970. [Google Scholar] [CrossRef]
- Nishida, Y.; Yamashita, E.; Miki, W. Quenching activities of common hydrophilic and lipophilic antioxidants against singlet oxygen using chemiluminescence detection system. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Mukai, K. Antioxidant Activity of Foods: Development of Singlet Oxygen Absorption Capacity (SOAC) Assay Method. J. Nutr. Sci. Vitaminol. 2019, 65, 285–302. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Et Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef]
- Conn, P.F.; Lambert, C.; Land, E.J.; Schalch, W.; Truscott, T.G. Carotene-Oxygen Radical Interactions. Free. Radic. Res. Commun. 1992, 16, 401–408. [Google Scholar] [CrossRef]
- Böhm, F.; Tinkler, J.; Truscott, T. Carotenoids protect against cell membrane damage by the nitrogen dioxide radical. Nat. Med. 1995, 1, 98–99. [Google Scholar] [CrossRef]
- Truscott, T.; McGARVEY, D.; Lambert, C.; Hill, T.; Tinkler, J.; Conn, P.; Böhm, F.; Land, E.; Schalch, W. The interaction of carotenoids with reactive oxy-species. Biochem. Soc. Trans. 1995, 23, 252S. [Google Scholar] [CrossRef] [PubMed]
- Everett, S.A.; Dennis, M.F.; Patel, K.B.; Maddix, S.; Kundu, S.C.; Willson, R.L. Scavenging of Nitrogen Dioxide, Thiyl, and Sulfonyl Free Radicals by the Nutritional Antioxidant beta-Carotene. J. Biol. Chem. 1996, 271, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.J.; Land, E.J.; McGarvey, D.J.; Schalch, W.; Tinkler, J.H.; Truscott, T.G. Interactions between Carotenoids and the CCl3O2.bul. Radical. J. Am. Chem. Soc. 2002, 117, 8322–8326. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Sampson, J.; Bramley, P.M.; Holloway, D.E. Why Do We Expect Carotenoids to be Antioxidantsin vivo? Free. Radic. Res. 1997, 26, 381–398. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett. 1997, 417, 261–266. [Google Scholar] [CrossRef]
- Han, R.-M.; Chen, C.-H.; Tian, Y.-X.; Zhang, J.-P.; Skibsted, L.H. Fast Regeneration of Carotenoids from Radical Cations by Isoflavonoid Dianions: Importance of the Carotenoid Keto Group for Electron Transfer. J. Phys. Chem. A 2009, 114, 126–132. [Google Scholar] [CrossRef]
- Terao, J. Antioxidant activity of β-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping β-Carotene and Retinol Supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef]
- World Health Organization. IARC Fruit and vegetables. In Handbooks of Cancer Prevention; IARC Press: Lyon, France, 2003; Volume 8. [Google Scholar]
- Wakai, K.; Sugawara, Y.; Tsuji, I.; Tamakoshi, A.; Shimazu, T.; Matsuo, K.; Nagata, C.; Mizoue, T.; Tanaka, K.; Inoue, M.; et al. Risk of lung cancer and consumption of vegetables and fruit in Japanese: A pooled analysis of cohort studies in Japan. Cancer Sci. 2015, 106, 1057–1065. [Google Scholar] [CrossRef]
- Narita, S.; Saito, E.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Ishihara, J.; Takachi, R.; Shibuya, K.; Inoue, M.; et al. Dietary consumption of antioxidant vitamins and subsequent lung cancer risk: The Japan Public Health Center-based prospective study. Int. J. Cancer 2018, 142, 2441–2460. [Google Scholar] [CrossRef] [PubMed]
- Focsan, A.L.; Pan, S.; Kispert, L.D. Electrochemical Study of Astaxanthin and Astaxanthin n-Octanoic Monoester and Diester: Tendency to Form Radicals. J. Phys. Chem. B 2014, 118, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Kado, T.; Takikawa, A.; Igarashi, Y.; Onogi, Y.; Wada, T.; Sasaoka, T.; Yamamoto, S.; Sasahara, M.; et al. Astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle via activation of AMPK pathway. J. Cachex- Sarcopenia Muscle 2020, 11, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N. When Carotenoid Biosynthesis Genes Met Escherichia coli: The Early Days and These Days. In Carotenoids: Biosynthetic and Biofunctional Approaches; Springer: Singapore, 2021. [Google Scholar]
- Harker, M.; Hirschberg, J.; Oren, A. Paracoccus marcusii sp. nov., an orange Gram-negative coccus. Int. J. Syst. Evol. Microbiol. 1998, 48 (Pt 2), 543–548. [Google Scholar] [CrossRef]
- Tsubokura, A.; Yoneda, H.; Mizuta, H. Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 1999, 49 (Pt 1), 277–282. [Google Scholar] [CrossRef]
- Takaichi, S.; Maoka, T.; Akimoto, N.; Khan, S.T.; Harayama, S. Major Carotenoid Isolated from Paracoccus schoinia NBRC 100637T Is Adonixanthin Diglucoside. J. Nat. Prod. 2006, 69, 1823–1825. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miki, W.; Izumida, H.; Shizuri, Y. New Trihydroxy-keto-carotenoids Isolated from an Astaxanthin-producing Marine Bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 200–203. [Google Scholar] [CrossRef]
- Asker, D.; Beppu, T.; Ueda, K. Sphingomonas astaxanthinifaciens sp. nov., a novel astaxanthin-producing bacterium of the family Sphingomonadaceae isolated from Misasa, Tottori, Japan. FEMS Microbiol. Lett. 2007, 273, 140–148. [Google Scholar] [CrossRef]
- Osanjo, G.; Muthike, E.; Tsuma, L.; Okoth, M.; Bulimo, W.; Lünsdorf, H.; Abraham, W.-R.; Dion, M.; Timmis, K.; Golyshin, P.; et al. A salt lake extremophile, Paracoccus bogoriensis sp. nov., efficiently produces xanthophyll carotenoids. Afr. J. Microbiol. Res. 2009, 3, 426–433. [Google Scholar]
- Matsumoto, M.; Iwama, D.; Arakaki, A.; Tanaka, A.; Tanaka, T.; Miyashita, H.; Matsunaga, T. Altererythrobacter ishigakiensis sp. nov., an astaxanthin-producing bacterium isolated from a marine sediment. Int. J. Syst. Evol. Microbiol. 2011, 61, 2956–2961. [Google Scholar] [CrossRef]
- Shahina, M.; Hameed, A.; Lin, S.-Y.; Hsu, Y.-H.; Liu, Y.-C.; Cheng, I.-C.; Lee, M.-R.; Lai, W.-A.; Lee, R.-J.; Young, C.-C. Sphingomicrobium astaxanthinifaciens sp. nov., an astaxanthin-producing glycolipid-rich bacterium isolated from surface seawater and emended description of the genus Sphingomicrobium. Int. J. Syst. Evol. Microbiol. 2013, 63, 3415–3422. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Purification and Identification of Astaxanthin and Its Novel Derivative Produced by Radio-tolerant Sphingomonas astaxanthinifaciens. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.-L., Eds.; Springer: New York, NY, USA, 2018; pp. 171–192. [Google Scholar] [CrossRef]
- Nishida, Y.; Adachi, K.; Kasai, H.; Shizuri, Y.; Shindo, K.; Sawabe, A.; Komemushi, S.; Miki, W.; Misawa, N. Elucidation of a Carotenoid Biosynthesis Gene Cluster Encoding a Novel Enzyme, 2,2′-β-Hydroxylase, from Brevundimonas sp. Strain SD212 and Combinatorial Biosynthesis of New or Rare Xanthophylls. Appl. Environ. Microbiol. 2005, 71, 4286–4296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, X.; Li, O.; Qian, C.; Gao, H. Cloning and Characterization of Genes Involved in Nostoxanthin Biosynthesis of Sphingomonas elodea ATCC 31461. PLoS ONE 2012, 7, e35099. [Google Scholar] [CrossRef]
- Masamoto, K.; Misawa, N.; Kaneko, T.; Kikuno, R.; Toh, H. r Carotene Hydroxylase Gene from the Cyanobacterium Synechocystis sp. PCC. Plant Cell Physiol. 1998, 39, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, B.; Sandmann, G.; Vioque, A. A New Type of Asymmetrically Acting β-Carotene Ketolase Is Required for the Synthesis of Echinenone in the Cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 1997, 272, 9728–9733. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Harada, H.; Ikenaga, H.; Matsuda, S.; Takaichi, S.; Shindo, K.; Sandmann, G.; Ogata, T.; Misawa, N. Characterization of Cyanobacterial Carotenoid Ketolase CrtW and Hydroxylase CrtR by Complementation Analysis in Escherichia coli. Plant Cell Physiol. 2008, 49, 1867–1878. [Google Scholar] [CrossRef]
- Sugiyama, K.; Takaichi, S. Carotenogenesis in cyanobacteria: CruA/CruP-type and CrtL-type lycopene cyclases. J. Gen. Appl. Microbiol. 2020, 66, 53–58. [Google Scholar] [CrossRef]
- Goodwin, T. The Biochemistry of the Carotenoids: Volume I Plants; Springer Science & Business Media: New York, NY, USA, 1980. [Google Scholar]
- Choi, S.-K.; Harada, H.; Matsuda, S.; Misawa, N. Characterization of two β-carotene ketolases, CrtO and CrtW, by complementation analysis in Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 75, 1335–1341. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takaki, A.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Single-Stage Astaxanthin Production Enhances the Nonmevalonate Pathway and Photosynthetic Central Metabolism in Synechococcus sp. PCC 7002. ACS Synth. Biol. 2019, 8, 2701–2709. [Google Scholar] [CrossRef]
- Yokoyama, A.; Adachi, K.; Shizuri, Y. New Carotenoid Glucosides, Astaxanthin Glucoside and Adonixanthin Glucoside, Isolated from the Astaxanthin-Producing Marine Bacterium, Agrobacterium aurantiacum. J. Nat. Prod. 1995, 58, 1929–1933. [Google Scholar] [CrossRef]
- Asker, D.; Amano, S.-I.; Morita, K.; Tamura, K.; Sakuda, S.; Kikuchi, N.; Furihata, K.; Matsufuji, H.; Beppu, T.; Ueda, K. Astaxanthin dirhamnoside, a new astaxanthin derivative produced by a radio-tolerant bacterium, Sphingomonas astaxanthinifaciens. J. Antibiot. 2009, 62, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, J.H.; Lee, B.Y.; Lee, P.C. The astaxanthin dideoxyglycoside biosynthesis pathway in Sphingomonas sp. PB 304. Appl. Microbiol. Biotechnol. 2014, 98, 9993–10003. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.H.; Kim, K.H.; Lee, P.C. Sphingomonas lacus sp. nov., an astaxanthin-dideoxyglycoside-producing species isolated from soil near a pond. Int. J. Syst. Evol. Microbiol. 2015, 65, 2824–2830. [Google Scholar] [CrossRef]
- Maruyama, T.; Kasai, H.; Choi, S.K.; Ramasamy, A.K.; Inomata, Y.; Misawa, N. Structure of a complete carotenoid biosynthesis gene cluster of marine bacterium Paracoccus sp. Strain NCarotenoid Sci. 2007, 11, 50–55. [Google Scholar]
- Yokoyama, A.; Shizuri, Y.; Misawa, N. Production of new carotenoids, astaxanthin glucosides, by Escherichia coli transformants carrying carotenoid biosynthesis genes. Tetrahedron Lett. 1998, 39, 3709–3712. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Isolation, Characterization, and Diversity of Novel Radiotolerant Carotenoid-Producing Bacteria. Methods Mol. Biol. 2012, 892, 21–60. [Google Scholar] [CrossRef] [PubMed]
- Calo, P.; De Miguel, T.; Sieiro, C.; Velazquez, J.; Villa, T. Ketocarotenoids in halobacteria: 3-hydroxy-echinenone and trans-astaxanthin. J. Appl. Bacteriol. 1995, 79, 282–285. [Google Scholar] [CrossRef]
- Alvares, J.J.; Furtado, I.J. Characterization of multicomponent antioxidants from Haloferax alexandrinus GUSF-1 (KF796625). 3 Biotech 2021, 11, 58. [Google Scholar] [CrossRef]
- Sandmann, G. Carotenoids and Their Biosynthesis in Fungi. Molecules 2022, 27, 1431. [Google Scholar] [CrossRef]
- Phaff, H.J.; Yoneyama, M.; Soneda, M. A comparative study of the yeast florae associated with trees on the Japanese islands and on the west coast of North America. In Proceedings of the IV IFS Fermentation Technology Today, Kyoto, Japan, 19–25 March 1972. [Google Scholar]
- Miller, M.W.; Yoneyama, M.; Soneda, M. Phaffia, a New Yeast Genus in the Deuteromycotina (Blastomycetes). Int. J. Syst. Evol. Microbiol. 1976, 26, 286–291. [Google Scholar] [CrossRef]
- Libkind, D.; Moliné, M.; de García, V.; Fontenla, S.; van Broock, M. Characterization of a novel South American population of the astaxanthin producing yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). J. Ind. Microbiol. Biotechnol. 2008, 35, 151–158. [Google Scholar] [CrossRef] [PubMed]
- David-Palma, M.; Libkind, D.; Brito, P.H.; Silva, M.; Bellora, N.; Coelho, M.A.; Heitman, J.; Gonçalves, P.; Sampaio, J.P. The Untapped Australasian Diversity of Astaxanthin-Producing Yeasts with Biotechnological Potential—Phaffia australis sp. nov. and Phaffia tasmanica sp. nov. Microorganisms 2020, 8, 1651. [Google Scholar] [CrossRef]
- Li, A.-H.; Yuan, F.-X.; Groenewald, M.; Bensch, K.; Yurkov, A.M.; Li, K.; Han, P.-J.; Guo, L.-D.; Aime, M.C.; Sampaio, J.; et al. Diversity and phylogeny of basidiomycetous yeasts from plant leaves and soil: Proposal of two new orders, three new families, eight new genera and one hundred and seven new species. Stud. Mycol. 2020, 96, 17–140. [Google Scholar] [CrossRef]
- David-Palma, M.; Libkind, D.; Sampaio, J.P. Global distribution, diversity hot spots and niche transitions of an astaxanthin-producing eukaryotic microbe. Mol. Ecol. 2014, 23, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.; Rodríguez-Sáiz, M.; de la Fuente, J.L.; Gudiña, E.J.; Godio, R.P.; Martín, J.F.; Barredo, J.L. The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome-P450 hydroxylase involved in the conversion of β-carotene into astaxanthin and other xanthophylls. Fungal Genet. Biol. 2006, 43, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Breitenbach, J.; Visser, H.; Setoguchi, Y.; Tabata, K.; Hoshino, T.; van den Berg, J.; Sandmann, G. Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a β-carotene 3-hydroxylase/4-ketolase. Mol. Genet. Genom. 2006, 275, 148–158. [Google Scholar] [CrossRef]
- Flores-Cotera, L.B.; Chávez-Cabrera, C.; Martínez-Cárdenas, A.; Sánchez, S.; García-Flores, O.U. Deciphering the mechanism by which the yeast Phaffia rhodozyma responds adaptively to environmental, nutritional, and genetic cues. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab048. [Google Scholar] [CrossRef]
- Green, J. The occurence of astaxanthin in the euglinoid Trachelomonas volvocina. Comp. Biochem. Physiol. 1963, 9, 313–316. [Google Scholar] [CrossRef]
- Czeczuga, B. Carotenoids in Euglena rubida Mainx. Comp. Biochem. Physiol. Part B Comp. Biochem. 1974, 48, 349–354. [Google Scholar] [CrossRef]
- Gerber, S.; Häder, D.-P. Effects of enhanced UV-B irradiation on the red coloured freshwater flagellate Euglena sanguinea. FEMS Microbiol. Ecol. 1994, 13, 177–184. [Google Scholar] [CrossRef]
- Watanabe, K.; Arafiles, K.H.V.; Higashi, R.; Okamura, Y.; Tajima, T.; Matsumura, Y.; Nakashimada, Y.; Matsuyama, K.; Aki, T. Isolation of High Carotenoid-producing Aurantiochytrium sp. Mutants and Improvement of Astaxanthin Productivity Using Metabolic Information. J. Oleo Sci. 2018, 67, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Cazzaniga, S.; Martini, F.; Paltrinieri, S.; Bossi, S.; Maffei, M.E.; Ballottari, M. Astaxanthin and eicosapentaenoic acid production by S4, a new mutant strain of Nannochloropsis gaditana. Microb. Cell Factories 2022, 21, 117. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Nakada, T. Is the scientific name of the astaxanthin-producing Haematococcus (Genus: Haematococcus; Chlorophyta), Haematococcus lacustris or Haematococcus pluvialis ? Jpn. J. Phycol. 2014, 62, 7–10. (In Japanese) [Google Scholar]
- Nakada, T.; Ota, S. What is the correct name for the type of Haematococcus Flot. (Volvocales, Chlorophyceae)? Taxon 2016, 65, 343–348. [Google Scholar] [CrossRef]
- Hazen, T.E. The Life History of Sphaerella lacustris (Haematococcus pluvialis ). Mem. Torrey Bot. Club 1899, 6, 211–246. [Google Scholar]
- Lang, N.J. Electron Microscopic Studies of Extraplastidic Astaxanthin in Haematococcus2. J. Phycol. 1968, 4, 12–19. [Google Scholar] [CrossRef]
- Buchheim, M.A.; Sutherland, D.M.; Buchheim, J.A.; Wolf, M. The blood alga: Phylogeny ofHaematococcus(Chlorophyceae) inferred from ribosomal RNA gene sequence data. Eur. J. Phycol. 2013, 48, 318–329. [Google Scholar] [CrossRef]
- Grung, M.; Metzger, P.; Liaaen-Jensen, S. Algal carotenoids 53; secondary carotenoids of algae 4; secondary carotenoids in the green alga Botryococcus braunii, race L, new strain. Biochem. Syst. Ecol. 1994, 22, 25–29. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ng, Y.K.; Phang, S.M. Composition and accumulation of secondary carotenoids in Chlorococcum sp. J. Appl. Phycol. 1997, 9, 147–155. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef]
- Orosa, M.; Valero, J.; Herrero, C.; Abalde, J. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol. Lett. 2001, 23, 1079–1085. [Google Scholar] [CrossRef]
- Remias, D.; Lütz-Meindl, U.; Lütz, C. Photosynthesis, pigments and ultrastructure of the alpine snow algaChlamydomonas nivalis. Eur. J. Phycol. 2005, 40, 259–268. [Google Scholar] [CrossRef]
- Fujii, K.; Imazato, E.; Nakashima, H.; Ooi, O.; Saeki, A. Isolation of the non-fastidious microalga with astaxanthin-accumulating property and its potential for application to aquaculture. Aquaculture 2006, 261, 285–293. [Google Scholar] [CrossRef]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of carotenoids and identification of aerial microalgae isolated from the surface of rocks in mountainous districts of Japan. Algal Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef]
- Hu, C.-W.; Chuang, L.-T.; Yu, P.-C.; Chen, C.-N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Kawasaki, S.; Yoshida, R.; Ohkoshi, K.; Toyoshima, H. Coelastrella astaxanthina sp. nov. (Sphaeropleales, Chlorophyceae), a novel microalga isolated from an asphalt surface in midsummer in Japan. Phycol. Res. 2019, 68, 107–114. [Google Scholar] [CrossRef]
- Singh, D.P.; Khattar, J.S.; Rajput, A.; Chaudhary, R.; Singh, R. High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS ONE 2019, 14, e0221930. [Google Scholar] [CrossRef]
- Tharek, A.; Mohamad, S.; Iwamoto, K.; Hara, H.; Ashyikin, N.; Binti Kaha, M.; Salleh, M.; Jamaluddin, H. Astaxanthin Production by Tropical Microalgae Strains Isolated from Environment in Malaysia. Asian J. Microbiol. Biotechnol. Environ. Sci. 2020, 22, 168–173. [Google Scholar]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined Production of Astaxanthin and β-Carotene in a New Strain of the Microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) Cultivated in Photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef]
- Doppler, P.; Kornpointner, C.; Halbwirth, H.; Remias, D.; Spadiut, O. Tetraedron minimum, First Reported Member of Hydrodictyaceae to Accumulate Secondary Carotenoids. Life 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Procházková, L.; Remias, D.; Holzinger, A.; Řezanka, T.; Nedbalová, L. Ecophysiological and ultrastructural characterisation of the circumpolar orange snow alga Sanguina aurantia compared to the cosmopolitan red snow alga Sanguina nivaloides (Chlorophyta). Polar Biol. 2021, 44, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1.40. under optimized culture conditions. J. Basic Microbiol. 2021, 62, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, T.; Xie, Y.; Zou, S.; Xu, S.; Lai, M.; Zuo, Z. Astaxanthin accumulation in Microcystis aeruginosa under different light quality. Bioresour. Technol. 2021, 346, 126629. [Google Scholar] [CrossRef]
- Zohir, W.F.; Kapase, V.U.; Kumar, S. Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin. Biology 2022, 11, 884. [Google Scholar] [CrossRef]
- Kleppel, G.; Lessard, E. Carotenoid pigments in microzooplankton. Mar. Ecol. Prog. Ser. 1992, 84, 211–218. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef]
- Andersson, M.; Van Nieuwerburgh, L.; Snoeijs, P. Pigment transfer from phytoplankton to zooplankton with emphasis on astaxanthin production in the Baltic Sea food web. Mar. Ecol. Prog. Ser. 2003, 254, 213–224. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-Dimensional Ultrastructural Study of Oil and Astaxanthin Accumulation during Encystment in the Green Alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef]
- Collins, A.M.; Jones, H.D.T.; Han, D.; Hu, Q.; Beechem, T.E.; Timlin, J.A. Carotenoid Distribution in Living Cells of Haematococcus pluvialis (Chlorophyceae). PLoS ONE 2011, 6, e24302. [Google Scholar] [CrossRef] [PubMed]
- Fagerer, S.R.; Schmid, T.; Ibáñez, A.J.; Pabst, M.; Steinhoff, R.; Jefimovs, K.; Urban, P.L.; Zenobi, R. Analysis of single algal cells by combining mass spectrometry with Raman and fluorescence mapping. Analyst 2013, 138, 6732–6736. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Morita, A.; Ohnuki, S.; Hirata, A.; Sekida, S.; Okuda, K.; Ohya, Y.; Kawano, S. Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci. Rep. 2018, 8, 5617. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Gu, W.; Jiang, L.; Zhu, Y.; Gong, A. Study on the Visualization of Pigment in Haematococcus pluvialis by Raman Spectroscopy Technique. Sci. Rep. 2019, 9, 12097. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Wang, B.; Zarka, A.; Trebst, A.; Boussiba, S. Astaxanthin Accumulation in Haematococcus pluvialis (Chlorophyceae) as An Active Photoprotective Process under High Irradiance1. J. Phycol. 2003, 39, 1116–1124. [Google Scholar] [CrossRef]
- Hoham, R.W.; Remias, D. Snow and Glacial Algae: A Review. J. Phycol. 2020, 56, 264–282. [Google Scholar] [CrossRef]
- Peled, E.; Pick, U.; Zarka, A.; Shimoni, E.; Leu, S.; Boussiba, S. Light-Induced Oil Globule Migration InHaematococcus Pluvialis (Chlorophyceae). J. Phycol. 2012, 48, 1209–1219. [Google Scholar] [CrossRef]
- Laza-Martínez, A.; Fernández-Marín, B.; García-Plazaola, J.I. Rapid colour changes inEuglena sanguinea(Euglenophyceae) caused by internal lipid globule migration. Eur. J. Phycol. 2018, 54, 91–101. [Google Scholar] [CrossRef]
- Toyoshima, H.; Takaichi, S.; Kawasaki, S. Water-soluble astaxanthin-binding protein (AstaP) from Coelastrella astaxanthina Ki-4 (Scenedesmaceae) involving in photo-oxidative stress tolerance. Algal Res. 2020, 50, 101988. [Google Scholar] [CrossRef]
- Cuttriss, A.J.; Chubb, A.C.; Alawady, A.; Grimm, B.; Pogson, B.J. Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Funct. Plant Biol. 2007, 34, 663–672. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga, B. Ketocarotenoids—Autumn carotenoids in Metasequoia glyptostroboides. Biochem. Syst. Ecol. 1987, 15, 303–306. [Google Scholar] [CrossRef]
- Ida, K.; Saito, F.; Takeda, S. Isomers of rhodoxanthin in reddish brown leaves of gymnosperms and effect of daylight intensity on the contents of pigments during autumnal coloration. J. Plant Res. 1991, 104, 157–169. [Google Scholar] [CrossRef]
- Ida, K.; Masamoto, K.; Maoka, T.; Fujiwara, Y.; Takeda, S.; Hasegawa, E. The leaves of the common box,Buxus sempervirens (Buxaceae), become red as the level of a red carotenoid, anhydroeschscholtzxanthin, increases. J. Plant Res. 1995, 108, 369–376. [Google Scholar] [CrossRef]
- Kamata, T.; Simpson, K.L. Study of astaxanthin diester extracted from Adonis aestivalis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 587–591. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Gantt, E. Elucidation of the Pathway to Astaxanthin in the Flowers of Adonis aestivalis. Plant Cell 2011, 23, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Etoh, T.; Kishimoto, S.; Sakata, S. Carotenoids and Their Fatty Acid Esters in the Petals of Adonis aestivalis. J. Oleo Sci. 2011, 60, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Takemura, M.; Maoka, T. Carotenoid Biosynthesis in Animals: Case of Arthropods. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Springer Singapore: Singapore, 2021; pp. 217–220. [Google Scholar]
- Hill, G.E.; Johnson, J.D. The Vitamin A–Redox Hypothesis: A Biochemical Basis for Honest Signaling via Carotenoid Pigmentation. Am. Nat. 2012, 180, E127–E150. [Google Scholar] [CrossRef]
- Tomášek, O.; Gabrielová, B.; Kačer, P.; Maršík, P.; Svobodová, J.; Syslová, K.; Vinkler, M.; Albrecht, T. Opposing effects of oxidative challenge and carotenoids on antioxidant status and condition-dependent sexual signalling. Sci. Rep. 2016, 6, 23546. [Google Scholar] [CrossRef]
- Weaver, R.J.; Santos, E.S.A.; Tucker, A.M.; Wilson, A.E.; Hill, G.E. Carotenoid metabolism strengthens the link between feather coloration and individual quality. Nat. Commun. 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.E.; Hill, G.E.; Sandercock, B. Do carotenoid-based ornaments entail resource trade-offs? An evaluation of theory and data. Funct. Ecol. 2018, 32, 1908–1920. [Google Scholar] [CrossRef]
- Hill, G.E. Evolution: The biochemistry of honest sexual signaling. Curr. Biol. 2022, 32, R1005–R1007. [Google Scholar] [CrossRef] [PubMed]
- Bezzerides, A.L.; McGraw, K.J.; Parker, R.S.; Husseini, J. Elytra color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 2007, 61, 1401–1408. [Google Scholar] [CrossRef]
- Stevens, M.; Mappes, J.; Sandre, S.-L. The effect of predator appetite, prey warning coloration and luminance on predator foraging decisions. Behaviour 2010, 147, 1121–1143. [Google Scholar] [CrossRef]
- Lindstedt, C.; Eager, H.; Ihalainen, E.; Kahilainen, A.; Stevens, M.; Mappes, J. Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav. Ecol. 2011, 22, 580–587. [Google Scholar] [CrossRef]
- Lindstedt, C.; Schroderus, E.; Lindström, L.; Mappes, T.; Mappes, J. Evolutionary constraints of warning signals: A genetic trade-off between the efficacy of larval and adult warning coloration can maintain variation in signal expression. Evolution 2016, 70, 2562–2572. [Google Scholar] [CrossRef]
- Stevens, M.; Ruxton, G.D. Linking the evolution and form of warning coloration in nature. Proc. R. Soc. B Boil. Sci. 2011, 279, 417–426. [Google Scholar] [CrossRef]
- Matsuno, T.; Maoka, T.; Katsuyama, M.; Ookubo, M.; Katagiri, K.; Jimura, H. The occurrence of enantiomeric and meso-astaxanthin in aquatic animals. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1589–1592. [Google Scholar] [CrossRef]
- Hertberg, S.; Liaaen-Jensenm, S.; Enzell, C.R.; Francis, G.W. Carotenoids. 3.* The Carotenoids of Actinia equina Structure Determination of Actinoerythrin and Violerythrin. Acta Chem. Scand. 1969, 23, 3290. [Google Scholar] [CrossRef]
- Czeczuga, B. Some carotenoids in the jelly-fish Aurelia aurita (Scyphozoa: Discomedusae). Mar. Biol. 1970, 5, 141–144. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N.; Tsushima, M.; Komemushi, S.; Mezaki, T.; Iwase, F.; Takahashi, Y.; Sameshima, N.; Mori, M.; Sakagami, Y. Carotenoids in Marine Invertebrates Living along the Kuroshio Current Coast. Mar. Drugs 2011, 9, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Matsuno, T.; Hiraoka, K.; Maoka, T. Carotenoids in the Gonad of Scallops. Nippon. Suisan Gakkaishi 1981, 47, 385–390. [Google Scholar] [CrossRef]
- Narita, M.; Maoka, T.; Ebitani, K.; Nishino, H. Characteristics of chemical constituents and red pigment of orange adductor muscle of scallop Mizuhopecten yessoensis in the Okhotsk Sea and anti-oxidative activity of the pigment. Nippon. Suisan Gakkaishi 2013, 79, 48–54. [Google Scholar] [CrossRef]
- Liu, H.; Tan, K.S.; Zhang, X.; Zhang, H.; Cheng, D.; Ting, Y.; Li, S.; Ma, H.; Zheng, H. Comparison of Gut Microbiota Between Golden and Brown Noble Scallop Chlamys nobilis and Its Association with Carotenoids. Front. Microbiol. 2020, 11, 36. [Google Scholar] [CrossRef]
- Han, J.; Lu, Y.; Zheng, H.; Liu, H.; Deng, H.; Zhang, B. Differential expression of CuZnSOD gene under low temperature stress in noble scallop Chlamys nobilis with different carotenoid content. Fish Shellfish. Immunol. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, B.; Ma, H.; Li, S.; Zheng, H. Oxidative stress responses of golden and brown noble scallops Chlamys nobilis to acute cold stress. Fish Shellfish. Immunol. 2019, 95, 349–356. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Lim, L.-S.; Ma, H.; Li, S.; Zheng, H. Roles of Carotenoids in Invertebrate Immunology. Front. Immunol. 2019, 10, 3041. [Google Scholar] [CrossRef]
- Maoka, T.; Yokoi, S.; Matsuno, T. Comparative biochemical studies of carotenoids in nine species of cephalopoda. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 92, 247–250. [Google Scholar] [CrossRef]
- Veerman, A. The carotenoid pigments of Schizonobia sycophanta Womersley (Acari: Tetranychidae). Comp. Biochem. Physiol. 1974, 48, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Prado-Cabrero, A.; Saefurahman, G.; Nolan, J.M. Stereochemistry of Astaxanthin Biosynthesis in the Marine Harpacticoid Copepod Tigriopus californicus. Mar. Drugs 2020, 18, 506. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Katsuyama, M.; Kaneko, N.; Matsuno, T. Stereochemical investigation of carotenoids in the antarctic krill Euphausia superba. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1671–1673. [Google Scholar] [CrossRef]
- Tlusty, M.F.; Metzler, A.; Huckabone, S.; Suanda, S.; Guerrier, S. Morphological colour change in the American lobster (Homarus americanus) in response to background colour and UV light. N. Z. J. Mar. Freshw. Res. 2009, 43, 247–255. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids of insects, grasshoppers, crickets, mantises, and stic insects. Carotenoid Sci. 2015, 20, 51–55. [Google Scholar]
- Twomey, E.; Johnson, J.D.; Castroviejo-Fisher, S.; Van Bocxlaer, I. A ketocarotenoid-based colour polymorphism in the Sira poison frog Ranitomeya sirensis indicates novel gene interactions underlying aposematic signal variation. Mol. Ecol. 2020, 29, 2004–2015. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.T.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef]
- Bryon, A.; Kurlovs, A.H.; Dermauw, W.; Greenhalgh, R.; Riga, M.; Grbić, M.; Tirry, L.; Osakabe, M.; Vontas, J.; Clark, R.M.; et al. Disruption of a horizontally transferred phytoene desaturase abolishes carotenoid accumulation and diapause in Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2017, 114, E5871–E5880. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral Transfer of Genes from Fungi Underlies Carotenoid Production in Aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef]
- Cobbs, C.; Heath, J.; Stireman, J.O., 3rd; Abbot, P. Carotenoids in unexpected places: Gall midges, lateral gene transfer, and carotenoid biosynthesis in animals. Mol. Phylogenet. Evol. 2013, 68, 221–228. [Google Scholar] [CrossRef]
- Takemura, M.; Maoka, T.; Koyanagi, T.; Kawase, N.; Nishida, R.; Tsuchida, T.; Hironaka, M.; Ueda, T.; Misawa, N. Elucidation of the whole carotenoid biosynthetic pathway of aphids at the gene level and arthropodal food chain involving aphids and the red dragonfly. BMC Zool. 2021, 6, 19. [Google Scholar] [CrossRef]
- Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 2011, 8, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Matsuno, T. Comparative biochemical studies of carotenoids in sea-urchins—I. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 801–810. [Google Scholar] [CrossRef]
- Chapter 8 Carotenoids in sea urchins. Developments in Aquaculture and Fisheries Science. In Edible Sea Urchins: Biology and Ecology; Tsushima, M. Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 37, pp. 159–166. [Google Scholar]
- Galasso, C.; Orefice, I.; Toscano, A.; Fernández, T.V.; Musco, L.; Brunet, C.; Sansone, C.; Cirino, P. Food Modulation Controls Astaxanthin Accumulation in Eggs of the Sea Urchin Arbacia lixula. Mar. Drugs 2018, 16, 186. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.W.; Upadhyay, R.R.; Liaaen-Lensen, S. Short Communications. Animal Carotenoids. 4.* The Carotenoids of Asterias rubens. Acta. Che. Scand. 1970, 24, 3050–3054. [Google Scholar] [CrossRef]
- Maoka, T.; Tsushima, M.; Matsuno, T. new acetylenic carotenoids from the starfishes Asterina pectinifera and Asterias amurensis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 829–834. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Suzuki, Y. Biochemical studies of the ascidian, Cynthia roretzi v Drasche IV Carotenoids in test. Tohoku J. Agric. Res. 1959, 10, 397–407. [Google Scholar]
- Ohkubo, M.; Tsushima, M.; Maoka, T.; Matsuno, T. Carotenoids and their metabolism in the goldfish Carassius auratus (Hibuna). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 124, 333–340. [Google Scholar] [CrossRef]
- Authority, E.F.S. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of use of colouring agents in animal nutrition-PART I. General Principles and Astaxanthin. EFSA J. 2005, 3, 291. [Google Scholar] [CrossRef]
- Matsui, K.; Takaichi, S.; Nakamura, M. Morphological and Biochemical Changes in Carotenoid Granules in the Ventral Skin during Growth of the Japanese Newt Cynops pyrrhogaster. Zool. Sci. 2003, 20, 435–440. [Google Scholar] [CrossRef]
- Matsui, K.; Asari, M.; Ichihara, N. Astaxanthin reduction pathway in an amphibian, as a research tool for ketocarotenoid metabolism. J. Azabu Univ. 2007, 13, 166. [Google Scholar]
- Brejcha, J.; Bataller, J.V.; Bosáková, Z.; Geryk, J.; Havlíková, M.; Kleisner, K.; Maršík, P.; Font, E. Body coloration and mechanisms of colour production in Archelosauria: The case of deirocheline turtles. R. Soc. Open Sci. 2019, 6, 190319. [Google Scholar] [CrossRef]
- McLean, C.A.; Lutz, A.; Rankin, K.J.; Elliott, A.; Moussalli, A.; Stuart-Fox, D. Red carotenoids and associated gene expression explain colour variation in frillneck lizards. Proc. R. Soc. B Boil. Sci. 2019, 286, 20191172. [Google Scholar] [CrossRef] [PubMed]
- de Mello, P.L.H.; Hime, P.M.; E Glor, R. Transcriptomic Analysis of Skin Color in Anole Lizards. Genome Biol. Evol. 2021, 13, evab110. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.N.; Clarke, J.A. Estimating the distribution of carotenoid coloration in skin and integumentary structures of birds and extinct dinosaurs. Evolution 2022, 76, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Amat, J.; Rendón, M. Flamingo coloration and its significance. In Flamingos, Behavior, Biology, and Relationship with Humans; Anderson, M.J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 77–95. [Google Scholar]
- Toomey, M.B.; Collins, A.M.; Frederiksen, R.; Cornwall, M.C.; Timlin, J.A.; Corbo, J.C. A complex carotenoid palette tunes avian colour vision. J. R. Soc. Interface 2015, 12, 20150563. [Google Scholar] [CrossRef]
- Wilby, D.; Roberts, N.W. Optical influence of oil droplets on cone photoreceptor sensitivity. J. Exp. Biol. 2017, 220, 1997–2004. [Google Scholar] [CrossRef]
- Toomey, M.B.; Corbo, J.C. Evolution, Development and Function of Vertebrate Cone Oil Droplets. Front. Neural Circuits 2017, 11, 97. [Google Scholar] [CrossRef]
- Schiedt, K. New Aspects of Carotenoid Metabolism in Animals. Carotenoids Chem. Biol. 1989, 247–268. [Google Scholar] [CrossRef]
- Emerling, C.A. Independent pseudogenization of CYP2J19 in penguins, owls and kiwis implicates gene in red carotenoid synthesis. Mol. Phylogenetics Evol. 2018, 118, 47–53. [Google Scholar] [CrossRef]
- Twyman, H.; Andersson, S.; Mundy, N.I. Evolution of CYP2J19, a gene involved in colour vision and red coloration in birds: Positive selection in the face of conservation and pleiotropy. BMC Evol. Biol. 2018, 18, 22. [Google Scholar] [CrossRef]
- Delhey, K.; Valcu, M.; Dale, J.; Kempenaers, B. The evolution of carotenoid-based plumage colours in passerine birds. J. Anim. Ecol. 2023, 92, 66–77. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of vertebrate visual pigments. Vis. Res. 2008, 48, 2022–2041. [Google Scholar] [CrossRef]
- Thoen, H.H.; How, M.J.; Chiou, T.-H.; Marshall, J. A Different Form of Color Vision in Mantis Shrimp. Science 2014, 343, 411–413. [Google Scholar] [CrossRef]
- Chiou, T.-H.; Place, A.R.; Caldwell, R.L.; Marshall, N.J.; Cronin, T.W. A novel function for a carotenoid: Astaxanthin used as a polarizer for visual signalling in a mantis shrimp. J. Exp. Biol. 2012, 215, 584–589. [Google Scholar] [CrossRef]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2010, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Luna-Flores, C.H.; Wang, A.; von Hellens, J.; Speight, R.E. Towards commercial levels of astaxanthin production in Phaffia rhodozyma. J. Biotechnol. 2022, 350, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Bocanegra, A.R.; Legarreta, I.G.; Jeronimo, F.M.; Campocosio, A.T. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2004, 92, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ishibashi, T.; Kuwahara, D.; Hirasawa, K. Commercial Production of Astaxanthin with Paracoccus carotinifaciens. In Carotenoids: Biosynthetic and Biofunctional Approaches; Misawa, N., Ed.; Springer: Singapore, 2021; pp. 11–20. [Google Scholar]
- Inc, A. Panaferd® the Natural Choice. Available online: https://www.panaferd.com/ (accessed on 1 February 2023).
- Ide, T.; Hoya, M.; Tanaka, T.; Harayama, S. Enhanced production of astaxanthin in Paracoccus sp. strain N-81106 by using random mutagenesis and genetic engineering. Biochem. Eng. J. 2012, 65, 37–43. [Google Scholar] [CrossRef]
- Choi, S.S.; Seo, Y.B.; Nam, S.-W.; Kim, G.-D. Enhanced Production of Astaxanthin by Co-culture of Paracoccus haeundaensis and Lactic Acid Bacteria. Front. Mar. Sci. 2021, 7. [Google Scholar] [CrossRef]
- Additives, E.P.o.; Products or Substances used in Animal, F.; Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fasmon Durjava, M.; Kouba, M.; Lopez-Alonso, M.; et al. Safety and efficacy of a feed additive consisting of astaxanthin-rich Phaffia rhodozyma for salmon and trout (Igene Biotechnology, Inc.). EFSA J. 2022, 20, e07161. [Google Scholar] [CrossRef]
- Li, Y.; Gong, F.; Guo, S.; Yu, W.; Liu, J. Adonis amurensis Is a Promising Alternative to Haematococcus as a Resource for Natural Esterified (3S,3′S)-Astaxanthin Production. Plants 2021, 10, 1059. [Google Scholar] [CrossRef]
- Liu, B.-H.; Lee, Y.-K. Composition and biosynthetic pathways of carotenoids in the astaxanthin-producing green alga Chlorococcum sp. Biotechnol. Lett. 1999, 21, 1007–1010. [Google Scholar] [CrossRef]
- Liu, B.-H.; Lee, Y.-K. Secondary carotenoids formation by the green alga Chlorococcum sp. J. Appl. Phycol. 2000, 12, 301–307. [Google Scholar] [CrossRef]
- Ma, R.Y.-N.; Chen, F. Enhanced production of free trans-astaxanthin by oxidative stress in the cultures of the green microalga Chlorococcum sp. Process. Biochem. 2001, 36, 1175–1179. [Google Scholar] [CrossRef]
- Leya, T.; Rahn, A.; Lutz, C.; Remias, D. Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol. Ecol. 2009, 67, 432–443. [Google Scholar] [CrossRef]
- Yamaoka, Y. Microorganism and Production of Carotinoid Compounds Thereby. US Patent 7,374,908 B2, 20 May 2008. [Google Scholar]
- Aki, T.; Hachida, K.; Yoshinaga, M.; Katai, Y.; Yamasaki, T.; Kawamoto, S.; Kakizono, T.; Maoka, T.; Shigeta, S.; Suzuki, O.; et al. Thraustochytrid as a potential source of carotenoids. J. Am. Oil Chem. Soc. 2003, 80, 789–794. [Google Scholar] [CrossRef]
- Carmona, M.L.; Naganuma, T.; Yamaoka, Y. Identification by HPLC-MS of Carotenoids of the Thraustochytrium CHN-1 Strain Isolated from the Seto Inland Sea. Biosci. Biotechnol. Biochem. 2003, 67, 884–888. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Carmona, M.L.; Oota, S. Growth and Carotenoid Production of Thraustochytrium sp. CHN-1 Cultured under Superbright Red and Blue Light-emitting Diodes. Biosci. Biotechnol. Biochem. 2004, 68, 1594–1597. [Google Scholar] [CrossRef]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; Valle, M.G.-D.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Grung, M.; Liaaen-Jensen, S. Algal carotenoids 52; secondary carotenoids of algae 3; carotenoids in a natural bloom of Euglena sanguinea. Biochem. Syst. Ecol. 1993, 21, 757–763. [Google Scholar] [CrossRef]
- Fox, D.L.; Crozier, G.F.; Smith, V.E. Carotenoid fractionation in the plumose anemone Metridium. Comp. Biochem. Physiol. 1967, 22, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.L.; Wilkie, D.W.; Haxo, F.T. Carotenoid fractionation in the plumose anemone Metridium—II. Search for dietary sources of ovarian astaxanthin. Comp. Biochem. Physiol. Part B Comp. Biochem. 1978, 59, 289–294. [Google Scholar] [CrossRef]
- West, H.H. Pigmentation in the sea anemone Corynactis californica. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979, 64, 195–200. [Google Scholar] [CrossRef]
- Maoka, T.; Kuwahara, T.; Narita, M. Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain. Mar. Drugs 2014, 12, 1460–1470. [Google Scholar] [CrossRef]
- Maoka, T.; Ochi, J.; Mori, M.; Sakagami, Y. Identification of Carotenoids in the Freshwater Shellfish Unio douglasiae nipponensis, Anodonta lauta, Cipangopaludina chinensis laeta, and Semisulcospira libertina. J. Oleo Sci. 2012, 61, 69–74. [Google Scholar] [CrossRef]
- Matsuno, T.; Katagiri, K.; Maoka, T.; Komori, T. Carotenoids of shellfishes-VII. Carotenoids of the spindle shell Fusinus perplexus. Nippon. Suisan Gakkaishi 1984, 50, 1583–1588. [Google Scholar] [CrossRef]
- Tsushima, M.; Katsuyama, M.; Matsuno, T. Metabolism of Carotenoids in the Apple Snail, Pomacea canaliculata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 118, 431–436. [Google Scholar] [CrossRef]
- Tushima, M.; Maoka, T.; Matsuno, T. Comparative biochemical studies of carotenoids in marine invertebrates— the first positive identification of ϵ,ϵ-carotene derivatives and isolation of two new carotenoids from chitons. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 665–671. [Google Scholar] [CrossRef]
- Charest, D.J.; Balaban, M.O.; Marshall, M.R.; Cornell, J.A. Astaxanthin Extraction from Crawfish Shells by Supercritical CO2with Ethanol as Cosolvent. J. Aquat. Food Prod. Technol. 2001, 10, 81–96. [Google Scholar] [CrossRef]
- Czeczuga, B. Composition and tissue distribution of carotenoids and vitamin A in the crayfish Astacus leptodactylus (Esch.) (Crustacea, decapoda). Comp. Biochem. Physiol. Part B Comp. Biochem. 1971, 39, 945–953. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N. Carotenoids and Their Fatty Acid Esters of Spiny Lobster Panulirus japonicus. J. Oleo Sci. 2008, 57, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.J.; A Cobine, P.; E Hill, G. On the bioconversion of dietary carotenoids to astaxanthin in the marine copepod, Tigriopus californicus. J. Plankton Res. 2018, 40, 142–150. [Google Scholar] [CrossRef]
- Yoshitomi, B.; Yamaguchi, H. Chemical composition of dried eyeballs from Euphausia superba and Euphausia pacifica. Fish. Sci. 2007, 73, 1186–1194. [Google Scholar] [CrossRef]
- Whyte, J.N.C.; Fisheries, C.D.o.; Oceans; Station, P.B. Deposition of Astaxanthin Isomers in Chinook Salmon (Oncorhynchus Tshawytscha) Fed Different Sources of Pigment; Fisheries and Oceans Canada: Vancouver, BC, Canada, 1998. [Google Scholar]
- Renstrøm, B.; Borch, G.; Liaaen-Jensen, S. Natural occurrence of enantiomeric and meso-astaxanthin 4. Ex shrimps (Pandalus borealis). Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 69, 621–624. [Google Scholar] [CrossRef]
- Dave, D.; Liu, Y.; Pohling, J.; Trenholm, S.; Murphy, W. Astaxanthin recovery from Atlantic shrimp (Pandalus borealis) processing materials. Bioresour. Technol. Rep. 2020, 11, 100535. [Google Scholar] [CrossRef]
- Maoka, T.; Kawashima, Y.; Takaki, M. Structures of Yellow Xanthophylls and Metabolism of Astaxanthin in the Prawn Penaeus japonicus. J. Oleo Sci. 2018, 67, 1425–1433. [Google Scholar] [CrossRef]
- Maoka, T. A new carotenoid, 5,6-dihydrocrustaxanthin, from prawns and the distribution of yellow xanthophylls in shrimps. Biochem. Syst. Ecol. 2020, 92, 104083. [Google Scholar] [CrossRef]
- Arifuku, I. Development of technology and applying high-quality fat-soluble functional materials(1st Report): Extraction technology of astaxanthin from red snow crab shell. Rep. Tottori Inst. Ind. Technol. 2013, 16, 28–31. (In Japanese) [Google Scholar]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Albino Antunes, S.; Ferreira, S.; Hense, H. Enzymatic Hydrolysis of Blue Crab (Callinectes Sapidus) Waste Processing to Obtain Chitin, Protein, and Astaxanthin-Enriched Extract. Int. J. Environ. Agric. Res. 2017, 3, 81–92. [Google Scholar]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Foss, P.; Renstrøm, B.; Liaaen-Jensen, S. Natural occurrence of enantiomeric and Meso astaxanthin 7∗-crustaceans including zooplankton. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 313–314. [Google Scholar] [CrossRef]
- Matsuno, T.; Maoka, T. Carotenoids of crustacea. VI. The carotenoids of crab Paralithodes brevipes (Hanasakigani in Japanese). Nippon. Suisan Gakkaishi 1988, 54, 1437–1442. [Google Scholar] [CrossRef]
- Osawa, A.; Ito, K.; Uchuiyama, A.; Suzuki, M.; Itoh, K.; Fukuo, N.; Maoka, T.; Shindo, K. Carotenoids in Crabs Belonging to the Decapoda Species. J. Home Econ. Jpn. 2013, 64, 443–450. (In Japanese) [Google Scholar] [CrossRef]
- Osawa, A.; Kato, Y.; Shimatani, M.; Nodera, W.; Hattori, N.; Maoka, T.; Shindo, K. Carotenoids in Coconut Crab (Birgus latro) and Their Antioxidative Activitie. J. Home Econ. Jpn. 2011, 62, 499–505. (In Japanese) [Google Scholar] [CrossRef]
- Czeczuga, B.; Czeczuga-Semeniuk, E.; Semeniuk, A. Carotenoids and carotenoproteins in Asellus aquaticus L. (Crustacea: Isopoda). Folia Biol. 2005, 53, 109–114. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Bjerkeng, B. Astaxanthin from the red crab langostilla (Pleuroncodes planipes): Optical R/S isomers and fatty acid moieties of astaxanthin esters. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 437–444. [Google Scholar] [CrossRef]
- Osakabe, M.; Shimano, S. The flashy red color of the red velvet mite Balaustium murorum (Prostigmata: Erythraeidae) is caused by high abundance of the keto-carotenoids, astaxanthin and 3-hydroxyechinenone. Exp. Appl. Acarol. 2023, 89, 1–14. [Google Scholar] [CrossRef]
- Atarashi, M.; Manabe, Y.; Kishimoto, H.; Sugawara, T.; Osakabe, M. Antioxidant Protection by Astaxanthin in the Citrus Red Mite (Acari: Tetranychidae). Environ. Èntomol. 2017, 46, 1143–1150. [Google Scholar] [CrossRef]
- Wybouw, N.; Kurlovs, A.H.; Greenhalgh, R.; Bryon, A.; Kosterlitz, O.; Manabe, Y.; Osakabe, M.; Vontas, J.; Clark, R.M.; Van Leeuwen, T. Convergent evolution of cytochrome P450s underlies independent origins of keto-carotenoid pigmentation in animals. Proc. R. Soc. B Boil. Sci. 2019, 286, 20191039. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Manabe, Y.; Sugawara, T.; Osakabe, M. Imaginal Feeding for Progression of Diapause Phenotype in the Two-Spotted Spider Mite (Acari: Tetranychidae). Environ. Èntomol. 2016, 45, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga, B. Astaxanthin—The carotenoid predominant in Eylais hamata (Koenike, 1897) (hydracarina, arachnoidea). Comp. Biochem. Physiol. Part B 1972, 42, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Green, J. Pigments of the hydracarine Eylais extendens (acari: Hydrachnellae). Comp. Biochem. Physiol. 1964, 13, 469–472. [Google Scholar] [CrossRef]
- Kitahara, T.; Saitoh, K. Biochemical Studies of Corotenoids in the Chum Salmon (Oncorhynchus keta) during Upstream Migration for Spawning V: Behavior of Carotenoids in the Kokanee (O. nerka) with the Advancement of Maturation. Sci. Rep. Hokkaido Salmon Hatch. 1977, 29–36. [Google Scholar]
- Matsuno, T.; Katsuyama, M.; Nagata, S. Comparative Biochemical Studies of Carotenoids in Fishes-XIX, Carotenoids of Chum Salmon, Coho Salmon, Biwa Trout, Red-spotted Masu Salmon, Masu Salmon, and Kokanee. Bull. Jpn. Soc. Sci. 1980, 46, 879–884. [Google Scholar] [CrossRef]
- Aas, G.H.; Bjerkeng, B.; Hatlen, B.; Storebakken, T. Idoxanthin, a major carotenoid in the flesh of Arctic charr (Salvelinus alpinus) fed diets containing astaxanthin. Aquaculture 1997, 150, 135–142. [Google Scholar] [CrossRef]
- Megdal, P.A.; Craft, N.A.; Handelman, G.J. A Simplified Method to Distinguish Farmed (Salmo salar) from Wild Salmon: Fatty Acid Ratios Versus Astaxanthin Chiral Isomers. Lipids 2009, 44, 569–576. [Google Scholar] [CrossRef]
- Kitahara, T. Carotenoid pigments in chum salmon-II. Behavior of carotenoids in the chum salmon Oncorhynchus keta during development. Nippon. Suisan Gakkaishi 1984, 50, 531–536. [Google Scholar] [CrossRef]
- Kitahara, T. Behaviour of carotenoids in the masu salmon Oncorhynchus masou during anadromus migration. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 253–255. [Google Scholar] [CrossRef]
- Hata, M.; Hata, M. Carotenoid pigments in rainbow trout, Salmo gairdneri irideus. Tohoku J. Agric. Res. 1975, 26, 35–40. [Google Scholar]
- Tsukuda, N. Studies on the discoloration of red fishes-I. Content of carotenoid pigments in eighteen species of red fishes. Bull. Jap. Soc. Sci. Fish. 1966, 32, 334–345. [Google Scholar] [CrossRef]
- Maoka, T.; Sato, W.; Nagai, H.; Takahashi, T. Carotenoids of Red, Brown, and Black Specimens of Plectropomus leopardus, the Coral Trout (Suziara in Japanese). J. Oleo Sci. 2017, 66, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Satake, M.; Watanabe, T.; Kitajima, C.; Miki, W.; Yamaguchi, K.; Konosu, S. Pigmentation of cultured red sea bream with astaxanthin diester purified from krill oil. Nippon. Suisan Gakkaishi 1983, 49, 1855–1861. [Google Scholar] [CrossRef]
- Tsushima, M.; Matsuno, T. Carotenoid Composition and Two New 4-ketolutein Isomers in the Integuments of the Red Tilefish Branchiostegus japonicus. Fish. Sci. 1998, 64, 464–468. [Google Scholar] [CrossRef]
- Czeczuga, B. Investigations on carotenoids in amphibia—II. Carotenoids occurring in various parts of the body of certain species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1980, 65, 623–630. [Google Scholar] [CrossRef]
- Czeczuga, B. Investigations on Carotenoids in Amphibia. III. Presence of Neothxanthin in Bufo viridis LAURENTI (Anura: Bufonidae). Amphibia-Reptilia 1982, 3, 53–56. [Google Scholar] [CrossRef]
- Bonansea, M.; Heit, C.; Vaira, M. Pigment composition of the bright skin in the poison toad Melanophryniscus rubriventris (Anura: Bufonidae) from Argentina. Salamandra 2017, 53, 142–147. [Google Scholar]
- Steffen, J.E.; Learn, K.M.; Drumheller, J.S.; Boback, S.M.; McGraw, K.J. Carotenoid Composition of Colorful Body Stripes and Patches in the Painted Turtle (Chrysemys picta) and Red-Eared Slider (Trachemys scripta). Chelonian Conserv. Biol. 2015, 14, 56–63. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, L.; de Blas, E.G.; Martínez-Padilla, J.; Mougeot, F.; Mateo, R. Carotenoid profile and vitamins in the combs of the red grouse (Lagopus lagopus scoticus): Implications for the honesty of a sexual signal. J. Ornithol. 2015, 157, 145–153. [Google Scholar] [CrossRef]
- Barbosa, A.; Palacios, M.J.; Negro, J.J.; Cuervo, J.J. Plasma carotenoid depletion during fasting in moulting penguins. J. Ornithol. 2012, 154, 559–562. [Google Scholar] [CrossRef]
- Itoh, K.; Mastumura, S.; Hayashi, S.; Kobayashi, Y.; Maoka, T. Supplementation of astaxanthin to the penguin diet under captivity and its accumulation in the blood. J. Jpn. Assoc. Zoos Aquar. 2018, 60, 25. (In Japanese) [Google Scholar]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic Aspects of Carotenoid Biosynthesis. Chem. Rev. 2013, 114, 164–193. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N. Carotenoid β-Ring Hydroxylase and Ketolase from Marine Bacteria—Promiscuous Enzymes for Synthesizing Functional Xanthophylls. Mar. Drugs 2011, 9, 757–771. [Google Scholar] [CrossRef]
- Choi, S.-K.; Nishida, Y.; Matsuda, S.; Adachi, K.; Kasai, H.; Peng, X.; Komemushi, S.; Miki, W.; Misawa, N. Characterization of β -Carotene Ketolases, CrtW, from Marine Bacteria by Complementation Analysis in Escherichia coli. Mar. Biotechnol. 2005, 7, 515–522. [Google Scholar] [CrossRef]
- Choi, S.-K.; Matsuda, S.; Hoshino, T.; Peng, X.; Misawa, N. Characterization of bacterial β-carotene 3,3′-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adonirubin production by gene combination in Escherichia coli. Appl. Microbiol. Biotechnol. 2006, 72, 1238–1246. [Google Scholar] [CrossRef]
- Fraser, P.D.; Miura, Y.; Misawa, N. In Vitro Characterization of Astaxanthin Biosynthetic Enzymes. J. Biol. Chem. 1997, 272, 6128–6135. [Google Scholar] [CrossRef]
- Fraser, P.D.; Shimada, H.; Misawa, N. Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur. J. Biochem. 1998, 252, 229–236. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Gantt, E. A study in scarlet: Enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J. 2005, 41, 478–492. [Google Scholar] [CrossRef]
- Mascia, F.; Girolomoni, L.; Alcocer, M.J.P.; Bargigia, I.; Perozeni, F.; Cazzaniga, S.; Cerullo, G.; D’andrea, C.; Ballottari, M. Functional analysis of photosynthetic pigment binding complexes in the green alga Haematococcus pluvialis reveals distribution of astaxanthin in Photosystems. Sci. Rep. 2017, 7, 16319. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Rmiki, N.-E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of Oleic Acid in Haematococcus pluvialis (Chlorophyceae) under Nitrogen Starvation or High Light is Correlated with That of Astaxanthin Esters . J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Zarka, A.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S.J.J. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J. Phycol. 2005, 41, 819–826. [Google Scholar] [CrossRef]
- Lei, A.; Chen, H.; Shen, G.; Hu, Z.; Chen, L.; Wang, J. Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol. Biofuels 2012, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef]
- Grunewald, K.; Eckert, M.; Hirschberg, J.; Hagen, C. Phytoene Desaturase Is Localized Exclusively in the Chloroplast and Up-Regulated at the mRNA Level during Accumulation of Secondary Carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol. 2000, 122, 1261–1268. [Google Scholar] [CrossRef]
- Grünewald, K.; Hirschberg, J.; Hagen, C. Ketocarotenoid Biosynthesis Outside of Plastids in the Unicellular Green Alga Haematococcus pluvialis. Pediatrics 2001, 276, 6023–6029. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Zhang, Z.; Wang, J.; Huang, J.; Fan, J.; Yu, A.; Wang, W.; Li, Y. Sequential Heterotrophy–Dilution–Photoinduction Cultivation of Haematococcus pluvialis for efficient production of astaxanthin. Bioresour. Technol. 2015, 198, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Waissman-Levy, N.; Leu, S.; Khozin-Goldberg, I.; Boussiba, S. Manipulation of trophic capacities in Haematococcus pluvialis enables low-light mediated growth on glucose and astaxanthin formation in the dark. Algal Res. 2019, 40, 101497. [Google Scholar] [CrossRef]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Shih, C.; Ehrhardt, D.; Grossman, A.R.; Apt, K.E. Trophic Conversion of an Obligate Photoautotrophic Organism Through Metabolic Engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Ding, W.; Mao, X.; Li, Y.; Gerken, H.; Liu, J. Astaxanthin Is Ketolated from Zeaxanthin Independent of Fatty Acid Synthesis in Chromochloris zofingiensis. Plant Physiol. 2020, 183, 883–897. [Google Scholar] [CrossRef]
- Lotan, T.; Hirschberg, J. Cloning and expression inEscherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett. 1995, 364, 125–128. [Google Scholar] [CrossRef]
- Huang, J.-C.; Chen, F.; Sandmann, G. Stress-related differential expression of multiple β-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. J. Biotechnol. 2006, 122, 176–185. [Google Scholar] [CrossRef]
- Luo, Q.; Bian, C.; Tao, M.; Huang, Y.; Zheng, Y.; Lv, Y.; Li, J.; Wang, C.; You, X.; Jia, B.; et al. Genome and Transcriptome Sequencing of the Astaxanthin-Producing Green Microalga, Haematococcus pluvialis. Genome Biol. Evol. 2019, 11, 166–173. [Google Scholar] [CrossRef]
- Linden, H. Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim. Biophys Acta 1999, 1446, 203–212. [Google Scholar] [CrossRef]
- Frassanito, R.; Cantonati, M.; Flaim, G.; Mancini, I.; Guella, G. A new method for the identification and the structural characterisation of carotenoid esters in freshwater microorganisms by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 3531–3539. [Google Scholar] [CrossRef]
- Morimoto, D.; Yoshida, T.; Sawayama, S. Draft Genome Sequence of the Astaxanthin-Producing Microalga Haematococcus lacustris Strain NIES-144. Genome Announc. 2020, 9, e00128-20. [Google Scholar] [CrossRef] [PubMed]
- Fábregas, J.; Domínguez, A.; Maseda, A.; Otero, A. Interactions between irradiance and nutrient availability during astaxanthin accumulation and degradation in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2003, 61, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lababpour, A.; Hada, K.; Shimahara, K.; Katsuda, T.; Katoh, S. Effects of nutrient supply methods and illumination with blue light emitting diodes (LEDs) on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2004, 98, 452–456. [Google Scholar] [CrossRef]
- Lababpour, A.; Shimahara, K.; Hada, K.; Kyoui, Y.; Katsuda, T.; Katoh, S. Fed-batch culture under illumination with blue light emitting diodes (LEDs) for astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2005, 100, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Shimahara, K.; Shiraishi, H.; Yamagami, K.; Ranjbar, R.; Katoh, S. Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. J. Biosci. Bioeng. 2006, 102, 442–446. [Google Scholar] [CrossRef]
- Yoshimura, S.; Ranjbar, R.; Inoue, R.; Katsuda, T.; Katoh, S. Effective utilization of transmitted light for astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2006, 102, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Complementary limiting factors of astaxanthin synthesis during photoautotrophic induction of Haematococcus pluvialis: C/N ratio and light intensity. Appl. Microbiol. Biotechnol. 2007, 74, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Shiraishi, H.; Ishizu, N.; Ranjbar, R.; Katoh, S. Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxanthin production by Haematococcus pluvialis. J. Biosci. Bioeng. 2008, 105, 216–220. [Google Scholar] [CrossRef]
- Vidhyavathi, R.; Venkatachalam, L.; Kamath, B.S.; Sarada, R.; Ravishankar, G.A. Differential expression of carotenogenic genes and associated changes in pigment profile during regeneration of Haematococcus pluvialis cysts. Appl. Microbiol. Biotechnol. 2007, 75, 879–887. [Google Scholar] [CrossRef]
- Vidhyavathi, R.; Venkatachalam, L.; Sarada, R.; Ravishankar, G.A. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J. Exp. Bot. 2008, 59, 1409–1418. [Google Scholar] [CrossRef]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J. Appl. Phycol. 2010, 22, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, G.; Chong, S.; Mak, N.-K.; Chen, F.; Jiang, Y. Ultraviolet-B radiation improves astaxanthin accumulation in green microalga Haematococcus pluvialis. Biotechnol. Lett. 2010, 32, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, J.; Sommerfeld, M.; Hu, Q. Susceptibility and Protective Mechanisms of Motile and Non Motile Cells of Haematococcus pluvialis (Chlorophyceae) To Photooxidative Stress1. J. Phycol. 2012, 48, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef]
- Gu, W.; Li, H.; Zhao, P.; Yu, R.; Pan, G.; Gao, S.; Xie, X.; Huang, A.; He, L.; Wang, G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014, 4, 6661. [Google Scholar] [CrossRef]
- Gwak, Y.; Hwang, Y.-S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.-G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Shi, M.; Niu, X.; Yu, X.; Gao, L.; Zhang, X.; Chen, L.; Zhang, W. Metabolomic and network analysis of astaxanthin-producing Haematococcus pluvialis under various stress conditions. Bioresour. Technol. 2014, 170, 522–529. [Google Scholar] [CrossRef]
- Wan, M.; Zhang, J.; Hou, D.; Fan, J.; Li, Y.; Huang, J.; Wang, J. The effect of temperature on cell growth and astaxanthin accumulation of Haematococcus pluvialis during a light–dark cyclic cultivation. Bioresour. Technol. 2014, 167, 276–283. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Hu, Q.; Sommerfeld, M.; Lu, Y.; Han, D. Cellular Capacities for High-Light Acclimation and Changing Lipid Profiles across Life Cycle Stages of the Green Alga Haematococcus pluvialis. PLoS ONE 2014, 9, e106679. [Google Scholar] [CrossRef]
- Sharma, K.K.; Ahmed, F.; Schenk, P.M.; Li, Y. UV-C mediated rapidcarotenoid induction and settling performance of Dunaliellasalina and Haematococcus pluvialis. Biotechnol. Bioeng. 2015, 112, 2106–2114. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, Z.-H.; Park, H.; Lee, C.-G. Specific light uptake rates can enhance astaxanthin productivity in Haematococcus lacustris. Bioprocess Biosyst. Eng. 2016, 39, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Kim, D.G.; Roh, S.W.; Choi, J.-S.; Choi, Y.-E. Enhancement of astaxanthin production using Haematococcus pluvialis with novel LED wavelength shift strategy. Appl. Microbiol. Biotechnol. 2016, 100, 6231–6238. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome Analysis in Haematococcus pluvialis: Astaxanthin Induction by High Light with Acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Ahn, J.-W.; Kim, J.-B.; Kim, J.Y.; Choi, Y.-E. Comparative transcriptome analysis of Haematococcus pluvialis on astaxanthin biosynthesis in response to irradiation with red or blue LED wavelength. World J. Microbiol. Biotechnol. 2018, 34, 96. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Alsenani, F.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Gene expression profiling of astaxanthin and fatty acid pathways in Haematococcus pluvialis in response to different LED lighting conditions. Bioresour. Technol. 2018, 250, 591–602. [Google Scholar] [CrossRef]
- Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Ke, H.; Zheng, X.; Chen, D.; Wang, C.; Wu, S.; et al. Differences between Motile and Nonmotile Cells of Haematococcus pluvialis in the Production of Astaxanthin at Different Light Intensities. Mar. Drugs 2019, 17, 39. [Google Scholar] [CrossRef]
- Pang, N.; Fu, X.; Fernandez, J.S.M.; Chen, S. Multilevel heuristic LED regime for stimulating lipid and bioproducts biosynthesis in Haematococcus pluvialis under mixotrophic conditions. Bioresour. Technol. 2019, 288, 121525. [Google Scholar] [CrossRef]
- Bilbao, P.G.S.; Garelli, A.; Díaz, M.; Salvador, G.A.; Leonardi, P.I. Crosstalk between sterol and neutral lipid metabolism in the alga Haematococcus pluvialis exposed to light stress. Biochim. et Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158767. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, D.; Li, A.; Hu, Z.; Gao, Z.; Yang, Y.; Wang, C. Transcriptome-based analysis of the effects of salicylic acid and high light on lipid and astaxanthin accumulation in Haematococcus pluvialis. Biotechnol. Biofuels 2021, 14, 82. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Gamma-aminobutyric acid facilitates the simultaneous production of biomass, astaxanthin and lipids in Haematococcus pluvialis under salinity and high-light stress conditions. Bioresour. Technol. 2021, 320, 124418. [Google Scholar] [CrossRef]
- Wang, X.; Meng, C.; Zhang, H.; Xing, W.; Cao, K.; Zhu, B.; Zhang, C.; Sun, F.; Gao, Z. Transcriptomic and Proteomic Characterizations of the Molecular Response to Blue Light and Salicylic Acid in Haematococcus pluvialis. Mar. Drugs 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamouda, M.; Kacem, A.; Achour, L.; Krichen, Y.; Legrand, J.; Grizeau, D.; Dupré, C. Comparative study on photosynthetic and antioxidant activities of Haematococcus pluvialis vegetative and resting cells: UVA light-induced stimulation. J. Appl. Microbiol. 2022, 132, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Erokhin, V.E.; Minyuk, G.S.; Gordienko, A.P.; Kapranov, S.V. Dynamics of luminescence characteristics of Haematococcus lacustris cultures in different cultivation conditions. Luminescence 2022, 37, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Sun, F.; Meng, C.; Xing, W.; Zhu, X.; Wang, C.; Cao, K.; Zhang, C.; Zhu, B.; Yao, T.; et al. Transcriptome Analysis of the Accumulation of Astaxanthin in Haematococcus pluvialis Treated with White and Blue Lights as well as Salicylic Acid. BioMed Res. Int. 2022, 2022, 4827595. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Yan, H.; Hu, Q.; Han, D. Regulation of Light Spectra on Cell Division of the Unicellular Green Alga Haematococcus pluvialis: Insights from Physiological and Lipidomic Analysis. Cells 2022, 11, 1956. [Google Scholar] [CrossRef]
- Yang, H.E.; Yu, B.S.; Sim, S.J. Enhanced astaxanthin production of Haematococcus pluvialis strains induced salt and high light resistance with gamma irradiation. Bioresour. Technol. 2023, 372, 128651. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, P.; Liu, S.; Gan, Q.; Cui, H.; Qin, S. Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of β-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour. Technol. 2010, 101, 6468–6474. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Miao, X.; Wang, Y.; Yang, L.; Lv, H.; Chen, L.; Ye, N. Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzym. Microb. Technol. 2012, 51, 225–230. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Zhao, Y.; Wang, Y.; Lv, H.; Yang, L.; Chen, L.; Ye, N. Differential Expression of Carotenogenic Genes, Associated Changes on Astaxanthin Production and Photosynthesis Features Induced by JA in H. pluvialis. PLoS ONE 2012, 7, e42243. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Gao, H.; Zhang, X.; Xu, D.; Su, Y.; Wang, Y.; Zhao, Y.; Ye, N. Analysis of mRNA expression profiles of carotenogenesis and astaxanthin production of Haematococcus pluvialis under exogenous 2, 4-epibrassinolide (EBR). Biol. Res. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Gao, H.; Li, Y.; Zhang, X.; Xu, N.; Zhou, S.; Liu, B.; Su, Y.; Ye, N. Carotenoid genes transcriptional regulation for astaxanthin accumulation in fresh water unicellular alga Haematococcus pluvialis by gibberellin A3 (GA3). Indian J. Biochem. Biophys. 2013, 50, 548–553. [Google Scholar] [PubMed]
- Gao, Z.; Li, Y.; Wu, G.; Li, G.; Sun, H.; Deng, S.; Shen, Y.; Chen, G.; Zhang, R.; Meng, C.; et al. Transcriptome Analysis in Haematococcus pluvialis: Astaxanthin Induction by Salicylic Acid (SA) and Jasmonic Acid (JA). PLoS ONE 2015, 10, e0140609. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-T.; Lee, C.; Han, S.-I.; Kim, J.Y.; Kim, S.; Choi, Y.-E. Effect of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid on different growth stages of Haematococcus pluvialis. Bioresour. Technol. 2016, 220, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Song, M.; Huang, D.; Hu, Z.; Wu, Y.; Wang, C. Haematococcus pluvialis Accumulated Lipid and Astaxanthin in a Moderate and Sustainable Way by the Self-Protection Mechanism of Salicylic Acid Under Sodium Acetate Stress. Front. Plant Sci. 2021, 12, 763742. [Google Scholar] [CrossRef]
- Wang, X.; Mou, J.-H.; Qin, Z.-H.; Hao, T.-B.; Zheng, L.; Buhagiar, J.; Liu, Y.-H.; Balamurugan, S.; He, Y.; Lin, C.S.K.; et al. Supplementation with rac-GR24 Facilitates the Accumulation of Biomass and Astaxanthin in Two Successive Stages of Haematococcus pluvialis Cultivation. J. Agric. Food Chem. 2022, 70, 4677–4689. [Google Scholar] [CrossRef]
- Xing, H.; Zhao, Y.; Li, T.; Han, B.; Zhao, P.; Yu, X. Enhancing astaxanthin and lipid coproduction in Haematococcus pluvialis by the combined induction of plant growth regulators and multiple stresses. Bioresour. Technol. 2022, 344, 126225. [Google Scholar] [CrossRef]
- Almutairi, A.W. Phenol phycoremediation by Haematococcus pluvialis coupled with enhanced astaxanthin and lipid production under rac-GR24 supplementation for enhanced biodiesel production. Saudi J. Biol. Sci. 2023, 30, 103681. [Google Scholar] [CrossRef]
- Ding, W.; Cui, J.; Zhao, Y.; Han, B.; Li, T.; Zhao, P.; Xu, J.-W.; Yu, X. Enhancing Haematococcus pluvialis biomass and γ-aminobutyric acid accumulation by two-step cultivation and salt supplementation. Bioresour. Technol. 2019, 285, 121334. [Google Scholar] [CrossRef]
- Li, F.; Zhang, N.; Zhang, Y.; Lian, Q.; Qin, C.; Qian, Z.; Wu, Y.; Yang, Z.; Li, C.; Huang, X.; et al. NaCl Promotes the Efficient Formation of Haematococcus pluvialis Nonmotile Cells under Phosphorus Deficiency. Mar. Drugs 2021, 19, 337. [Google Scholar] [CrossRef]
- Li, Q.; You, J.; Qiao, T.; Zhong, D.-B.; Yu, X. Sodium chloride stimulates the biomass and astaxanthin production by Haematococcus pluvialis via a two-stage cultivation strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Zharova, D.A.; Ivanova, A.N.; Drozdova, I.V.; Belyaeva, A.I.; Boldina, O.N.; Voitsekhovskaja, O.V.; Tyutereva, E.V. Role of Autophagy in Haematococcus lacustris Cell Growth under Salinity. Plants 2022, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-I.; Chang, S.H.; Lee, C.; Jeon, M.S.; Heo, Y.M.; Kim, S.; Choi, Y.-E. Astaxanthin biosynthesis promotion with pH shock in the green microalga, Haematococcus lacustris. Bioresour. Technol. 2020, 314, 123725. [Google Scholar] [CrossRef]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-E.; Hwang, S.K.; Chang, W.S.; Kim, B.W.; Lee, J.; Sim, S.J. Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl. Microbiol. Biotechnol. 2015, 99, 5203–5215. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Z.; Acheampong, A.; Huang, Q. Low-temperature plasma promotes growth of Haematococcus pluvialis and accumulation of astaxanthin by regulating histone H3 lysine 4 tri-methylation. Bioresour. Technol. 2022, 343, 126095. [Google Scholar] [CrossRef]
- Chekanov, K.; Zaytseva, A.; Mamedov, I.; Solovchenko, A.; Lobakova, E. The Dynamics of the Bacterial Community of the Photobioreactor-Cultivated Green Microalga Haematococcus lacustris During Stress-Induced Astaxanthin Accumulation. Biology 2021, 10, 115. [Google Scholar] [CrossRef]
- Lee, S.-A.; Kim, M.; Esterhuizen, M.; Van Le, V.; Kang, M.; Ko, S.-R.; Oh, H.-M.; Kim, Y.J.; Ahn, C.-Y. An acceleration of carotenoid production and growth of Haematococcus lacustris induced by host-microbiota network interaction. Microbiol. Res. 2022, 262, 127097. [Google Scholar] [CrossRef]
- Zhao, N.; Yi, L.; Ren, S.; Yin, Q.; Xiang, W.; Zhang, X.; Xie, B. Algicidal interaction between Paenibacillus polymyxa MEZ6 and microalgae. J. Appl. Microbiol. 2022, 133, 646–655. [Google Scholar] [CrossRef]
- Yan, H.; Ding, M.; Lin, J.; Zhao, L.; Han, D.; Hu, Q. Folate-mediated one-carbon metabolism as a potential antifungal target for the sustainable cultivation of microalga Haematococcus pluvialis. Biotechnol. Biofuels Bioprod. 2023, 16, 104. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, Z.; Hou, Y.; Liu, C.; Gao, F.; Zheng, Y.; Chen, F. Ethanol induced astaxanthin accumulation and transcriptional expression of carotenogenic genes in Haematococcus pluvialis. Enzym. Microb. Technol. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Shang, M.; Ding, W.; Zhao, Y.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Enhanced astaxanthin production from Haematococcus pluvialis using butylated hydroxyanisole. J. Biotechnol. 2016, 236, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhao, Y.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Reiter, R.J.; Yu, X. Melatonin: A Multifunctional Molecule That Triggers Defense Responses against High Light and Nitrogen Starvation Stress in Haematococcus pluvialis. J. Agric. Food Chem. 2018, 66, 7701–7711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, C.; Ding, W.; Li, T.; Xu, J.-W.; Zhao, P.; Ma, H.; Yu, X. Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour. Technol. 2018, 266, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Alimujiang, A.; Wang, X.; Luo, S.-W.; Balamurugan, S.; Yang, W.-D.; Liu, J.-S.; Zhang, L.; Li, H.-Y. Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xing, H.; Li, X.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Physiological and Metabolomics Analyses Reveal the Roles of Fulvic Acid in Enhancing the Production of Astaxanthin and Lipids in Haematococcus pluvialis under Abiotic Stress Conditions. J. Agric. Food Chem. 2019, 67, 12599–12609. [Google Scholar] [CrossRef]
- Cui, J.; Yu, C.; Zhong, D.-B.; Zhao, Y.; Yu, X. Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour. Technol. 2020, 305, 123069. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Yu, C.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Integration of physiological and metabolomic profiles to elucidate the regulatory mechanisms underlying the stimulatory effect of melatonin on astaxanthin and lipids coproduction in Haematococcus pluvialis under inductive stress conditions. Bioresour. Technol. 2021, 319, 124150. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, J.; Li, Q.; Qiao, T.; Zhong, D.-B.; Zhao, P.; Yu, X. A joint strategy comprising melatonin and 3-methyladenine to concurrently stimulate biomass and astaxanthin hyperaccumulation by Haematococcus pluvialis. Bioresour. Technol. 2021, 341, 125784. [Google Scholar] [CrossRef]
- Hassanpour, H.; Pourhabibian, R. Impact of sodium pyrophosphate and static magnetic field on Haematococcus pluvialis: Enhancement of astaxanthin accumulation, PAL, and antioxidant enzyme activities. Physiol. Mol. Biol. Plants 2022, 28, 1207–1216. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Zhao, Y.; Gao, H.; Li, L.; Zhang, Y.; Yu, X. Myo-inositol facilitates astaxanthin and lipid coproduction in Haematococcus pluvialis by regulating oxidative stress and ethylene signalling. Bioresour. Technol. 2022, 366, 128222. [Google Scholar] [CrossRef]
- Sun, J.; Zan, J.; Zang, X. Research of Fluridone’s Effects on Growth and Pigment Accumulation of Haematococcus pluvialis Based on Transcriptome Sequencing. Int. J. Mol. Sci. 2022, 23, 3122. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Xu, R.; Yu, W.; Liu, J. A strategy for promoting carbon flux into fatty acid and astaxanthin biosynthesis by inhibiting the alternative oxidase respiratory pathway in Haematococcus pluvialis. Bioresour. Technol. 2022, 344, 126275. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Zhang, L. Development of a 5-aminolevulinic acid feeding strategy capable of enhancing Haematococcus pluvialis biomass, astaxanthin, and fatty acid yields. Bioresour. Technol. 2023, 368, 128319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Zhu, Y.; Yang, W.; Zhou, J.; Cen, K. Transcriptome sequencing and metabolic pathways of astaxanthin accumulated in Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 228, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chekanov, K.; Schastnaya, E.; Solovchenko, A.; Lobakova, E. Effects of CO2 enrichment on primary photochemistry, growth and astaxanthin accumulation in the chlorophyte Haematococcus pluvialis. J. Photochem. Photobiol. B Biol. 2017, 171, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cheng, J.; Lu, H.; Yang, W.; Zhou, J.; Cen, K. Transcriptome-based analysis on carbon metabolism of Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 233, 313–321. [Google Scholar] [CrossRef]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.-A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, J.; Zheng, L.; Teng, Z.; Zhang, Q.; Qiu, D.; Song, L. Disodium 2-oxoglutarate promotes carbon flux into astaxanthin and fatty acid biosynthesis pathways in Haematococcus. Bioresour. Technol. 2020, 299, 122612. [Google Scholar] [CrossRef]
- Yu, C.; Wang, H.-P.; Qiao, T.; Zhao, Y.; Yu, X. A fed-batch feeding with succinic acid strategy for astaxanthin and lipid hyper-production in Haematococcus pluviualis. Bioresour. Technol. 2021, 340, 125648. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Zhao, J.; Liu, J. Exogenous sodium fumarate enhances astaxanthin accumulation in Haematococcus pluvialis by enhancing the respiratory metabolic pathway. Bioresour. Technol. 2021, 341, 125788. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Zhao, J.; Liu, J. Enhancement of astaxanthin accumulation in Haematococcus pluvialis by exogenous oxaloacetate combined with nitrogen deficiency. Bioresour. Technol. 2022, 345, 126484. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, H.-P.; Yu, X. The associative induction of succinic acid and hydrogen sulfide for high-producing biomass, astaxanthin and lipids in Haematococcus pluvialis. Bioresour. Technol. 2022, 358, 127397. [Google Scholar] [CrossRef] [PubMed]
- Joun, J.; Sirohi, R.; Sim, S.J. The effects of acetate and glucose on carbon fixation and carbon utilization in mixotrophy of Haematococcus pluvialis. Bioresour. Technol. 2023, 367, 128218. [Google Scholar] [CrossRef] [PubMed]
- Recht, L.; Töpfer, N.; Batushansky, A.; Sikron, N.; Gibon, Y.; Fait, A.; Nikoloski, Z.; Boussiba, S.; Zarka, A. Metabolite Profiling and Integrative Modeling Reveal Metabolic Constraints for Carbon Partitioning under Nitrogen Starvation in the Green Algae Haematococcus pluvialis. Pediatrics 2014, 289, 30387–30403. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zheng, L.; Liu, J.; Dai, J.; Song, L. A novel fed-batch strategy to boost cyst cells production based on the understanding of intracellular carbon and nitrogen metabolism in Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121744. [Google Scholar] [CrossRef]
- Shi, J.; Zang, X.; Cong, X.; Hou, L.; He, B.; Ding, Y.; Dong, M.; Sun, D.; Guo, Y.; Zhang, F.; et al. Cloning of nitrite reductase gene from Haematococcus pluvialis and transcription and enzymatic activity analysis at different nitrate and phosphorus concentration. Gene 2019, 697, 123–130. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Chai, W.; Liu, Z.; Wang, X.; He, C.; Hu, Z.; Chen, S.; Wang, W.; Chen, F. Transcriptome analysis of Haematococcus pluvialis of multiple defensive systems against nitrogen starvation. Enzym. Microb. Technol. 2020, 134, 109487. [Google Scholar] [CrossRef]
- Hoys, C.; Romero-Losada, A.B.; del Río, E.; Guerrero, M.G.; Romero-Campero, F.J.; García-González, M. Unveiling the underlying molecular basis of astaxanthin accumulation in Haematococcus through integrative metabolomic-transcriptomic analysis. Bioresour. Technol. 2021, 332, 125150. [Google Scholar] [CrossRef]
- Li, Y.; Sommerfeld, M.; Chen, F.; Hu, Q. Consumption of oxygen by astaxanthin biosynthesis: A protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J. Plant Physiol. 2008, 165, 1783–1797. [Google Scholar] [CrossRef]
- Tran, N.-P.; Park, J.-K.; Hong, S.-J.; Lee, C.-G. Proteomics of proteins associated with astaxanthin accumulation in the green algae Haematococcus lacustris under the influence of sodium orthovanadate. Biotechnol. Lett. 2009, 31, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Tao, M.; Li, J.; Hu, Z. Effects of selenite on green microalga Haematococcus pluvialis: Bioaccumulation of selenium and enhancement of astaxanthin production. Aquat. Toxicol. 2017, 183, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, T.; Zhao, Y.; Yu, X. Role of copper in the enhancement of astaxanthin and lipid coaccumulation in Haematococcus pluvialis exposed to abiotic stress conditions. Bioresour. Technol. 2021, 335, 125265. [Google Scholar] [CrossRef] [PubMed]
- Nasri, N.; Keyhanfar, M.; Behbahani, M.; Dini, G. Enhancement of astaxanthin production in Haematococcus pluvialis using zinc oxide nanoparticles. J. Biotechnol. 2021, 342, 72–78. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Xu, X.; Cheng, J.; Chen, S.; Tian, J.; Yang, W.; Crocker, M. Simultaneous promotion of photosynthesis and astaxanthin accumulation during two stages of Haematococcus pluvialis with ammonium ferric citrate. Sci. Total Environ. 2021, 750, 141689. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, Y.H.; Jeon, M.S.; Kim, S.; Choi, Y.-E. Polyethylenimine linked with chitosan improves astaxanthin production in Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2023, 107, 569–580. [Google Scholar] [CrossRef]
- Venckus, P.; Endriukaitytė, I.; Čekuolytė, K.; Gudiukaitė, R.; Pakalniškis, A.; Lastauskienė, E. Effect of Biosynthesized Silver Nanoparticles on the Growth of the Green Microalga Haematococcus pluvialis and Astaxanthin Synthesis. Nanomaterials 2023, 13, 1618. [Google Scholar] [CrossRef]
- Eom, H.; Lee, C.-G.; Jin, E. Gene expression profile analysis in astaxanthin-induced Haematococcus pluvialis using a cDNA microarray. Planta 2005, 223, 1231–1242. [Google Scholar] [CrossRef]
- Meng, C.X.; Wei, W.; Su, Z.L.; Qin, S. Characterization of carotenoid hydroxylase gene promoter in Haematococcus pluvialis. Indian J. Biochem. Biophys. 2006, 43, 284–288. [Google Scholar]
- Park, J.-K.; Tran, P.N.; Kim, J.D.; Hong, S.J.; Lee, C.G. Carotenogenesis in Haematococcus lacustris: Role of Protein Tyrosine Phosphatases. J. Microbiol. Biotechnol. 2009, 19, 918–921. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Rhee, J.-K.; Kim, H.-S.; Ahn, J.-W.; Hwang, H.; Yang, J.-W. Chemical Genetics Approach Reveals Importance of cAMP and MAP Kinase Signaling to Lipid and Carotenoid Biosynthesis in Microalgae. J. Microbiol. Biotechnol. 2015, 25, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Gusbeth, C.; Frey, W.; Nick, P. Nanosecond pulsed electric fields modulate the expression of the astaxanthin biosynthesis genes psy, crtR-b and bkt 1 in Haematococcus pluvialis. Sci. Rep. 2020, 10, 15508. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; Gujar, A.; Zhang, H.; Xu, W.; Zhao, C.; Zhu, X.; Xue, J.; Zhang, C.; Ji, C.; Qin, S.; et al. Cloning, expression, and characterization of a novel plant type cryptochrome gene from the green alga Haematococcus pluvialis. Protein Expr. Purif. 2020, 172, 105633. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, C.; Huang, D.; Li, H.; Wang, C. The Papain-like Cysteine Protease HpXBCP3 from Haematococcus pluvialis Involved in the Regulation of Growth, Salt Stress Tolerance and Chlorophyll Synthesis in Microalgae. Int. J. Mol. Sci. 2021, 22, 11539. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, K.; Ning, J.; Luo, Q.; Yang, Y.; Huang, D.; Li, H. Transcription Factors from Haematococcus pluvialis Involved in the Regulation of Astaxanthin Biosynthesis Under High Light-Sodium Acetate Stress. Front. Bioeng. Biotechnol. 2021, 9, 650178. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, W.; Hu, Q.; Li, H.; Wang, C. The Histone Acetyltransferase HpGCN5 Involved in the Regulation of Abiotic Stress Responses and Astaxanthin Accumulation in Haematococcus pluvialis. Front. Plant Sci. 2022, 13, 903764. [Google Scholar] [CrossRef]
- Jin, H.; Lao, Y.M.; Zhou, J.; Cai, Z.H. Identification of a RelA/SpoT Homolog and Its Possible Role in the Accumulation of Astaxanthin in Haematococcus pluvialis. Front. Plant Sci. 2022, 13, 796997. [Google Scholar] [CrossRef]
- Song, Y.; Luo, T.; Zhao, C.; Ji, C.; Zhang, C.; Ma, R.; Cui, H.; Li, R. Identification and expression analysis of WRKY gene family in eukaryotic algae. Chin. J. Biotechnol. 2022, 38, 1965–1980. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.; Han, T.; Sui, J.; Wang, H. Cryptochrome-mediated blue-light signal contributes to carotenoids biosynthesis in microalgae. Front. Microbiol. 2022, 13, 1083387. [Google Scholar] [CrossRef]
- Ahirwar, A.; Meignen, G.; Khan, M.J.; Sirotiya, V.; Harish; Scarsini, M.; Roux, S.; Marchand, J.; Schoefs, B.; Vinayak, V. Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: A review. Bioresour. Technol. 2021, 340, 125707. [Google Scholar] [CrossRef]
- Kayani, S.-I.; -Rahman, S.-U.; Shen, Q.; Cui, Y.; Liu, W.; Hu, X.; Zhu, F.; Huo, S. Molecular approaches to enhance astaxanthin biosynthesis; future outlook: Engineering of transcription factors in Haematococcus pluvialis. Crit. Rev. Biotechnol. 2023, 1–16. [Google Scholar] [CrossRef]
- Wilawan, B.; Chan, S.S.; Ling, T.C.; Show, P.L.; Ng, E.-P.; Jonglertjunya, W.; Phadungbut, P.; Khoo, K.S. Advancement of Carotenogenesis of Astaxanthin from Haematococcus pluvialis: Recent Insight and Way Forward. Mol. Biotechnol. 2023, 1–22. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.-C.; Fukatsu, T. Symbiotic Bacterium Modifies Aphid Body Color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Aoyama, K.; Motone, K.; Aburaya, S.; Yamashiro, H.; Miura, N.; Inoue, K. Mutualistic Interactions between Dinoflagellates and Pigmented Bacteria Mitigate Environmental Stress. Microbiol. Spectr. 2023, 11, e0246422. [Google Scholar] [CrossRef]
- Schiedt, K.; Bischof, S.; Glinz, E. Recent progress on carotenoid metabolism in animals. Pure Appl. Chem. 1991, 63, 89–100. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, H.; Tan, K.; Ma, H.; Li, S.; Zheng, H. Identification of a key gene StAR-like-3 responsible for carotenoids accumulation in the noble scallop Chlamys nobilis. Food Chem. Mol. Sci. 2022, 4, 100072. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, H.; Zhang, H.; Deng, L.; Liu, W.; Wang, S.; Meng, F.; Wang, Y.; Guo, Z.; Li, S.; et al. A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration. BMC Genom. 2015, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Xun, X.; Zhang, M.; Wang, S.; Li, H.; Zhao, L.; Fu, Q.; Wang, H.; Li, T.; et al. A carotenoid oxygenase is responsible for muscle coloration in scallop. Biochim. et Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 966–975. [Google Scholar] [CrossRef]
- Tan, K.; Guo, Z.; Zhang, H.; Ma, H.; Li, S.; Zheng, H. Carotenoids regulation in polymorphic noble scallops Chlamys nobilis under different light cycle. Aquaculture 2021, 531, 735937. [Google Scholar] [CrossRef]
- Wan, S.; Li, Q.; Yu, H.; Liu, S.; Kong, L. Transcriptome analysis based on dietary beta-carotene supplement reveals genes potentially involved in carotenoid metabolism in Crassostrea gigas. Gene 2022, 818, 146226. [Google Scholar] [CrossRef]
- Wan, S.; Li, Q.; Yu, H.; Liu, S.; Kong, L. A nuclear receptor heterodimer, CgPPAR2-CgRXR, acts as a regulator of carotenoid metabolism in Crassostrea gigas. Gene 2022, 827, 146473. [Google Scholar] [CrossRef] [PubMed]
- Ytrestøyl, T.; Afanasyev, S.; Ruyter, B.; Hatlen, B.; Østbye, T.-K.; Krasnov, A. Transcriptome and functional responses to absence of astaxanthin in Atlantic salmon fed low marine diets. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 39, 100841. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, J.; Verlhac-Trichet, V.; Madaro, A.; Lall, S.P.; Torrissen, O.; Olsen, R.E. Molecular Mechanism Involved in Carotenoid Metabolism in Post-Smolt Atlantic Salmon: Astaxanthin Metabolism During Flesh Pigmentation and Its Antioxidant Properties. Mar. Biotechnol. 2021, 23, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Osada, K.; Hatano, M.; Saneyoshi, M. Comparison of carotenoids in muscle and ovary from four genera of salmonid fishes. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 503–508. [Google Scholar] [CrossRef]
- Sundvold, H.; Helgeland, H.; Baranski, M.; Omholt, S.W.; Våge, D. Characterisation of a novel paralog of scavenger receptor class B member I (SCARB1) in Atlantic salmon (Salmo salar). BMC Genet. 2011, 12, 52. [Google Scholar] [CrossRef]
- Schiedt, K.; Leuenberger, F.J.; Vecchi, M.; Glinz, E. Absorption, retention and metabolic transformations of carotenoids in rainbow trout, salmon and chicken. Pure Appl. Chem. 1985, 57, 685–692. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Hatlen, B.; Jobling, M. Astaxanthin and its metabolites idoxanthin and crustaxanthin in flesh, skin, and gonads of sexually immature and maturing Arctic charr (Salvelinus alpinus (L.)). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 395–404. [Google Scholar] [CrossRef]
- Mundy, N.I.; Stapley, J.; Bennison, C.; Tucker, R.; Twyman, H.; Kim, K.-W.; Burke, T.; Birkhead, T.R.; Andersson, S.; Slate, J. Red Carotenoid Coloration in the Zebra Finch Is Controlled by a Cytochrome P450 Gene Cluster. Curr. Biol. 2016, 26, 1435–1440. [Google Scholar] [CrossRef]
- Lopes, R.J.; Johnson, J.D.; Toomey, M.B.; Ferreira, M.S.; Araujo, P.M.; Melo-Ferreira, J.; Andersson, L.; Hill, G.E.; Corbo, J.C.; Carneiro, M. Genetic Basis for Red Coloration in Birds. Curr. Biol. 2016, 26, 1427–1434. [Google Scholar] [CrossRef]
- Twyman, H.; Valenzuela, N.; Literman, R.; Andersson, S.; Mundy, N.I. Seeing red to being red: Conserved genetic mechanism for red cone oil droplets and co-option for red coloration in birds and turtles. Proc. R. Soc. B Boil. Sci. 2016, 283, 20161208. [Google Scholar] [CrossRef]
- Friedman, N.R.; McGraw, K.J.; Omland, K.E. Evolution of Carotenoid Pigmentation in Caciques and Meadowlarks (Icteridae): Repeated Gains of Red Plumage Coloration by Carotenoid C4-Oxygenation. Evolution 2013, 68, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lewis, V.M.; Foster, T.N.; Toomey, M.B.; Corbo, J.C.; Parichy, D.M. Development and genetics of red coloration in the zebrafish relative Danio albolineatus. eLife 2021, 10, e70253. [Google Scholar] [CrossRef] [PubMed]
- Toomey, M.B.; Marques, C.I.; Araújo, P.M.; Huang, D.; Zhong, S.; Liu, Y.; Schreiner, G.D.; Myers, C.A.; Pereira, P.; Afonso, S.; et al. A mechanism for red coloration in vertebrates. Curr. Biol. 2022, 32, 4201–4214.e4212. [Google Scholar] [CrossRef] [PubMed]
- Córdova, P.; Gonzalez, A.-M.; Nelson, D.R.; Gutiérrez, M.-S.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Characterization of the cytochrome P450 monooxygenase genes (P450ome) from the carotenogenic yeast Xanthophyllomyces dendrorhous. BMC Genom. 2017, 18, 540. [Google Scholar] [CrossRef] [PubMed]
- Weaver, R.J.; Gonzalez, B.K.; Santos, S.R.; Havird, J.C. Red Coloration in an Anchialine Shrimp: Carotenoids, Genetic Variation, and Candidate Genes. Biol. Bull. 2020, 238, 119–130. [Google Scholar] [CrossRef]
- Mojib, N.; Amad, M.; Thimma, M.; Aldanondo, N.; Kumaran, M.; Irigoien, X. Carotenoid metabolic profiling and transcriptome-genome mining reveal functional equivalence among blue-pigmented copepods and appendicularia. Mol. Ecol. 2014, 23, 2740–2756. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Cooper, J.P.; Hwang, K.; Singh, H.; Wang, D.; Reynolds, C.P.; Jr, R.W.C.; Williams, S.C.; Maurer, B.J.; Kang, M.H. Fenretinide metabolism in humans and mice: Utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br. J. Pharmacol. 2011, 163, 1263–1275. [Google Scholar] [CrossRef]
- Illingworth, N.; Boddy, A.; Daly, A.; Veal, G. Characterization of the metabolism of fenretinide by human liver microsomes, cytochrome P450 enzymes and UDP-glucuronosyltransferases. Br. J. Pharmacol. 2011, 162, 989–999. [Google Scholar] [CrossRef]
- Surmatis, J.D.; Thommen, R. A total synthesis of astaxanthin dimethyl ether. J. Org. Chem. 1967, 32, 180–184. [Google Scholar] [CrossRef]
- Leftwick, A.P.; Weedon, B.C.L. Total synthesis of astaxanthin and hydroxyechinenone. Chem. Commun. 1967, 49–50. [Google Scholar] [CrossRef]
- Bernhard, K. Synthetic Astaxanthin. The Route of a Carotenoid from Research to Commercialisation. In Carotenoids; Krinsky, N.I., Mathews-Roth, M.M., Taylor, R.F., Eds.; Springer: Boston, MA, USA, 1989; pp. 337–363. [Google Scholar]
- Rodríguez-deLeón, E.; Jiménez-Halla, J.O.C.; Báez, J.E.; Bah, M.M. A Simple and Efficient Method for the Partial Synthesis of Pure (3R,3′S)-Astaxanthin from (3R,3′R,6′R)-Lutein and Lutein Esters via (3R,3′S)-Zeaxanthin and Theoretical Study of Their Formation Mechanisms. Molecules 2019, 24, 1386. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Kimura, F.; Satoh, A.; Miyazawa, T. Plasma Carotenoid Concentrations before and after Supplementation with Astaxanthin in Middle-Aged and Senior Subjects. Biosci. Biotechnol. Biochem. 2011, 75, 1856–1858. [Google Scholar] [CrossRef] [PubMed]
- Virchenko, O.; Stefánsson, T. Light Increases Astaxanthin Absorbance in Acetone Solution through Isomerization Reactions. Molecules 2023, 28, 847. [Google Scholar] [CrossRef] [PubMed]
- Schiedt, K.; Bischof, S.; Glinz, E. [15] Metabolism of carotenoids and in Vivo racemization of (3S,3′S)-Astaxanthin in the crustacean Penaeus. In Carotenoids Part B Metabolism, Genetics, and Biosynthesis; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1993; Volume 214, pp. 148–168. [Google Scholar]
- Woollard, D.C.; Bensch, A.; Indyk, H.; McMahon, A. Determination of vitamin A and vitamin E esters in infant formulae and fortified milk powders by HPLC: Use of internal standardisation. Food Chem. 2016, 197, 457–465. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R.; Llewellyn, C.; Bjornland, T.; Repeta, D.; Welschmeyer, N. Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183–196. [Google Scholar] [CrossRef]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.; de Oliveira, L.F. Raman spectra of carotenoids in natural products. Spectrochim. Acta Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Hikima, J.-I.; Ando, M.; Hamaguchi, H.-O.; Sakai, M.; Maita, M.; Yazawa, K.; Takeyama, H.; Aoki, T. On-site Direct Detection of Astaxanthin from Salmon Fillet Using Raman Spectroscopy. Mar. Biotechnol. 2017, 19, 157–163. [Google Scholar] [CrossRef]
- Cannizzaro, C.; Rhiel, M.; Marison, I.; von Stockar, U. On-line monitoring of Phaffia rhodozyma fed-batch process with in situ dispersive raman spectroscopy. Biotechnol. Bioeng. 2003, 83, 668–680. [Google Scholar] [CrossRef]
- Wold, J.P.; Marquardt, B.J.; Dable, B.K.; Robb, D.; Hatlen, B. Rapid Quantification of Carotenoids and Fat in Atlantic Salmon (Salmo Salar L.) by Raman Spectroscopy and Chemometrics. Appl. Spectrosc. 2004, 58, 395–403. [Google Scholar] [CrossRef]
- Landry, J.D.; Torley, P.J.; Blanch, E.W. Quantitation of carotenoids and fatty acids from Atlantic salmon using a portable Raman device. Anal. 2022, 147, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Radwan, B.; Adamczyk, A.; Tott, S.; Czamara, K.; Kaminska, K.; Matuszyk, E.; Baranska, M. Labeled vs. Label-Free Raman Imaging of Lipids in Endothelial Cells of Various Origins. Molecules 2020, 25, 5752. [Google Scholar] [CrossRef] [PubMed]
- Czamara, K.; Adamczyk, A.; Stojak, M.; Radwan, B.; Baranska, M. Astaxanthin as a new Raman probe for biosensing of specific subcellular lipidic structures: Can we detect lipids in cells under resonance conditions? Cell. Mol. Life Sci. 2020, 78, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Uragami, C.; Yamashita, E.; Gall, A.; Robert, B.; Hashimoto, H. Application of resonance Raman microscopy to in vivo carotenoid. Acta Biochim. Pol. 2012, 59, 53–56. [Google Scholar] [CrossRef]
- Osterrothová, K.; Culka, A.; Němečková, K.; Kaftan, D.; Nedbalová, L.; Procházková, L.; Jehlička, J. Analyzing carotenoids of snow algae by Raman microspectroscopy and high-performance liquid chromatography. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019, 212, 262–271. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. The nature and role of pigments of marine invertebrates. Nat. Prod. Rep. 2006, 23, 223–255. [Google Scholar] [CrossRef]
- Gherardi, F. Towards a sustainable human use of freshwater crayfish (Crustacea, Decapoda, Astacidea). Knowl. Manag. Aquat. Ecosyst. 2011, 401, 02. [Google Scholar] [CrossRef]
- Sweden, V. Experience a Swedish crayfish party. Available online: https://visitsweden.com/what-to-do/culture-history-and-art/swedish-traditions/more-traditions/crayfish-party/ (accessed on 1 January 2023).
- Dardar, T.M.; Bruce, C. Women-Chiefs and Crawfish Warriors: A Brief History of the Houma People; United Houma Nation: Houma, LA, USA, 2000. [Google Scholar]
- Comby, H. Origin Story. In The Origin of the Houma Tribe; The University of Southern Mississippi: Hattiesburg, MI, USA, 2014; Volume 51, pp. 176–177. [Google Scholar]
- d’Oney, J.D. A Kingdom of Water; University of Nebraska Press: Lincoln, NE, USA, 2020. [Google Scholar]
- Louisiana Statutes. State Crustacean, Title 49, Section RS 49:Translated by Clint Bruce. 2000. Available online: http://legis.la.gov/legis/Law.aspx?d=103562 (accessed on 1 August 2023).
- Moro-Abadía, O.; Garate, D. The beginnings of European Upper Paleolithic art: A critical review. N. Atl. Archaeol. 2010, 2, 1–18. [Google Scholar]
- Crétin, C. «Les Eyzies-de-Tayac-Sireuil–Abri du Poisson, Gorge d’Enfer» [Notice Archéologique], ADLFI. Archéologie de la France–Informations [En ligne]. Available online: http://journals.openedition.org/adlfi/101468 (accessed on 5 January 2023).
- Amoss, P.T. The Fish God Gave Us: The First Salmon Ceremony Revived. Arct. Anthropol. 1987, 24, 56–66. [Google Scholar]
- O’Dwyer, R. Reviewed Work: Fionn Mac Cumhaill: Celtic Myth in English Literature by James MacKillop Can. J. Ir. Stud. 1989, 14, 75–78. [Google Scholar] [CrossRef]
- Kurlansky, M. Salmon: A Fish, The Earth, and The History of A Common Fate; Ventura, C.A., Ed.; Patagonia: Ventura, CA, USA, 2020. [Google Scholar]
- Murakami-Shi-Shi (History of Murakami City), Tsuushi-Hen (Edition of General History) 1; Murakami-Shi: Murakami-Shi, Japan, 1989.
- NIIKEI. “Salted Salmon for Daijosai: Craftsmen in Murakami Put Their Heart and Soul into Collaborative Production” in Japanese. Available online: https://www.niikei.jp/24511/ (accessed on 30 January 2023).
- Corporation, M.N.M.N. Salmon Museum. Available online: https://www.maruha-nichiro.co.jp/salmon/culture/c01.html (accessed on 31 January 2023).
- Iwasaki-Goodman, M.; Nomoto, M. Revitalizing the relationship between Ainu and salmon: Salmon rituals in the present. Senri Ethnol. Stud. 2001, 59, 27–46. [Google Scholar]
- Dunne, J.; Mercuri, A.M.; Evershed, R.P.; Bruni, S.; di Lernia, S. Earliest direct evidence of plant processing in prehistoric Saharan pottery. Nat. Plants 2016, 3, 16194. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Dillehay, T.D.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte Verde: Seaweed, Food, Medicine, and the Peopling of South America. Science 2008, 320, 784–786. [Google Scholar] [CrossRef]
- Mayes, C. Medicalization of Eating and Feeding. In Encyclopedia of Food and Agricultural Ethics; Thompson, P.B., Kaplan, D.M., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–8. [Google Scholar]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2022, 850, 2691–2706. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- European, C.; Directorate-General for Education, Y.S. Culture. Stormy times: Nature and Humans: Cultural Courage for Change: 11 Messages for and From Europe; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Olabi, A.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of microalgae in achieving sustainable development goals and circular economy. Sci. Total. Environ. 2022, 854, 158689. [Google Scholar] [CrossRef]
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.-W.; Huang, C.-Y.; Chang, J.-S. Microalgae as sustainable food and feed sources for animals and humans–Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Cristaldi, M.; Anfuso, C.D.; Spampinato, G.; Rusciano, D.; Lupo, G. Comparative Efficiency of Lutein and Astaxanthin in the Protection of Human Corneal Epithelial Cells In Vitro from Blue-Violet Light Photo-Oxidative Damage. Appl. Sci. 2022, 12, 1268. [Google Scholar] [CrossRef]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Muhammad, G.; Butler, T.O.; Chen, B.; Lv, Y.; Xiong, W.; Zhao, X.; Solovchenko, A.E.; Zhao, A.; Mofijur, M.; Xu, J.; et al. Sustainable production of lutein—An underexplored commercially relevant pigment from microalgae. Biomass-Convers. Biorefinery 2022, 1–22. [Google Scholar] [CrossRef]
- Kurnia, A.; Satoh, S.; Kuramoto, D.; Hanzawa, S. Effect of Different Astaxanthin Sources on Skin Pigmentation of Red Sea Bream (Pagrus major). Aquac. Sci. 2007, 55, 441–447. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Johnson, E.A.; Lewis, M.J.; Grau, C. Pigmentation of Egg Yolks with Astaxanthin from the Yeast Phaffia rhodozyma. Poult. Sci. 1980, 59, 1777–1782. [Google Scholar] [CrossRef]
- An, G.-H.; Song, J.-Y.; Chang, K.-S.; Lee, B.-D.; Chae, H.-S.; Jang, B.-G. Pigmentation and Delayed Oxidation of Broiler Chickens by the Red Carotenoid, Astaxanthin, from Chemical Synthesis and the Yeast, Xanthophyllomyces dendrorhous. Asian-Australasian J. Anim. Sci. 2004, 17, 1309–1314. [Google Scholar] [CrossRef]
- Inoue, H.; Shimamoto, S.; Takahashi, H.; Kawashima, Y.; Wataru, S.; Ijiri, D.; Ohtsuka, A. Effects of astaxanthin-rich dried cell powder from Paracoccus carotinifaciens on carotenoid composition and lipid peroxidation in skeletal muscle of broiler chickens under thermo-neutral or realistic high temperature conditions. Anim. Sci. J. 2019, 90, 229–236. [Google Scholar] [CrossRef]
- Ao, X.; Kim, I. Effects of astaxanthin produced by Phaffia rhodozyma on growth performance, antioxidant activities, and meat quality in Pekin ducks. Poult. Sci. 2019, 98, 4954–4960. [Google Scholar] [CrossRef]
- Sun, T.; Yin, R.; Magnuson, A.D.; Tolba, S.A.; Liu, G.; Lei, X.G. Dose-Dependent Enrichments and Improved Redox Status in Tissues of Broiler Chicks under Heat Stress by Dietary Supplemental Microalgal Astaxanthin. J. Agric. Food Chem. 2018, 66, 5521–5530. [Google Scholar] [CrossRef]
- Perenlei, G.; Tojo, H.; Okada, T.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary astaxanthin rich yeast, Phaffia rhodozyma, on meat quality of broiler chickens. Anim. Sci. J. 2014, 85, 895–903. [Google Scholar] [CrossRef]
- Saito, F.; Kita, K. Maternal Intake of Astaxanthin Improved Hatchability of Fertilized Eggs Stored at High Temperature. J. Poult. Sci. 2011, 48, 33–39. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Effect of astaxanthin produced by Phaffia rhodozyma on growth performance, meat quality, and fecal noxious gas emission in broilers. Poult. Sci. 2014, 93, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.; Sun, T.; Yin, R.; Liu, G.; Tolba, S.; Shinde, S.; Lei, X. Supplemental microalgal astaxanthin produced coordinated changes in intrinsic antioxidant systems of layer hens exposed to heat stress. Algal Res. 2018, 33, 84–90. [Google Scholar] [CrossRef]
- Toyes-Vargas, E.; Ortega-Pérez, R.; Espinoza-Villavicencio, J.L.; Arellano-Pérez, M.; Civera, R.; Palacios, E. Effect of marine by-product meals on hen egg production parameters, yolk lipid composition and sensory quality. J. Anim. Physiol. Anim. Nutr. 2018, 102, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, G.Y.; Kang, H.K.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Tolba, S.A.; Magnuson, A.D.; Sun, T.; Lei, X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020, 99, 4853–4860. [Google Scholar] [CrossRef]
- Dansou, D.M.; Wang, H.; Nugroho, R.D.; He, W.; Zhao, Q.; Zhang, J. Assessment of Response to Moderate and High Dose Supplementation of Astaxanthin in Laying Hens. Animals 2021, 11, 1138. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, L.; Ge, J.; Wu, X.; Peng, Y.; Zhang, T.; Jiang, M. Astaxanthin supplementation enriches productive performance, physiological and immunological responses in laying hens. Anim. Biosci. 2021, 34, 443–448. [Google Scholar] [CrossRef]
- He, W.; Wang, H.; Tang, C.; Zhao, Q.; Zhang, J. Dietary supplementation with astaxanthin alleviates ovarian aging in aged laying hens by enhancing antioxidant capacity and increasing reproductive hormones. Poult. Sci. 2023, 102, 102258. [Google Scholar] [CrossRef]
- Pertiwi, H.; Mahendra, M.Y.N.; Kamaludeen, J. Astaxanthin as a Potential Antioxidant to Improve Health and Production Performance of Broiler Chicken. Vet. Med. Int. 2022, 2022, 4919442. [Google Scholar] [CrossRef]
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin ameliorates heat stress-induced impairment of blastocyst development In Vitro: Astaxanthin colocalization with and action on mitochondria. J. Assist. Reprod. Genet. 2013, 30, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tani, C.; Maoka, T.; Tani, M.; Moritomo, Y.; Okada, T.; Kitahara, G.; Katamoto, H. Accumulation of Xanthophylls from the Phaffia Yeast (Xanthophyllomyces dendrorhrous) in Calves. J. Oleo Sci. 2014, 63, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Ispada, J.; Rodrigues, T.A.; Risolia, P.H.B.; Lima, R.S.; Gonçalves, D.R.; Rettori, D.; Nichi, M.; Feitosa, W.B.; Paula-Lopes, F.F. Astaxanthin counteracts the effects of heat shock on the maturation of bovine oocytes. Reprod. Fertil. Dev. 2018, 30, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahu, D.S.; Chandra, G.; Yadav, S.P.; Kumar, R.; Ali, N.; Roy, D.; Maurya, P.S. Effect of Astaxanthin and Copper Supplementation on Growth, Immunity, Antioxidant, and Blood Biochemical Status of Growing Murrah Buffalo Heifers. Biol. Trace Elem. Res. 2022, 200, 5052–5063. [Google Scholar] [CrossRef]
- Namekawa, T.; Ikeda, S.; Sugimoto, M.; Kume, S. Effects of Astaxanthin-containing Oil on Development and Stress-related Gene Expression of Bovine Embryos Exposed to Heat Stress. Reprod. Domest. Anim. 2010, 45, e387–e391. [Google Scholar] [CrossRef]
- Jang, H.; Ji, S.; Kim, Y.; Lee, H.; Shin, J.; Cheong, H.; Kim, J.; Park, I.; Kong, H.; Park, C.; et al. Antioxidative Effects of Astaxanthin against Nitric Oxide-Induced Oxidative Stress on Cell Viability and Gene Expression in Bovine Oviduct Epithelial Cell and the Developmental Competence of Bovine IVM/IVF Embryos. Reprod. Domest. Anim. 2010, 45, 967–974. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, J.; Pang, W.; Yang, G.; Chu, G. Astaxanthin improves preserved semen quality at 17 °C by enhancing sperm antioxidant and motility properties. Reprod. Domest. Anim. 2022, 57, 1187–1197. [Google Scholar] [CrossRef]
- Szczepanik, K.; Furgał-Dierżuk, I.; Gala, L.; Świątkiewicz, M. Effects of Hermetia illucens Larvae Meal and Astaxanthin as Feed Additives on Health and Production Indices in Weaned Pigs. Animals 2022, 13, 163. [Google Scholar] [CrossRef]
- Lei, Y.; Kim, I.H. Effect of Phaffia rhodozyma on performance, nutrient digestibility, blood characteristics, and meat quality in finishing pigs. J. Anim. Sci. 2014, 92, 171–176. [Google Scholar] [CrossRef]
- Do, L.T.K.; Luu, V.V.; Morita, Y.; Taniguchi, M.; Nii, M.; Peter, A.T.; Otoi, T. Astaxanthin present in the maturation medium reduces negative effects of heat shock on the developmental competence of porcine oocytes. Reprod. Biol. 2015, 15, 86–93. [Google Scholar] [CrossRef]
- Abdel-Ghani, M.A.; Yanagawa, Y.; Balboula, A.Z.; Sakaguchi, K.; Kanno, C.; Katagiri, S.; Takahashi, M.; Nagano, M. Astaxanthin improves the developmental competence of in vitro-grown oocytes and modifies the steroidogenesis of granulosa cells derived from bovine early antral follicles. Reprod. Fertil. Dev. 2019, 31, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Chelenga, M.; Sakaguchi, K.; Abdel-Ghani, M.A.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Effect of increased oxygen availability and astaxanthin supplementation on the growth, maturation and developmental competence of bovine oocytes derived from early antral follicles. Theriogenology 2020, 157, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.-Y.; Xiang, D.-C.; Shao, Q.-Y.; Zhang, B.; Liu, S.-N.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Inhibitory effects of astaxanthin on postovulatory porcine oocyte aging in vitro. Sci. Rep. 2020, 10, 20217. [Google Scholar] [CrossRef]
- Xiang, D.-C.; Jia, B.-Y.; Fu, X.-W.; Guo, J.-X.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Z.; Liu, S.; Gao, F.; Zhang, J.; Peng, Z.; Wang, L.; Pan, X. Astaxanthin improves the development of the follicles and oocytes through alleviating oxidative stress induced by BPA in cultured follicles. Sci. Rep. 2022, 12, 7853. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Somsak, P.; Piromlertamorn, W.; Sanmee, U. Effects of astaxanthin supplementation in fertilization medium and/or culture medium on the fertilization and development of mouse oocytes. Clin. Exp. Reprod. Med. 2022, 49, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kükürt, A.; Karapehlivan, M. Protective effect of astaxanthin on experimental ovarian damage in rats. J. Biochem. Mol. Toxicol. 2022, 36, e22966. [Google Scholar] [CrossRef]
- Toktay, E.; Tastan, T.B.; Gurbuz, M.A.; Erbas, E.; Demir, O.; Ugan, R.A.; Selli, J. Potential protective effect of astaxanthin on ovary ischemia-reperfusion injury. Iran. J. Basic. Med. Sci. 2022, 25, 173–178. [Google Scholar] [CrossRef]
- Guo, H.-T.; Wang, J.-R.; Sun, L.-Z.; Jin, X.-H.; Shi, X.-Y.; Lin, J.-Y.; Yue, S.-L.; Zhou, J.-B. Effects of astaxanthin on plasma membrane function and fertility of boar sperm during cryopreservation. Theriogenology 2021, 164, 58–64. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Bang, S.; Shin, S.T.; Cho, J. The effect of astaxanthin supplementation on the post-thaw quality of dog semen. Reprod. Domest. Anim. 2020, 55, 1163–1171. [Google Scholar] [CrossRef]
- Basioura, A.; Tsakmakidis, I.; Martinez, E.; Roca, J.; Li, J.; Molina, M.; Theodoridis, A.; Boscos, C.; Parrilla, I. Effect of astaxanthin in extenders on sperm quality and functional variables of frozen-thawed boar semen. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, D. Effects of Astaxanthin on Miniature Pig Sperm Cryopreservation. BioMed Res. Int. 2018, 2018, 6784591. [Google Scholar] [CrossRef] [PubMed]
- Gharaei, R.; Alyasin, A.; Mahdavinezhad, F.; Samadian, E.; Ashrafnezhad, Z.; Amidi, F. Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J. Assist. Reprod. Genet. 2022, 39, 995–1008. [Google Scholar] [CrossRef]
- Jabarpour, M.; Aleyasin, A.; Nashtaei, M.S.; Lotfi, S.; Amidi, F. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: A randomized clinical trial. Sci. Rep. 2023, 13, 3376. [Google Scholar] [CrossRef]
- Rostami, S.; Alyasin, A.; Saedi, M.; Nekoonam, S.; Khodarahmian, M.; Moeini, A.; Amidi, F. Astaxanthin ameliorates inflammation, oxidative stress, and reproductive outcomes in endometriosis patients undergoing assisted reproduction: A randomized, triple-blind placebo-controlled clinical trial. Front. Endocrinol. 2023, 14, 1144323. [Google Scholar] [CrossRef]
- Le-Feuvre, R.; Moraga-Suazo, P.; Gonzalez, J.; Martin, S.S.; Henríquez, V.; Donoso, A.; Agurto-Muñoz, C. Biotechnology applied to Haematococcus pluvialis Fotow: Challenges and prospects for the enhancement of astaxanthin accumulation. J. Appl. Phycol. 2020, 32, 3831–3852. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Bin Ariff, A.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]
- Huy, M.; Vatland, A.K.; Kumar, G. Nutraceutical productions from microalgal derived compounds via circular bioeconomy perspective. Bioresour. Technol. 2022, 347, 126575. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Oslan, S.N.; Mohamad, R.; Tan, J.S.; Yusoff, A.H.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Bioprocess Strategy of Haematococcus lacustris for Biomass and Astaxanthin Production Keys to Commercialization: Perspective and Future Direction. Fermentation 2022, 8, 179. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Hu, Q.; Sommerfeld, M.; Li, Y.; Han, D. A new paradigm for producing astaxanthin from the unicellular green algaHaematococcus pluvialis. Biotechnol. Bioeng. 2016, 113, 2088–2099. [Google Scholar] [CrossRef]
- Zhang, X.W.; Gong, X.-D.; Chen, F. Kinetic models for astaxanthin production by high cell density mixotrophic culture of the microalga Haematococcus pluvialis. J. Ind. Microbiol. Biotechnol. 1999, 23, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Goksan, T.; Ak, I.; Gokpinar, S. An alternative approach to the traditional mixotrophic cultures of Haematococcus pluvialis Flotow (Chlorophyceae). J. Microbiol. Biotechnol. 2010, 20, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: Strains screening and significance evaluation of environmental factors. Bioresour. Technol. 2011, 102, 10861–10867. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.O.; McDougall, G.J.; Campbell, R.; Stanley, M.S.; Day, J.G. Media Screening for Obtaining Haematococcus pluvialis Red Motile Macrozooids Rich in Astaxanthin and Fatty Acids. Biology 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kucek, L.A.; Hill, E.; Pinchuk, G.E.; Mundree, S.G.; Beliaev, A.S. Conversion of stranded waste-stream carbon and nutrients into value-added products via metabolically coupled binary heterotroph-photoautotroph system. Bioresour. Technol. 2018, 260, 68–75. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, X.; Zhang, W.; Chen, L.; Liu, T. Protoplast preparation from enriched flagellates and resting cells of Haematococcus pluvialis. J. Appl. Microbiol. 2018, 124, 469–479. [Google Scholar] [CrossRef]
- Fan, F.; Fei, Z.; Wan, M.; Huang, J.; Wang, W.; Bai, W.; He, M.; Li, Y. The optimization of centrifugal pump driving horizontal tubular photobioreactor for enhancing astaxanthin production using heterotrophic Haematococcus pluvialis. J. Biotechnol. 2021, 341, 168–174. [Google Scholar] [CrossRef]
- Khazi, M.I.; Shi, L.; Liaqat, F.; Yang, Y.; Li, X.; Yang, D.; Li, J. Sequential Continuous Mixotrophic and Phototrophic Cultivation Might Be a Cost-Effective Strategy for Astaxanthin Production from the Microalga Haematococcus lacustris. Front. Bioeng. Biotechnol. 2021, 9, 740533. [Google Scholar] [CrossRef]
- Lv, R.; Liu, K.; Chen, F.; Xing, H.; Xu, N.; Sun, X.; Hu, C. Buffering culture solution significantly improves astaxanthin production efficiency of mixotrophic Haematococcus pluvialis. Bioresour. Technol. 2022, 354, 127175. [Google Scholar] [CrossRef]
- Khazi, M.I.; Liaqat, F.; Gu, W.; Mohamed, B.; Zhu, D.; Li, J. Astaxanthin production from the microalga Haematococcus lacustris with a dual substrate mixotrophy strategy. Biotechnol. J. 2023, e2300095. [Google Scholar] [CrossRef]
- Wang, B.; Pan, X.; Wang, F.; Liu, L.; Jia, J. Photoprotective carbon redistribution in mixotrophic Haematococcus pluvialis under high light stress. Bioresour. Technol. 2022, 362, 127761. [Google Scholar] [CrossRef] [PubMed]
- Sharon-Gojman, R.; Maimon, E.; Leu, S.; Zarka, A.; Boussiba, S. Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales). Algal Res. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Yuan, G.; Xu, X.; Zhang, W.; Zhang, W.; Cui, Y.; Qin, S.; Liu, T. Biolistic Transformation of Haematococcus pluvialis With Constructs Based on the Flanking Sequences of Its Endogenous Alpha Tubulin Gene. Front. Microbiol. 2019, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Anwar, M.; Mei, R.; Li, X.; Zhao, D.; Jiang, Y.; Zhuang, J.; Liu, C.; Wang, C.; Hu, Z. Establishment and optimization of PEG-mediated protoplast transformation in the microalga Haematococcus pluvialis. J. Appl. Phycol. 2022, 34, 1595–1605. [Google Scholar] [CrossRef]
- Shin, S.-E.; Lim, J.-M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.-S.; Lee, B.; Hwangbo, K.; et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef]
- Jeon, S.; Lim, J.-M.; Lee, H.-G.; Shin, S.-E.; Kang, N.K.; Park, Y.-I.; Oh, H.-M.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Current status and perspectives of genome editing technology for microalgae. Biotechnol. Biofuels 2017, 10, 267. [Google Scholar] [CrossRef]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol. Bioeng. 2018, 115, 719–728. [Google Scholar] [CrossRef]
- Patel, V.K.; Soni, N.; Prasad, V.; Sapre, A.; Dasgupta, S.; Bhadra, B. CRISPR–Cas9 System for Genome Engineering of Photosynthetic Microalgae. Mol. Biotechnol. 2019, 61, 541–561. [Google Scholar] [CrossRef]
- Honda, M.; Nishida, Y. In Vitro Evaluation of Skin-Related Physicochemical Properties and Biological Activities of Astaxanthin Isomers. ACS Omega 2023, 8, 19311–19319. [Google Scholar] [CrossRef]
- Nakamura, S.; Maoka, T.; Tsuji, S.; Hayashi, M.; Shimazawa, M.; Hara, H. Central Nervous System Migration of Astaxanthin and Adonixanthin Following Their Oral Administration in Cynomolgus Monkeys. J. Nutr. Sci. Vitaminol. 2020, 66, 488–494. [Google Scholar] [CrossRef]
- Izumi-Nagai, K.; Nagai, N.; Ohgami, K.; Satofuka, S.; Ozawa, Y.; Tsubota, K.; Ohno, S.; Oike, Y.; Ishida, S. Inhibition of Choroidal Neovascularization with an Anti-Inflammatory Carotenoid Astaxanthin. Investig. Opthalmology Vis. Sci. 2008, 49, 1679–1685. [Google Scholar] [CrossRef]
- Inoue, Y.; Shimazawa, M.; Nagano, R.; Kuse, Y.; Takahashi, K.; Tsuruma, K.; Hayashi, M.; Ishibashi, T.; Maoka, T.; Hara, H. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017, 134, 147–157. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Shimazawa, M.; Otsubo, K.; Ishibashi, T.; Hara, H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J. Pharm. Pharmacol. 2008, 60, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Shimazawa, M.; Inoue, Y.; Nakano, Y.; Ojino, K.; Izawa, H.; Tsuruma, K.; Ishibashi, T.; Hara, H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016, 41, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, R.; Aihara, M. Neuroprotective effect of astaxanthin against rat retinal ganglion cell death under various stresses that induce apoptosis and necrosis. Mol. Vis. 2014, 20, 1796–1805. [Google Scholar]
- Nagaki, Y.; Mihara, M.; Takahashi, J.; Kitamura, A.; Horita, Y.; Sugiura, Y.; Tsukahara, H. The effect of astaxanthin on retinal capillary blood flow in normal volunteers. J. Clin. Ther. Med. 2005, 21, 5. [Google Scholar]
- Saito, M.; Yoshida, K.; Saito, W.; Fujiya, A.; Ohgami, K.; Kitaichi, N.; Tsukahara, H.; Ishida, S.; Ohno, S. Astaxanthin increases choroidal blood flow velocity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 239–245. [Google Scholar] [CrossRef]
- Miyawaki, H.; Takahashi, J.; Tsukahara, H.; Takehara, I. Effects of Astaxanthin on Human Blood Rheology. J. Clin. Biochem. Nutr. 2008, 43, 69–74. [Google Scholar] [CrossRef]
- Takamura, T. Hepatokine Selenoprotein P-Mediated Reductive Stress Causes Resistance to Intracellular Signal Transduction. Antioxidants Redox Signal. 2020, 33, 517–524. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid. Redox Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef]
- Food Safety Commission of Japan (FSCJ); Chair Man, M.T. FS/281-2/2004, Notification of the Results of the Assessment Food Health Effects Pertaining to Food Safety No. 0825002 Issued by Food Safety Comity of Ministry of Health, Labour and Welfare. Available online: https://www.fsc.go.jp/fsciis/evaluationDocument/show/kya20030825089 (accessed on 30 November 2022).
- Yamashita, E. Suppression of post-UVB hyperpigmentation. Fragr. J. 1995, 14, 180–185. [Google Scholar]
- Rad, N.R.; Movahedian, A.; Feizi, A.; Aminorroaya, A.; Aarabi, M.H. Antioxidant effects of astaxanthin and metformin combined therapy in type 2 diabetes mellitus patients: A randomized double-blind controlled clinical trial. Res. Pharm. Sci. 2022, 17, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, S.; Kato, T.; Kasai, T.; Sato, A.; Yatsu, S.; Matsumoto, H.; Shitara, J.; Murata, A.; Shimizu, M.; Suda, S.; et al. Changes in self-reported physical activity and health-related quality of life following 3-month astaxanthin supplementation in patients with heart failure: Results from a pilot study. Ann. Palliat. Med. 2021, 10, 1396–1403. [Google Scholar] [CrossRef]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Kasai, T.; Sato, A.; Ishiwata, S.; Yatsu, S.; Matsumoto, H.; Shitara, J.; Murata, A.; Shimizu, M.; Suda, S.; et al. Effects of 3-Month Astaxanthin Supplementation on Cardiac Function in Heart Failure Patients with Left Ventricular Systolic Dysfunction-A Pilot Study. Nutrients 2020, 12, 1896. [Google Scholar] [CrossRef]
- Coombes, J.S.; E Sharman, J.; Fassett, R.G. Astaxanthin has no effect on arterial stiffness, oxidative stress, or inflammation in renal transplant recipients: A randomized controlled trial (the XANTHIN trial). Am. J. Clin. Nutr. 2016, 103, 283–289. [Google Scholar] [CrossRef]
- Fassett, R.G.; Healy, H.; Driver, R.; Robertson, I.K.; Geraghty, D.P.; E Sharman, J.; Coombes, J.S. Astaxanthin vs placebo on arterial stiffness, oxidative stress and inflammation in renal transplant patients (Xanthin): A randomised controlled trial. BMC Nephrol. 2008, 9, 17. [Google Scholar] [CrossRef]
- Takemoto, M.; Yamaga, M.; Furuichi, Y.; Yokote, K. Astaxanthin Improves Nonalcoholic Fatty Liver Disease in Werner Syndrome with Diabetes Mellitus. J. Am. Geriatr. Soc. 2015, 63, 1271–1273. [Google Scholar] [CrossRef]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of Astaxanthin on Oxidative Stress in Overweight and Obese Adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Iwabayashi, M.; Fujioka, N.; Nomoto, K.; Miyazaki, R.; Takahashi, H.; Hibino, S.; Takahashi, Y.; Nishikawa, K.; Nishida, M.; Yonei, Y. Efficacy and safety of eight-week treatment with astaxanthin in individuals screened for increased oxidative stress burden. Anti-Aging Med. 2009, 6, 15–21. [Google Scholar] [CrossRef]
- Kim, Y.K.; Chyun, J.H. The Effects of Astaxanthin Supplements on Lipid Peroxidation and Antioxidant Status in Postmenopausal Women. Nutr. Sci. 2004, 7, 41–46. [Google Scholar]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with Dietary Astaxanthin Combined with Collagen Hydrolysate Improves Facial Elasticity and Decreases Matrix Metalloproteinase-1 and -12 Expression: A Comparative Study with Placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef]
- Satoh, A.; Kawamura, T.; Horibe, N.; Ito, T.; Yagi, H.; Hashizume, H.; Takigawa, M. Effect of the intake of astaxanthin-containing Haematococcus pluvialis extract on the severity, immunofunction and physiological function in patients with atopic dermatitis. J. Environ. Dermatol. Cutan. Allergol. 2009, 3, 429–438. (In Japanese) [Google Scholar]
- Hashimoto, H.; Arai, K.; Takahashi, J.; Chikuda, M. The effect of aging on the antioxidative activity of astaxanthin in human aqueous humor. J. Clin. Biochem. Nutr. 2021, 68, 169–172. [Google Scholar] [CrossRef]
- Hashimoto, H.A.K.; Okamoto, Y.; Takahashi, J.; Chikuda, M.; Obara, Y. Effect of astaxanthin consumption on hydroperoxides in the aqueous. Rinsho Ganka (Jpn. J. Clin. Ophthalmol.) 2011, 65, 465–470. [Google Scholar] [CrossRef]
- Hashimoto, H.; Arai, K.; Takahashi, J.; Chikuda, M. Effects of astaxanthin on VEGF level and antioxidation in human aqueous humor: Difference by sex. J. Clin. Biochem. Nutr. 2019, 65, 47–51. [Google Scholar] [CrossRef]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M. The effect of astaxanthin on vascular endothelial growth factor (VEGF) levels and peroxidation reactions in the aqueous humor. J. Clin. Biochem. Nutr. 2016, 59, 10–15. [Google Scholar] [CrossRef]
- Hashimoto, H.; Arai, K.; Hayashi, S.; Okamoto, H.; Takahashi, J.; Chikuda, M.; Obara, Y. Effects of astaxanthin on antioxidation in human aqueous humor. J. Clin. Biochem. Nutr. 2013, 53, 1–7. [Google Scholar] [CrossRef]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Kuwahata, M.; Higashi, A. Astaxanthin-, β-Carotene-, and Resveratrol-Rich Foods Support Resistance Training-Induced Adaptation. Antioxidants 2021, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Mettler, J.A.; Patek, K.; Butawan, M.; Bloomer, R.J. Astaxanthin Supplementation Increases Glutathione Concentrations but Does Not Impact Fat Oxidation During Exercise in Active Young Men. Int. J. Sport Nutr. Exerc. Metab. 2021, 32, 8–15. [Google Scholar] [CrossRef]
- Baralic, I.; Andjelkovic, M.; Djordjevic, B.; Dikic, N.; Radivojevic, N.; Suzin-Zivkovic, V.; Radojevic-Skodric, S.; Pejic, S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evid.-Based Complement. Altern. Med. 2015, 2015, 78376. [Google Scholar] [CrossRef] [PubMed]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of Astaxanthin Supplementation on Paraoxonase 1 Activities and Oxidative Stress Status in Young Soccer Players. Phytother. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52, 382–392. [Google Scholar]
- Klinkenberg, L.J.J.; Res, P.T.; Haenen, G.R.; Bast, A.; van Loon, L.J.C.; van Dieijen-Visser, M.P.; Meex, S.J. Effect of Antioxidant Supplementation on Exercise-Induced Cardiac Troponin Release in Cyclists: A Randomized Trial. PLoS ONE 2013, 8, e79280. [Google Scholar] [CrossRef]
- Res, P.T.; Cermak, N.M.; Stinkens, R.; Tollakson, T.J.; Haenen, G.R.; Bast, A.; VAN Loon, L.J.C. Astaxanthin Supplementation Does Not Augment Fat Use or Improve Endurance Performance. Med. Sci. Sports Exerc. 2013, 45, 1158–1165. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Fry, A.; Schilling, B.; Chiu, L.; Hori, N.; Weiss, L. Astaxanthin Supplementation Does Not Attenuate Muscle Injury Following Eccentric Exercise in Resistance-Trained Men. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 401–412. [Google Scholar] [CrossRef]
- Nakanishi, R.; Kanazashi, M.; Tanaka, M.; Tanaka, M.; Fujino, H. Impacts of Astaxanthin Supplementation on Walking Capacity by Reducing Oxidative Stress in Nursing Home Residents. Int. J. Environ. Res. Public Health 2022, 19, 13492. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Kyle, N.H.; Bashmakov, Y.K. Markers of Hypoxia and Oxidative Stress in Aging Volunteers Ingesting Lycosomal Formulation of Dark Chocolate Containing Astaxanthin. J. Nutr. Health Aging 2018, 22, 1092–1098. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid β Levels in Human Red Blood Cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y.; Takahashi, J.; Tominaga, K.; Miura, N. Daily fatigue-reducing effect of astaxanthin—A randomized, placebo-controlled, double-blind, parallel-group study. Jpn. Pharmacol. Ther. (Yakuri Chiryo) 2017, 45, 61–72. [Google Scholar]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Hosoi, M.; Feragalli, B.; Cotellese, R.; Cosentino, V.; Cosentino, M.; Eggenhoffner, R.; et al. A natural pharma standard supplement formulation to control treatment-related toxicity and oxidative stress in genitourinary cancer: A preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4196–4202. [Google Scholar]
- Yagi, H.; Kawaguchi, M.; Nishio, K.; Sato, R.; Kobori, Y.; Arai, G.; Okada, H. Effect of Astaxanthin for Anticholinergic Agent-resistant Overactive Bladder. Anti-Aging Medicine [in Japanese] 2013, 9, 612–616. [Google Scholar]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.-K.; Kim, J.T.; Oh, J.M.; Shin, W.G.; Park, J.-J.; Lee, B.J.; Joo, M.K.; et al. Protective Effects of Haematococcus Astaxanthin on Oxidative Stress in Healthy Smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Yamada, T.; Ryo, K.; Tai, Y.; Tamaki, Y.; Inoue, H.; Mishima, K.; Tsubota, K.; Saito, I. Evaluation of Therapeutic Effects of Astaxanthin on Impairments in Salivary Secretion. J. Clin. Biochem. Nutr. 2010, 47, 130–137. [Google Scholar] [CrossRef]
- Chen, J.-T.; Kotani, K.; Bui, T.T.; Piao, C.H.; Kim, S.M.; Song, C.H.; Shin, H.S.; Lee, C.-H.; Chai, O.H.; Tennant, K.G.; et al. Effects of Astaxanthin on Liver and Leukocyte Parameters in Healthy Climacteric Women: Preliminary Data. J. Med. Food 2017, 20, 724–725. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Gajewska, A.; Macierzyńska-Piotrowska, E.; Pawelczyk, T.; Bartosz, G.; Szemraj, J. Enhanced Antioxidant Capacity and Anti-Ageing Biomarkers after Diet Micronutrient Supplementation. Molecules 2014, 19, 14794–14808. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kimura, F.; Satoh, A.; Miyazawa, T. Erythrocytes Carotenoids after Astaxanthin Supplementation in Middle-Aged and Senior Japanese Subjects. J. Oleo Sci. 2011, 60, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhao, P.; Li, B.; Zhang, J.; Huang, C. Antioxidant effects and impact on human health of astaxanthin. Chin. J. Food Hyg. 2011, 23, 313–316. [Google Scholar]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Rissanen, T.H.; Nyyssönen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ghantabpour, T.; Nashtaei, M.S.; Nekoonam, S.; Rezaei, H.; Amidi, F. The Effect of Astaxanthin on Motility, Viability, Reactive Oxygen Species, Apoptosis, and Lipid Peroxidation of Human Spermatozoa During the Freezing–Thawing Process. Biopreservation Biobanking 2022, 20, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.H.; El Garem, Y.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef]
- Sudo, M.; Yamada, K.; Yazawa, K. The effects of supplement intake on female floorball athletes. Kokushikan Soc. Sport Sci. 2020, 20, 39–46. (In Japanese) [Google Scholar]
- Sudo, A.; Yamada, K.; Yazawa, K. Effects of Consuming A Supplement with Antioxidant Action on Physical Fatigue and Sports Performance. Jpn. J. Complement. Altern. Med. 2019, 16, 21–26. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef]
- Tsukahara, H.; Matsuyama, A.; Abe, T.; Kyo, H.; Ohta, T.; Suzuki, N. Effects of Intake of Astaxanthin Contained Drink on Skin Condition. Jpn. J. Complement. Altern. Med. 2016, 13, 57–62. [Google Scholar] [CrossRef]
- Winther, K.; Wongsuphasawat, K.; Phetcharat, L. The effectiveness of a standardized rose hip powder, containing seeds and shells of Rosa canina, on cell longevity, skin wrinkles, moisture, and elasticity. Clin. Interv. Aging 2015, 10, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, K.; Shiobara, M.; Yuki Sato, Y.; Nakanuma, C.; Maekawa, T.; Ohtsuki, M.; Yazawa, K.; Imokawa, G. Anti-aging and functional improvement effects for the skin by functional foods intakes: Clinical effects on skin by oral ingestion of preparations containing Astaxanthin and Vitamins C and E. Jichi Med. Univ. J. 2012, 35, 25–33. [Google Scholar]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.-M.; Floriach, N.; Sala, E.; Aguilera, J. Increase in minimal erythemal dose following oral administration of an antioxidant complex based on a mix of carotenoids: Double-blind, placebo-controlled trial. Photodermatol. Photoimmunol. Photomed. 2017, 33, 284–286. [Google Scholar] [CrossRef]
- Satoh, A.; Mitarai, A.; Kanno, T.; Hori, Y.; Takeda, R. Effects of the lntake of Astaxanthin on the Reduction of Skin Darkling Induced by UV lrradiation in adult women. Pharmacomet. (OyoYakuri) 2011, 80, 7–11. (In Japanse) [Google Scholar]
- Yamashita, E. The Effects of a Dietary Supplement Containing. Astaxanthin on Skin Condition. Carotenoid Sci. 2006, 10, 91–95. [Google Scholar]
- Yamashita, E. Cosmetic benefit of dietary supplements containing astaxanthin and tocotrienol on human skin. Food Style 21 2002, 6, 112–117. (In Japanese) [Google Scholar]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Miura, N. Effects of diet containing astaxanthin on visual function in healthy individuals: A randomized, double-blind, placebo-controlled, parallel study. J. Clin. Biochem. Nutr. 2023, 72, 74–81. [Google Scholar] [CrossRef]
- Kizawa, Y.; Sekikawa, T.; Kageyama, M.; Tomobe, H.; Kobashi, R.; Yamada, T. Effects of anthocyanin, astaxanthin, and lutein on eye functions: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2021, 69, 77–90. [Google Scholar] [CrossRef]
- Sudo, M.; Yamada, K.; Yazawa, K. Effects of consuming a supplement with antioxidant action on physical fatigue and the state of the skin in middle-aged women. Kokushikan Soc. Sport Sci. 2019, 19, 21–26. (In Japanese) [Google Scholar]
- Kono, K.; Shimizu, Y.; Takahashi, S.; Matsuoka, S.; Yui, K. Effect of multiple dietary supplement containing lutein, astaxanthin, cyanidin-3-glucoside, and DHA on accommodative ability. Immunol. Endocr. Metab. Agents Med. Chem. (Former. Curr. Med. Chem. -Immunol. Endocr. Metab. Agents) 2014, 14, 114–125. [Google Scholar] [CrossRef]
- Seya, Y.; Takahashi, J.; Imanaka, K. Relationships Between Visual Fatigue and Reaction Times −Effects of a Repetition of a Visual Task and Long-term Intake of a Supplementation Food Including Astaxanthin on Reaction Time. Jpn. J. Physiol. Anthropol. 2009, 14, 59–66. (In Japanese) [Google Scholar] [CrossRef]
- Tsukahara, H.; Koikeda, T.; Arai, T.; Hayashi, H.; Ohno, S.; Suzuki, N. Supplementation Effect of Astaxanthin on Blood Flow and Shoulder Stiffness -A Preliminary Pilot Study-. Jpn. J. Complement. Altern. Med. 2008, 5, 49–56. [Google Scholar] [CrossRef]
- Yoshida, K.; Sakai, O.; Honda, T.; Kikuya, T.; Takeda, R.; Sawabe, A.; Inaba, M.; Koike, C. Effects of Astaxanthin, Lutein, and Zeaxanthin on Eye–Hand Coordination and Smooth-Pursuit Eye Movement after Visual Display Terminal Operation in Healthy Subjects: A Randomized, Double-Blind Placebo-Controlled Intergroup Trial. Nutrients 2023, 15, 1459. [Google Scholar] [CrossRef] [PubMed]
- D’aloisio, R.; Di Antonio, L.; Toto, L.; Rispoli, M.; Di Iorio, A.; Delvecchio, G.; Mastropasqua, R. Choroidal Changes in Blood Flow in Patients with Intermediate AMD after Oral Dietary Supplement Based on Astaxanthin, Bromelain, Vitamin D3, Folic Acid, Lutein, and Antioxidants. Medicina 2022, 58, 1092. [Google Scholar] [CrossRef]
- Tian, L.; Wen, Y.; Li, S.; Zhang, P.; Wang, Y.; Wang, J.; Cao, K.; Du, L.; Wang, N.; Jie, Y. Benefits and Safety of Astaxanthin in the Treatment of Mild-To-Moderate Dry Eye Disease. Front. Nutr. 2021, 8, 796951. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Huang, J.-Y.; Yeh, P.-T. A randomized, double-blind, placebo-controlled study of oral antioxidant supplement therapy in patients with dry eye syndrome. Clin. Ophthalmol. 2016, 10, 813–820. [Google Scholar] [CrossRef]
- The Carmis Study Group; Piermarocchi, S.; Saviano, S.; Parisi, V.; Tedeschi, M.; Panozzo, G.; Scarpa, G.; Boschi, G.; Giudice, G.L.; Sartore, M.; et al. Carotenoids in Age-Related Maculopathy Italian Study (CARMIS): Two-Year Results of a Randomized Study. Eur. J. Ophthalmol. 2012, 22, 216–225. [Google Scholar] [CrossRef]
- Parisi, V.; Tedeschi, M.; Gallinaro, G.; Varano, M.; Saviano, S.; Piermarocchi, S. Carotenoids and Antioxidants in Age-Related Maculopathy Italian Study: Multifocal Electroretinogram Modifications after 1 Year. Ophthalmology 2008, 115, 324–333.e322. [Google Scholar] [CrossRef]
- Sawaki, K.; Yoshigi, H.; Aoki, K.; Koikawa, N.; Azumane, A.; Kaneko, K.; Yamaguchi, M. Sports Performance Benefits from Taking Natural Astaxanthin Characterized by Visual Acuity and Muscle Fatigue Improvement in Humans. J. Clin. Ther. Med. 2002, 18, 1085–1100. (In Japanese) [Google Scholar]
- Ciaraldi, T.P.; Boeder, S.C.; Mudaliar, S.R.; Giovannetti, E.R.; Henry, R.R.; Pettus, J.H. Astaxanthin, a natural antioxidant, lowers cholesterol and markers of cardiovascular risk in individuals with prediabetes and dyslipidaemia. Diabetes Obes. Metab. 2023, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, A.; Nouri-Habashi, A.; Razi, O.; Ataeinosrat, A.; Rahmani, H.; Mollabashi, S.S.; Bagherzadeh-Rahmani, B.; Aghdam, S.M.; Khalajzadeh, L.; Al Kiyumi, M.H.; et al. Astaxanthin Supplemented with High-Intensity Functional Training Decreases Adipokines Levels and Cardiovascular Risk Factors in Men with Obesity. Nutrients 2023, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Wika, A.A.; Reason, K.W.; Green, J.M.; Killen, L.G.; Mcallister, M.J.; Waldman, H.S. Astaxanthin Reduces Heart Rate and Carbohydrate Oxidation Rates During Exercise in Overweight Individuals. Int. J. Exerc. Sci. 2023, 16, 252–266. [Google Scholar]
- Urakaze, M.; Kobashi, C.; Satou, Y.; Shigeta, K.; Toshima, M.; Takagi, M.; Takahashi, J.; Nishida, H. The Beneficial Effects of Astaxanthin on Glucose Metabolism and Modified Low-Density Lipoprotein in Healthy Volunteers and Subjects with Prediabetes. Nutrients 2021, 13, 4381. [Google Scholar] [CrossRef]
- Birudaraju, D.; Cherukuri, L.; Kinninger, A.; Chaganti, B.T.; Shaikh, K.; Hamal, S.; Flores, F.; Roy, S.K.; Budoff, M.J. A combined effect of Cavacurcumin, Eicosapentaenoic acid (Omega-3s), Astaxanthin and Gamma –linoleic acid (Omega-6) (CEAG) in healthy volunteers—A randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 2020, 35, 174–179. [Google Scholar] [CrossRef]
- Chan, K.-C.; Chen, S.-C.; Chen, P.-C. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J. Funct. Foods 2019, 53, 22–27. [Google Scholar] [CrossRef]
- Landi, F.; Martone, A.M.; Salini, S.; Zazzara, B.; Calvani, R.; Marzetti, E.; Nesci, A.; Di Giorgio, A.; Giupponi, B.; Santoro, L.; et al. Effects of a New Combination of Medical Food on Endothelial Function and Lipid Profile in Dyslipidemic Subjects: A Pilot Randomized Trial. BioMed Res. Int. 2019, 2019, 1970878. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar]
- Sarkkinen, E.S.; Savolainen, M.J.; Taurio, J.; Marvola, T.; Bruheim, I. Prospective, randomized, double-blinded, placebo-controlled study on safety and tolerability of the krill powder product in overweight subjects with moderately elevated blood pressure. Lipids Health. Dis. 2018, 17, 287. [Google Scholar] [CrossRef]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef]
- Maki, K.; Geohas, J.; Dicklin, M.; Huebner, M.; Udani, J. Safety and lipid-altering efficacy of a new omega-3 fatty acid and antioxidant-containing medical food in men and women with elevated triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids 2015, 99, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Okada, Y. Clinical efficacy of astaxanthin-containing Haematococcus pluvialis extract for the volunteers at risk of metabolic syndrome. J. Clin. Biochem. Nutr. 2008, 43, 38–43. [Google Scholar]
- Heidari, M.; Chaboksafar, M.; Alizadeh, M.; Sohrabi, B.; Kheirouri, S. Effects of Astaxanthin supplementation on selected metabolic parameters, anthropometric indices, Sirtuin1 and TNF-α levels in patients with coronary artery disease: A randomized, double-blind, placebo-controlled clinical trial. Front. Nutr. 2023, 10, 1104169. [Google Scholar] [CrossRef]
- Marazzi, G.; Campolongo, G.; Pelliccia, F.; Quattrino, S.; Vitale, C.; Cacciotti, L.; Massaro, R.; Volterrani, M.; Rosano, G. Comparison of Low-Dose Statin Versus Low-Dose Statin + Armolipid Plus in High-Intensity Statin-Intolerant Patients with a Previous Coronary Event and Percutaneous Coronary Intervention (ADHERENCE Trial). Am. J. Cardiol. 2017, 120, 893–897. [Google Scholar] [CrossRef]
- Takami, M.; Aoi, W.; Terajima, H.; Tanimura, Y.; Wada, S.; Higashi, A. Effect of dietary antioxidant-rich foods combined with aerobic training on energy metabolism in healthy young men. J. Clin. Biochem. Nutr. 2019, 64, 79–85. [Google Scholar] [CrossRef]
- Fukamauchi, M. Food functionality of astaxanthin-10: Synergistic effects of astaxanthin intake and aerobic exercise. Food Style 21 (JP) 2007, 11, 22–24. [Google Scholar]
- Barker, G.A.; Parten, A.L.; Lara, D.A.; Hannon, K.E.; McAllister, M.J.; Waldman, H.S. Astaxanthin Supplementation Reduces Subjective Markers of Muscle Soreness following Eccentric Exercise in Resistance-Trained Men. Muscles 2023, 2, 228–237. [Google Scholar] [CrossRef]
- Nieman, D.C.; Woo, J.; Sakaguchi, C.A.; Omar, A.M.; Tang, Y.; Davis, K.; Pecorelli, A.; Valacchi, G.; Zhang, Q. Astaxanthin supplementation counters exercise-induced decreases in immune-related plasma proteins. Front. Nutr. 2023, 10, 1143385. [Google Scholar] [CrossRef] [PubMed]
- Waldman, H.S.; Bryant, A.R.; Parten, A.L.; Grozier, C.D.; McAllister, M.J. Astaxanthin Supplementation Does Not Affect Markers of Muscle Damage or Inflammation After an Exercise-Induced Muscle Damage Protocol in Resistance-Trained Males. J. Strength Cond. Res. 2023, 37, e413–e421. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Warner, A.R.; Deb, S.K.; Gough, L.A.; Sparks, S.A.; McNaughton, L.R. The effect of astaxanthin supplementation on performance and fat oxidation during a 40 km cycling time trial. J. Sci. Med. Sport 2021, 24, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Horowitz, M.; Yanovich, R.; Raz, H.; Heled, Y. Asthaxanthin Improves Aerobic Exercise Recovery Without Affecting Heat Tolerance in Humans. Front. Sports Act. Living 2019, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.H.D.; Capelli, B.; Ding, L.; Li, Y.; Artaria, C. Effect of Astaxanthin Supplementation on Cardiorespiratory Function in Runners. EC Nutrition 2016, 11, 253–259. [Google Scholar] [CrossRef]
- Earnest, C.; Lupo, M.; White, K.; Church, T. Effect of Astaxanthin on Cycling Time Trial Performance. Int. J. Sports Med. 2011, 32, 882–888. [Google Scholar] [CrossRef]
- Malmstena, C.L.L.A. Dietary Supplementation with Astaxanthin-Rich Algal Meal Improves Strength Endurance–A Double Blind Placebo Controlled Study on Male Students. Carotenoid Sci. 2008, 13, 20–22. [Google Scholar]
- Tajima, T.; Nagata, A. Effects of astaxanthin ingestion on exercise-induced physiological changes. Health Behav. Sci. 2004, 3, 5–10. [Google Scholar] [CrossRef]
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D.J. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. Physiol. Rep. 2021, 9, e14887. [Google Scholar] [CrossRef]
- Liu, S.Z.; Ali, A.S.; Campbell, M.D.; Kilroy, K.; Shankland, E.G.; Roshanravan, B.; Marcinek, D.J.; Conley, K.E. Building strength, endurance, and mobility using an astaxanthin formulation with functional training in elderly. J. Cachex-Sarcopenia Muscle 2018, 9, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Benassi-Evans, B.; Bednarz, J.; Vincent, A.D.; Hall, S.; Hill, C.L. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: A 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 116, 672–685. [Google Scholar] [CrossRef] [PubMed]
- MacDermid, J.C.; Vincent, J.I.; Gan, B.S.; Grewal, R. A Blinded Placebo-Controlled Randomized Trial on the Use of Astaxanthin as an Adjunct to Splinting in the Treatment of Carpal Tunnel Syndrome. Hand 2011, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Hongo, N.; Tominaga, K.; Okuda, J.; Suzuki, M.; Miura, N. Effects of Astaxanthin on Cognitive Function, Physical Function and Mood Condition in Elderly People-A Randomized, Double-blind, Placebo-controlled, Parallel-group, Comparative Study-. Jpn. Pharmacol. Ther. (Yakuri Chiryo) 2018, 46, 2043–2062. (In Japanse) [Google Scholar]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer’s Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef]
- Zanotta, D.; Puricelli, S.; Bonoldi, G. Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: A noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 2014, 10, 225–230. [Google Scholar] [CrossRef]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich Haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef]
- Yamazaki, I.; Egawa, K.; Tanaka, K.; Rogi, T.; Nakai, M.; Miura, N. Effects of Sesame Lignans and Astaxanthin Supplementation on Daily Fatigue ―A Randomized, Double-blind, Placebo-controlled, Parallel-group Comparison Study. Jpn. Pharmacol. Ther. (Yakuri Chiryo) 2022, 50, 1111–1119. [Google Scholar]
- Hayashi, M.; Kawamura, M.; Kawashima, Y.; Uemura, T.; Maoka, T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on the status of stress and sleep in adults. J. Clin. Biochem. Nutr. 2020, 66, 92–102. [Google Scholar] [CrossRef]
- Saito, H.; Cherasse, Y.; Suzuki, R.; Mitarai, M.; Ueda, F.; Urade, Y. Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res. 2017, 61, 28019085. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T. Effects of Astaxanthin on Menstrual cramps and Hypermenorrhea. Obstet. Gynecol. Pract. 2015, 64, 961–966. (In Japanese) [Google Scholar]
- Dede, G.; Saylan, A. The effect of astaxanthin on human sperm parameters after cryopreservation. Can. Urol. Assoc. J. 2022, 16, E552–E557. [Google Scholar] [CrossRef]
- Kumalic, S.I.; Klun, I.V.; Bokal, E.V.; Pinter, B. Effect of the oral intake of astaxanthin on semen parameters in patients with oligo-astheno-teratozoospermia: A randomized double-blind placebo-controlled trial. Radiol. Oncol. 2020, 55, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin Improves Human Sperm Capacitation by Inducing Lyn Displacement and Activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef]
- Donà, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of Astaxanthin on Human Sperm Capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef]
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. 2018, 17, 38–42. [Google Scholar]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of Astaxanthin in Haematococcus Algal Extract: The Effects of Timing of Diet and Smoking Habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef]
- Odeberg, J.M.; Lignell, Å.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Østerlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J. Nutr. Biochem. 2000, 11, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Kishimoto, Y.; Suzuki, R.; Kawai, Y.; Tateya, I.; Hirano, S. Protective Effect of Astaxanthin on Vocal Fold Injury and Inflammation Due to Vocal Loading: A Clinical Trial. J. Voice 2017, 31, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Kupcinskas, L.; Lafolie, P.; Lignell, A.; Kiudelis, G.; Jonaitis, L.; Adamonis, K.; Andersen, L.P.; Wadström, T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008, 15, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Holck, S.; Kupcinskas, L.; Kiudelis, G.; Jonaitis, L.; Janciauskas, D.; Permin, H.; Wadström, T. Gastric inflammatory markers and interleukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunol. Med. Microbiol. 2007, 50, 244–248. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Failla, M.L. Hydrolysis of Zeaxanthin Esters by Carboxyl Ester Lipase during Digestion Facilitates Micellarization and Uptake of the Xanthophyll by Caco-2 Human Intestinal Cells. J. Nutr. 2006, 136, 588–594. [Google Scholar] [CrossRef]
- E Breithaupt, D.; Bamedi, A.; Wirt, U. Carotenol fatty acid esters: Easy substrates for digestive enzymes? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 721–728. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Sugawara, T.; Yamashita, K.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Esterification of xanthophylls by human intestinal Caco-2 cells. Arch. Biochem. Biophys. 2009, 483, 205–212. [Google Scholar] [CrossRef]
- Wingerath, T.; Sies, H.; Stahl, W. Xanthophyll Esters in Human Skin. Arch. Biochem. Biophys. 1998, 355, 271–274. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Ryan, L.; O’Brien, N. Comparison of the uptake and secretion of carotene and xanthophyll carotenoids by Caco-2 intestinal cells. Br. J. Nutr. 2007, 98, 38–44. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. Lycopene bioavailability is associated with a combination of genetic variants. Free. Radic. Biol. Med. 2015, 83, 238–244. [Google Scholar] [CrossRef]
- Herron, K.L.; McGrane, M.M.; Waters, D.; E Lofgren, I.; Clark, R.M.; Ordovas, J.M.; Fernandez, M.L. The ABCG5 Polymorphism Contributes to Individual Responses to Dietary Cholesterol and Carotenoids in Eggs. J. Nutr. 2006, 136, 1161–1165. [Google Scholar] [CrossRef]
- Lobo, G.P.; Hessel, S.; Eichinger, A.; Noy, N.; Moise, A.R.; Wyss, A.; Palczewski, K.; Von Lintig, J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production. FASEB J. 2010, 24, 1656–1666. [Google Scholar] [CrossRef]
- Teicher, V.B.; Kucharski, N.; Martin, H.-D.; van der Saag, P.; Sies, H.; Stahl, W. Biological Activities of Apo-canthaxanthinoic Acids Related to Gap Junctional Communication. Arch. Biochem. Biophys. 1999, 365, 150–155. [Google Scholar] [CrossRef]
- Ip, B.C.; Liu, C.; Lichtenstein, A.H.; von Lintig, J.; Wang, X.-D. Lycopene and Apo-10′-lycopenoic Acid Have Differential Mechanisms of Protection against Hepatic Steatosis in β-Carotene-9′,10′-oxygenase Knockout Male Mice. J. Nutr. 2015, 145, 268–276. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Costa, L.; Serra, A.T.; Bronze, M.R.; Valero-Cases, E.; Pérez-Llamas, F.; Candela, M.E.; Arnao, M.B.; Barberán, F.T.; Villalba, R.G.; et al. Saffron against Neuro-Cognitive Disorders: An Overview of Its Main Bioactive Compounds, Their Metabolic Fate and Potential Mechanisms of Neurological Protection. Nutrients 2022, 14, 5368. [Google Scholar] [CrossRef]
- Ashraf, A.; Ijaz, M.U.; Muzammil, S.; Nazir, M.M.; Zafar, S.; Zihad, S.N.K.; Uddin, S.J.; Hasnain, S.; Nayak, A.K. The role of bixin as antioxidant, anti-inflammatory, anticancer, and skin protecting natural product extracted from Bixa orellana L. Fitoterapia 2023, 169, 105612. [Google Scholar] [CrossRef]
- Takatani, N.; Beppu, F.; Yamano, Y.; Maoka, T.; Miyashita, K.; Hosokawa, M. Preparation of Apoastaxanthinals and Evaluation of Their Anti-inflammatory Action against Lipopolysaccharide-Stimulated Macrophages and Adipocytes. ACS Omega 2022, 7, 22341–22350. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Fang, T.; Zheng, J.; Guo, M. Physicochemical Properties and Cellular Uptake of Astaxanthin-Loaded Emulsions. Molecules 2019, 24, 727. [Google Scholar] [CrossRef] [PubMed]
- Affandi, M.M.M.; Julianto, T.; Majeed, A. Enhanced Oral Bioavailability of Astaxanthin with Droplet Size Reduction. Food Sci. Technol. Res. 2012, 18, 549–554. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Effects of Selected Polysorbate and Sucrose Ester Emulsifiers on the Physicochemical Properties of Astaxanthin Nanodispersions. Molecules 2013, 18, 768–777. [Google Scholar] [CrossRef]
- Anarjan, N.; Nehdi, I.A.; Tan, C.P. Protection of Astaxanthin in Astaxanthin Nanodispersions Using Additional Antioxidants. Molecules 2013, 18, 7699–7710. [Google Scholar] [CrossRef]
- Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Mar. Drugs 2019, 17, 627. [Google Scholar] [CrossRef]
- Abuhassira-Cohen, Y.; Edelman, R.; Abbas, R.; Kurnik, D.; Shibel, R.; Livney, Y.D. Enhancing the oral bioavailability of natural astaxanthin using plant-based micro- and nano-encapsulation materials: Results of an In vitro evaluation and a cross-over study in humans. Precis. Nanomed. 2020, 3, 641–655. [Google Scholar] [CrossRef]
- Takahashi, J.; Tsukahara, H.; Minato, S. Toxicological studies of astaxanthin from Haematococcus pluvialis –Ames test, oral single dose and 90-days subchronic toxicity studies in rats. J. Clin. Therap. Med. 2004, 20, 867–881. (In Japanse) [Google Scholar]
- Edwards, J.A.; Bellion, P.; Beilstein, P.; Rümbeli, R.; Schierle, J. Review of genotoxicity and rat carcinogenicity investigations with astaxanthin. Regul. Toxicol. Pharmacol. 2016, 75, 5–19. [Google Scholar] [CrossRef]
- Takahashi, J.; Tsukahara, H.; Horita, Y. Toxicological Studies of Astaxanthin from Haematococcus pluvialis: II: In Vivo Micronucleus Test in Mice and In Vitro Chromosome Aberration Test Using Mammalian Cultured Cells of AstaREAL Oil 50F. J. Clin. Therap. Med. (Rinsho-iyaku) 2010, 26, 287–295. [Google Scholar]
- Stewart, J.S.; Lignell, Å.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety assessment of astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats. Food Chem. Toxicol. 2008, 46, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhou, J.; Wang, F.; Xuan, R.; Chen, J.; Wu, W.; Chen, H. Safety assessment of astaxanthin from Haematococcus pluvialis: Acute toxicity, genotoxicity, distribution and repeat-dose toxicity studies in gestation mice. Regul. Toxicol. Pharmacol. 2020, 115, 104695. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, T.; Ishibashi, T.; Kyle, D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicol. Rep. 2014, 1, 582–588. [Google Scholar] [CrossRef]
- Buesen, R.; Schulte, S.; Strauss, V.; Treumann, S.; Becker, M.; Gröters, S.; Carvalho, S.; van Ravenzwaay, B. Safety assessment of [3S, 3′S]-astaxanthin–Subchronic toxicity study in rats. Food Chem. Toxicol. 2015, 81, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vega, K.; Edwards, J.; Beilstein, P. Subchronic (13-week) toxicity and prenatal developmental toxicity studies of dietary astaxanthin in rats. Regul. Toxicol. Pharmacol. 2015, 73, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, N.; Minenaka, Y.; Ichimura, M. Physiological and biochemical effects of carotenoids (β-carotene and astaxanthin) on experimental animals. Bull. Koshien Univ. A 1997, 25, 19–25. (In Japanese) [Google Scholar]
- Petri, D.; Lundebye, A.-K. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 202–209. [Google Scholar] [CrossRef]
- Jewell, C.; O’Brien, N.M. Effect of dietary supplementation with carotenoids on xenobiotic metabolizing enzymes in the liver, lung, kidney and small intestine of the rat. Br. J. Nutr. 1999, 81, 235–242. [Google Scholar] [CrossRef]
- Ohno, M.; Darwish, W.S.; Ikenaka, Y.; Miki, W.; Ishizuka, M. Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: Induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem. Toxicol. 2011, 49, 1285–1291. [Google Scholar] [CrossRef]
- Astorg, P.; Gradelet, S.; Leclerc, J.; Siess, M. Effects of provitamin a or non-provitamin a carotenoids on liver xenobiotic-metabolizing enzymes in mice. Nutr. Cancer 1997, 27, 245–249. [Google Scholar] [CrossRef]
- Kistler, A.; Liechti, H.; Pichard, L.; Wolz, E.; Oesterhelt, G.; Hayes, A.; Maurel, P. Metabolism and CYP-inducer properties of astaxanthin in man and primary human hepatocytes. Arch. Toxicol. 2002, 75, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Kikkawa, T.; Fukuyama, S.; Kyo, H.; Suzuki, N. Inhibitory and Inducing Effects of Astaxanthin on CYP. Jpn. J. Complement. Altern. Med. 2015, 12, 51–54. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Kwon, M.J.; Park, J.B.; Choi, H.D.; Shin, W.G.; Bae, S.K. Inhibitory effects of astaxanthin, β-cryptoxanthin, canthaxanthin, lutein, and zeaxanthin on cytochrome P450 enzyme activities. Food Chem. Toxicol. 2013, 59, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Efsa Panel on Nutrition, N.F.; Food, A.; Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Use of Lycopene as a food colour—Scientific Opinion of the Panel on Food additives, Flavourings, Processing Aids and Materials in Contact with Food. EFSA J. 2008, 6, 674. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Food, N.S.a.t. Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 16781. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, N. Allergies. Statement on the safety of synthetic zeaxanthin as an ingredient in food supplements. EFSA J. 2012, 10, 2891. [Google Scholar] [CrossRef]
- Kozuki, Y.; Miura, Y.; Yagasaki, K. Inhibitory effects of carotenoids on the invasion of rat ascites hepatoma cells in culture. Cancer Lett. 2000, 151, 111–115. [Google Scholar] [CrossRef]
- Song, X.-D.; Zhang, J.-J.; Wang, M.-R.; Liu, W.-B.; Gu, X.-B.; Lv, C.-J. Astaxanthin Induces Mitochondria-Mediated Apoptosis in Rat Hepatocellular Carcinoma CBRH-7919 Cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef]
- Li, J.; Dai, W.; Xia, Y.; Chen, K.; Li, S.; Liu, T.; Zhang, R.; Wang, J.; Lu, W.; Zhou, Y.; et al. Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/Β-Catenin in Vitro. Mar. Drugs 2015, 13, 6064–6081. [Google Scholar] [CrossRef]
- Andrei, S.; Gal, A.; Pintea, A.; Bedecean, I.; Arion, A.; Baba, A. Influence of astaxanthin administration on hepatic oxidative stress markers in rats injected with methylnitrosurea. Bull. UASVM Vet. Med. 2008, 65, 161–164. [Google Scholar]
- Ajithkumar, P.; Jeganathan, N.; Balamurugan, K. Evaluation of hepatoprotective and antioxidant activity of Astaxanthin–A lipid-solid dispersions formulation against acetaminophen induced liver injury in rats. Int. J. Toxicol. Pharmacol. Res. 2012, 4, 36–39. [Google Scholar]
- Rao, A.R.; Sarada, R.; Shylaja, M.D.; Ravishankar, G.A. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Technol. 2015, 52, 6703–6710. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Al Mamun, M.A.; Faruk, M.; Ul Islam, M.T.; Rahman, M.M.; Alam, M.N.; Rahman, A.; Reza, H.M.; Alam, M.A. Astaxanthin Ameliorates Hepatic Damage and Oxidative Stress in Carbon Tetrachloride-administered Rats. Pharmacogn. Res 2017, 9, S84–S91. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chang, C.-C.; Lin, S.-T.; Chyau, C.-C.; Peng, R.Y. Improved Hepatoprotective Effect of Liposome-Encapsulated Astaxanthin in Lipopolysaccharide-Induced Acute Hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef]
- Monmeesil, P.; Fungfuang, W.; Tulayakul, P.; Pongchairerk, U. The effects of astaxanthin on liver histopathology and expression of superoxide dismutase in rat aflatoxicosis. J. Veter-Med. Sci. 2019, 81, 1162–1172. [Google Scholar] [CrossRef]
- Jia, Y.; Kim, J.-Y.; Jun, H.-J.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Hwang, K.-Y.; Um, S.-J.; Chang, H.I.; Lee, S.-J. The natural carotenoid astaxanthin, a PPAR-α agonist and PPAR-γ antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef]
- Yang, J.-P.; Shin, J.-H.; Seo, S.-H.; Kim, S.-G.; Lee, S.H.; Shin, E.-H. Effects of Antioxidants in Reducing Accumulation of Fat in Hepatocyte. Int. J. Mol. Sci. 2018, 19, 2563. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Liu, T.; Wang, J.; Dai, W.; Wang, F.; Zheng, Y.; Chen, K.; Li, S.; Abudumijiti, H.; et al. Protective Effects of Astaxanthin on ConA-Induced Autoimmune Hepatitis by the JNK/p-JNK Pathway-Mediated Inhibition of Autophagy and Apoptosis. PLoS ONE 2015, 10, e0120440. [Google Scholar] [CrossRef]
- Shen, M.; Chen, K.; Lu, J.; Cheng, P.; Xu, L.; Dai, W.; Wang, F.; He, L.; Zhang, Y.; Chengfen, W.; et al. Protective Effect of Astaxanthin on Liver Fibrosis through Modulation of TGF-β1 Expression and Autophagy. Mediat. Inflamm. 2014, 2014, 954502. [Google Scholar] [CrossRef]
- Tsukahara, H.; Fukuhara, I.; Takehara, I. Evaluation of long-term safty of natural astaxanthin in healthy volunteers. Journal of Nutr. Food 2005, 8, 1–11. (In Japanese) [Google Scholar]
- Matsuyama, A.; Takahashi, J.; Itakura, H. A Safety Study on the Long-Term Consumption of Astaxanthin in Healthy Human Volunteers. Jpn. J. Complement. Altern. Med. 2010, 7, 43–50. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Nitta, T.; Shinmei, Y.; Chin, S.; Yoshida, K.; Tsukahara, H.; Ohno, S. Study on the Safety of High Dose Administration of Astaxanthin. J. Clin. Ther. Med. 2005, 21, 651–659. (In Japanese) [Google Scholar]
- Kajita, M.; Tsukahara, H.; Kato, M.; Yoshimoto, T. Safety of excessive intake of astaxanthin. Journal of Clinical Therapeutics & Medicines [in Japanese] 2009, 25, 691–698. [Google Scholar]
- Kajita, M.; Kato, T.; Yoshimoto, T.; Masuda, K. Study on the safety of high dose administration of astaxanthin. Folia Jpn. De Ophthalmol. Clin. 2010, 4, 365–370. (In Japanese) [Google Scholar]
- Park, D.Y.; Kim, Y.S.; Ryu, S.H.; Jin, Y.S. The association between sedentary behavior, physical activity and hyperuricemia. Vasc. Health Risk Manag. 2019, 15, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, J.; Liu, F.; Lee, C.; Lee, H.; Ho, Y.; Hsieh, T.; Wu, C.; Wang, C. Astaxanthin attenuates joint inflammation induced by monosodium urate crystals. FASEB J. 2020, 34, 11215–11226. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Saleh, D.O.; Jaleel, G.A.A.; Hussein, R.A.; Hassan, A. Heamatococcus pluvialis ameliorates bone loss in experimentally-induced osteoporosis in rats via the regulation of OPG/RANKL pathway. BioMedicine 2019, 116, 109017. [Google Scholar] [CrossRef]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.-D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Leslie, J.H.; Braun, K.L.; Novotny, R.; Mokuau, N. Factors affecting healthy eating and physical activity behaviors among multiethnic blue- and white-collar workers: A case study of one healthcare institution. Hawai’i J. Med. Public Health A J. Asia Pac. Med. Public Heath 2013, 72, 300–306. [Google Scholar]
- DiFrancisco-Donoghue, J.; Balentine, J.; Schmidt, G.; Zwibel, H. Managing the health of the eSport athlete: An integrated health management model. BMJ Open Sport Exerc. Med. 2019, 5, e000467. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, V.H.; Lessard, A.; Piché, F.; Tétreau, C.; Descarreaux, M. Video games and their associations with physical health: A scoping review. BMJ Open Sport Exerc. Med. 2020, 6, e000832. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Hiwatashi, N.; Tateya, I.; Kanemaru, S.I.; Nakamura, T.; Ito, J. Effect of astaxanthin on vocal fold wound healing. Laryngoscope 2014, 124, E1–E7. [Google Scholar] [CrossRef]
- Manciula, L.-G.; Berce, C.; Tabaran, F.; Trombitaș, V.; Albu, S. The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. J. Clin. Med. 2019, 8, 1941. [Google Scholar] [CrossRef]
- Pratap, K.; Majzoub, M.E.; Taki, A.C.; Hernandez, S.M.; Magnusson, M.; Glasson, C.R.K.; de Nys, R.; Thomas, T.; Lopata, A.L.; Kamath, S.D. The Algal Polysaccharide Ulvan and Carotenoid Astaxanthin Both Positively Modulate Gut Microbiota in Mice. Foods 2022, 11, 565. [Google Scholar] [CrossRef]
- Haasbroek, K.; Takabe, W.; Yagi, M.; Yonei, Y. High-fat Diet Induced Dysbiosis & Amelioration by Astaxanthin. Rad Hrvat. Akad. Znan. Umjet. Med. Znan. 2019, 58–66. [Google Scholar] [CrossRef]
- Lignell, A.; Surace, R.; Bottiger, P.; Borody, T.J. Symptom improvement in Helicobacter pylori positive non-ulcer dyspeptic patient after treatment with the carotenoid astaxanthin. In Proceedings of the International Carotenoid Symposium, Cairns, Australia, 18–23 July 1999. [Google Scholar]
- Baldi, E.; Muratori, M. Genetic Damage in Human Spermatozoa. In Genetic Damage in Human Spermatozoa; Baldi, E., Muratori, M., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- World Health Organization. Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 31 January 2023).
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Terai, K.; Horie, S.; Fukuhara, S.; Miyagawa, Y.; Kobayashi, K.; Tsujimura, A. Combination therapy with antioxidants improves total motile sperm counts: A Preliminary Study. Reprod. Med. Biol. 2020, 19, 89–94. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Silva, J.V.; Santos, M.A.S.; Fardilha, M. Fighting Bisphenol A-Induced Male Infertility: The Power of Antioxidants. Antioxidants 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Souter, I.; Smith, K.W.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013, 42, 224–231. [Google Scholar] [CrossRef]
- Eslami, M.; Esfandyari, S.; Aghahosseini, M.; Rashidi, Z.; Hosseinishental, S.H.; Brenjian, S.; Sobhani, A.; Amidi, F. Astaxanthin Protects Human Granulosa Cells against Oxidative Stress through Activation of NRF2/ARE Pathway and Its Downstream Phase II Enzymes. Cell J. 2021, 23, 319–328. [Google Scholar] [CrossRef]
- American Heart Association. What is Metabolic Syndrome? Available online: https://www.heart.org/en/health-topics/metabolic-syndrome/about-metabolic-syndrome (accessed on 31 January 2023).
- National Heart, Lung, and Blood Institute. What Is Metabolic Syndrome? Available online: https://www.nhlbi.nih.gov/health/metabolic-syndrome (accessed on 31 January 2023).
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, A.K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of Divergent Effects of Astaxanthin on Insulin Signaling in L6 Cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin in Obese Mice Fed a High-Fat Diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.; Guan, S.; Luo, T.; Zhao, C.; Cai, G.; Zheng, Y.; Jia, X.; Di, J.; Li, R.; et al. Astaxanthin from Haematococcus pluvialis alleviates obesity by modulating lipid metabolism and gut microbiota in mice fed a high-fat diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in Cardiovascular Health and Disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Le, D.; Ngo, M.-T.; Vo, M.-T.; Tran, T.L.; Tran-Van, H. Effects of Astaxanthin on Body and Liver Weight of High-Fat Diet through Regulation of Peroxisome Proliferator-Activated Receptors (PPARs). SSR Inst. Int. J. Life Sci. 2020, 6, 2502–2508. [Google Scholar] [CrossRef]
- Imai, S.-I. The NAD World 2.0: The importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. npj Syst. Biol. Appl. 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Baur, J.A.; Imai, S.-I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Nishida, Y.; Takikawa, A.; Fujisaka, S.; Kado, T.; Aminuddin, A.; Bilal, M.; Jeelani, I.; Aslam, M.R.; Nishimura, A.; et al. Astaxanthin, a Marine Carotenoid, Maintains the Tolerance and Integrity of Adipose Tissue and Contributes to Its Healthy Functions. Nutrients 2021, 13, 4374. [Google Scholar] [CrossRef]
- Union, A.o.w.o.t.E. The Impact of Demographic Change in Europe. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/new-push-european-democracy/impact-demographic-change-europe_en#impacts-of-demographic-change (accessed on 25 December 2022).
- United Nations Department of Economic ans Social Affairs Population Division. World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 28 February 2023).
- OECD. Japan will Need Reforms to Ease Economic Blow of a Shrinking Workforce. Available online: https://www.oecd.org/pensions/japan-will-need-reforms-to-ease-economic-blow-of-a-shrinking-workforce.htm (accessed on 30 January 2023).
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- The UN Decade of Healthy Ageing 2021–2030 in a Climate-Changing World. 2021–2030. Available online: https://cdn.who.int/media/docs/default-source/decade-of-healthy-ageing/decade-connection-series-climatechange.pdf?sfvrsn=e926d220_3&download=true#:~:text=Climate%20change%20in%20an%20ageing%20world&text=The%20number%20of%20older%20people,are%20more%20likely%20(26) (accessed on 25 December 2022).
- Alliance, T.P.H. Planetary Health. Available online: https://www.planetaryhealthalliance.org/planetary-health (accessed on 25 December 2022).
- Luthman, O.; Jonell, M.; Rönnbäck, P.; Troell, M. Strong and weak sustainability in Nordic aquaculture policies. Aquaculture 2022, 550, 737841. [Google Scholar] [CrossRef]
- Kamada, H.; Akagi, S.; Watanabe, S. Astaxanthin increases progesterone production in cultured bovine luteal cells. J. Vet. Med. Sci. 2017, 79, 1103–1109. [Google Scholar] [CrossRef]
- Wan, F.-C.; Zhang, C.; Jin, Q.; Wei, C.; Zhao, H.-B.; Zhang, X.-L.; You, W.; Liu, X.-M.; Liu, G.-F.; Liu, Y.-F.; et al. Protective effects of astaxanthin on lipopolysaccharide-induced inflammation in bovine endometrial epithelial cells†. Biol. Reprod. 2020, 102, 339–347. [Google Scholar] [CrossRef]
- Kjellstrom, T.; Briggs, D.; Freyberg, C.; Lemke, B.; Otto, M.; Hyatt, O. Heat, Human Performance, and Occupational Health: A Key Issue for the Assessment of Global Climate Change Impacts. Annu. Rev. Public Health 2016, 37, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Mora, C.; Dousset, B.; Caldwell, I.R.; Powell, F.E.; Geronimo, R.C.; Bielecki, C.R.; Counsell, C.W.W.; Dietrich, B.S.; Johnston, E.T.; Louis, L.V.; et al. Global risk of deadly heat. Nat. Clim. Chang. 2017, 7, 501–506. [Google Scholar] [CrossRef]
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated Physiological Mechanisms of Exercise Performance, Adaptation, and Maladaptation to Heat Stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J. Cell. Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, S.; Bhan, S. Effect of dietary supplementation of astaxanthin (potent antioxidant) on growth rate, DMI, FCR and metabolic changes in Karan Fries heifers during heat stress. J. Agrometeorol. 2019, 21, 80–88. [Google Scholar] [CrossRef]
- European Union. Commission Proposes Action to Fully Harness the Potential of Algae in Europe for Healthier Diets, Lower CO2 Emissions, and Addressing Water Pollution. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_6899 (accessed on 25 December 2022).
- OECD. OECD Work on Green Growth 2019. Available online: https://issuu.com/oecd.publishing/docs/gg_brochure_2019_web (accessed on 25 December 2022).
- European, C.; Joint Research, C.; Vazquez Calderon, F.; Sanchez Lopez, J. An Overview of the Algae Industry in Europe: Producers, Production Systems, Species, Biomass Uses, Other Steps in the Value Chain and Socio-Economic Data; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Talukdar, J.; Bhadra, B.; Dattaroy, T.; Nagle, V.; Dasgupta, S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. BioMedicine 2020, 132, 110886. [Google Scholar] [CrossRef]















| Administration | Subject | Dose/ Number of Subjects | Duration | Outcomes | Ref. |
|---|---|---|---|---|---|
| Long-term | Healthy Japanese (Mean 33 yrs.) | 6 mg/day (n = 15) | 12 weeks |
| [870] |
| Healthy Japanese (Mean 39 yrs.) | 9 mg/day (n = 15) | 12 weeks |
| [871] | |
| Overdose | Healthy Japanese | 20 mg/day (n = 16) | 4 weeks |
| [774] |
| Healthy Japanese | 30 mg/day (n = 10) | 4 weeks |
| [872] | |
| Healthy Japanese (Mean 42 yrs.) | 30 mg/day (n = 11) Placebo (n = 12) | 4 weeks |
| [873] | |
| Healthy Japanese (Mean 41 yrs.) | 45 mg/day (n = 15) Placebo (n = 7) | 4 weeks |
| [874] |
| Dose **,# | Repeated Intake Period * (Weeks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 6 | 8 | 12 | 16 | 20 | ≥24 | |
| <1 mg/day | [737] | [767] | [710] | [791] | [770,777] | ||||
| 2 mg/day | [746] | [703] | [757] | ||||||
| 3 mg/day | [745] | [739] | [730,800] | ||||||
| 4 mg/day | [39,788] | [751,778,786,796] | [713,714,740,744,792] | [702,712,714,717,782,787] | [758,759] | ||||
| 6 mg/day | [43,44,706] | [42,45,47,684,711,719,722,752,753,756,760] | [742,749,779] | [652,743,754,798] | [653,705,795] | [741] | |||
| 8 mg/day | [651,781] | [724] | [693,701,732,768,793] | [733] | |||||
| 9 mg/day | [46] | [748] | |||||||
| 10 mg/day | [691] | ||||||||
| 12 mg/day | [727] | [40,41,685,704,763,765,780,784] | [700,725,729,766,771,776,785,799] | [692,694,721,723,728,764,774,790,794,797,801] | [738] | [695,697,755,761,772] | |||
| 16 mg/day | [649,735,775,803] | ||||||||
| 18 mg/day | [773] | ||||||||
| 20 mg/day | [698] | [715,716,774,812] | [699] | [762] | |||||
| 24 mg/day | [811] | [718] | |||||||
| 40 mg/day | [726] | [813] | [731] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. https://doi.org/10.3390/md21100514
Nishida Y, Berg PC, Shakersain B, Hecht K, Takikawa A, Tao R, Kakuta Y, Uragami C, Hashimoto H, Misawa N, et al. Astaxanthin: Past, Present, and Future. Marine Drugs. 2023; 21(10):514. https://doi.org/10.3390/md21100514
Chicago/Turabian StyleNishida, Yasuhiro, Pernilla Christina Berg, Behnaz Shakersain, Karen Hecht, Akiko Takikawa, Ruohan Tao, Yumeka Kakuta, Chiasa Uragami, Hideki Hashimoto, Norihiko Misawa, and et al. 2023. "Astaxanthin: Past, Present, and Future" Marine Drugs 21, no. 10: 514. https://doi.org/10.3390/md21100514
APA StyleNishida, Y., Berg, P. C., Shakersain, B., Hecht, K., Takikawa, A., Tao, R., Kakuta, Y., Uragami, C., Hashimoto, H., Misawa, N., & Maoka, T. (2023). Astaxanthin: Past, Present, and Future. Marine Drugs, 21(10), 514. https://doi.org/10.3390/md21100514