Sclerotioloids A–C: Three New Alkaloids from the Marine-Derived Fungus Aspergillus sclerotiorum ST0501
Abstract
:1. Introduction
2. Results and Discussion
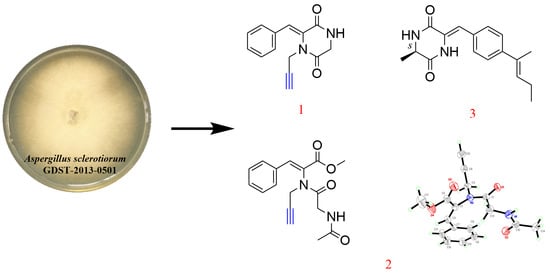
2.1. Elucidation of Chemical Structures
2.2. Bioassays of Compounds
2.2.1. Cytotoxicity Assay
2.2.2. Anti-Microbial Activity Assay
2.2.3. Anti-Oxidant Activity Assays
2.2.4. Anti-Inflammatory Activity Assays
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. LC-MS/MS and Molecular Networking Analysis
3.5. Spectroscopic and Spectrometric Data
3.6. Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Carroll, A.R.; Davis, R.A.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natoli, M.; Herzig, P.; Pishali, B.E.; Buchi, M.; Ritschard, R.; Heinzelmann, V.; Trub, M.; Zippelius, A.; Kashyap, A.S.; Natoli, M. Plinabulin, a Distinct Microtubule-Targeting Chemotherapy, Promotes M1-Like Macrophage Polarization and Anti-tumor Immunity. Front. Oncol. 2021, 11, 644608. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.R.; Fiebig, H.H.; Giavazzi, R.; Langdon, S.P.; Jimeno, J.M.; Faircloth, G.T. High antitumour activity of ET743 against human tumour xenografts from melanoma, non-small-cell lung and ovarian cancer. Ann. Oncol. 1999, 10, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Abdallah, H.M.; Mohamed, G.A.; Deshmukh, S.K. Exploring Potential of Aspergillus sclerotiorum: Secondary Metabolites and Biotechnological Relevance. Mycol. Prog. 2022, 22, 8. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Q.; Liu, J.; Wu, J.; Jia, H.; Liu, D.; Gu, Y.; Liu, J.; Huang, J.; Fan, A. Sclerotiamides C-H, Notoamides from a Marine Gorgonian-Derived Fungus with Cytotoxic Activities. J. Nat. Prod. 2022, 85, 1067–1078. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, J.-K.; Qu, H.-J.; Liu, P.-P.; Wang, Y.; Zhu, W.-M. A new cytotoxic indole-3-ethenamide from the halotolerant fungus Aspergillus sclerotiorum PT06-1. J. Antibiot. 2011, 64, 679–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, R.; Hou, X.M.; Xu, W.F.; Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Targeted Isolation of Asperheptatides from a Coral-Derived Fungus Using LC-MS/MS-Based Molecular Networking and Antitubercular Activities of Modified Cinnamate Derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, C.-L.; Fu, X.-M.; Xu, R.-F.; Zheng, J.-J.; Zhao, D.-L.; She, Z.-G.; Wang, C.-Y. Bioactive indole alkaloids and phenyl ether derivatives from a marine-derived Aspergillus sp. Fungus. J. Nat. Prod. 2013, 76, 547–553. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.-Y.; Shao, C.-L.; Wang, C.-Y. Metabolites from marine invertebrates and their symbiotic microorganisms: Molecular diversity discovery, mining, and application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-S.; Yao, G.-S.; Shi, X.-H.; Rehman, S.U.; Fu, X.-M.; Zhang, X.-L.; Wang, C.-Y. Epigenetic Agents Trigger the Production of Bioactive Nucleoside Derivatives and Bisabolane Sesquiterpenes from the Marine-Derived Fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-S.; Shi, X.-H.; Yao, G.-S.; Shao, C.-L.; Fu, X.-M.; Zhang, X.-L.; Guan, H.-S.; Wang, C.-Y. New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus Terreus. Mar. Drugs 2020, 18, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-H.; Geng, C.; Zhang, X.-W.; Zhu, H.-J.; Shao, C.-L.; Cao, F.; Wang, C.-Y. Discovery of bioactive indole-diketopiperazines from the marine-derived fungus Penicillium brasilianum aided by genomic information. Mar. Drugs 2019, 17, 514. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-Y.; Liu, X.-H.; Zheng, Y.-Y.; Ning, X.-Y.; Zhang, Y.-H.; Fu, X.-M.; Li, X.; Shao, C.-L.; Wang, C.-Y. 2,5-Diketopiperazines from a Sponge-Derived Fungus Aspergillus sclerotiorum. Front. Microbiol. 2022, 13, 808532. [Google Scholar] [CrossRef]
- Wang, B.; Park, E.M.; King, J.B.; Mattes, A.O.; Nimmo, S.L.; Clendinen, C.; Edison, A.S.; Anklin, C.; Cichewicz, R.H. Transferring Fungi to a Deuterium-Enriched Medium Results in Assorted, Conditional Changes in Secondary Metabolite Production. J. Nat. Prod. 2015, 78, 1415–1421. [Google Scholar] [CrossRef]
- Whyte, A.C.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Sclerotiamide: A new member of the paraherquamide class with potent antiinsectan activity from the sclerotia of Aspergillus sclerotiorum. J. Nat. Prod. 1996, 59, 1093–1095. [Google Scholar] [CrossRef]
- He, W.; Xu, Y.; Fu, P.; Zuo, M.; Liu, W.; Jiang, Y.; Wang, L.; Zhu, W. Cytotoxic Indolyl Diketopiperazines from the Aspergillus sp. GZWMJZ-258, Endophytic with the Medicinal and Edible Plant Garcinia multiflora. J. Agric. Food Chem. 2019, 67, 10660–10666. [Google Scholar] [CrossRef]
- Qian-Cutrone, J.; Huang, S.; Shu, Y.-Z.; Vyas, D.; Fairchild, C.; Menendez, A.; Krampitz, K.; Dalterio, R.; Klohr, S.E.; Gao, Q. Stephacidin A and B: Two structurally novel, selective inhibitors of the testosterone-dependent prostate LNCaP cells. J. Am. Chem. Soc. 2002, 124, 14556–14557. [Google Scholar] [CrossRef]
- Kato, H.; Yoshida, T.; Tokue, T.; Nojiri, Y.; Hirota, H.; Ohta, T.; Williams, R.M.; Tsukamoto, S. Notoamides A-D: Prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew. Chem. Int. Ed. 2007, 46, 2254–2256. [Google Scholar] [CrossRef]
- Fu, P.; Liu, P.; Qu, H.; Wang, Y.; Chen, D.; Wang, H.; Li, J.; Zhu, W. α-Pyrones and diketopiperazine derivatives from the marine-derived actinomycete Nocardiopsis dassonvillei HR10-5. J. Nat. Prod. 2011, 74, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.-G.; Kato, H.; Yonezawa, Y.; Hayakawa, M.; Yoshimura, J. Synthesis and structural assignment of naturally occurring 3-benzyl-6-benzylidene-2,5-piperazinedione. Heterocycles 1980, 14, 1767–1770. [Google Scholar] [CrossRef]
- Marcuccio, S.M.; Elix, J.A. Pyrazine chemistry. V. Synthesis of methylanhydropicroroccellin and dimethylpicroroccellin. Aust. J. Chem. 1985, 38, 1785–1796. [Google Scholar] [CrossRef]
- Sterns, M.; Patrick, J.M.; Patrick, V.A.; White, A.H. Conformational studies of some piperazine-2,5-diones: Crystal structures of three isomeric forms of methylanhydropicroroccellin (derivative of a lichen diketopiperazine) and a derivative bromohydrin. Aust. J. Chem. 1989, 42, 349–364. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Aquino, R.; Morelli, S.; Lauro, M.R.; Abdo, S.; Saija, A.; Tomaino, A. Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J. Nat. Prod. 2001, 64, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Luo, P.; Hua, P.; Ding, P.; Li, C.; Xu, J.; Zhou, H.; Gu, Q. Discovery of a New Pterocarpan-Type Antineuroinflammatory Compound from Sophora tonkinensis through Suppression of the TLR4/NFκB/MAPK Signaling Pathway with PU.1 as a Potential Target. ACS Chem. Neurosci. 2019, 10, 295–303. [Google Scholar] [CrossRef] [PubMed]






| Position | δC, mult. | ΔH, mult. (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | NH | 8.42 (s) | H-6 | C-3 |
| 2 | 163.4, C | C-6, C-10 | ||
| 3 | 129.9, C | 1-NH, C-7 | ||
| 4 | N | |||
| 5 | 165.7, C | C-6, C-7 | ||
| 6 | 44.5, CH2 | 4.01, d, (2.4) | 1-NH | C-2, C-5 |
| 7 | 32.9, CH2 | 4.13, d, (2.4) | C-3, C-5, C-9 | |
| 8 | 78.2, C | |||
| 9 | 74.5, CH | 3.10, t, (2.4) | C-7 | |
| 10 | 120.8, CH | 7.10 (s) | C-2, C-16 | |
| 11 | 133.4, C | C-16 | ||
| 12 | 128.6, CH | 7.35–7.40 (m) | H-13 | |
| 13 | 129.4, CH | 7.35–7.40 (m) | H-12, H-14 | |
| 14 | 128.8, CH | 7.35–7.40 (m) | H-13, H-15 | |
| 15 | 129.4, CH | 7.35–7.40 (m) | H-14, H-16 | |
| 16 | 128.6, CH | 7.35–7.40 (m) | H-15 | C-10, C-11 |
| Position | δC, mult. | ΔH, mult. (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 21.9, CH3 | 1.77 (s) | C-2 | |
| 2 | 169.0, C | 1-NH | ||
| 3 | NH | 8.06 (s) | H-4 | C-2 |
| 4 | 40.4, CH2 | 3.56, dd, (17.0, 5.7) 3.66, dd, (17.1, 5.7) | 1-NH | C-5 |
| 5 | 168.4, C | C-4 | ||
| 6 | N | |||
| 7 | 35.5, CH2 | 4.24, dd, (17.6, 2.5) 4.38, dd, (17.5, 2.5) | C-8, C-9 | |
| 8 | 77.5, C | C-7 | ||
| 9 | 75.8, CH | 3.15 (s) | C-7 | |
| 10 | 126.6, C | |||
| 11 | 164.5, C | C-12, C-13 | ||
| 12 | 52.5, OCH3 | 3.81 (s) | C-11 | |
| 13 | 139.5 CH | 7.84 (s) | C-11, C-14 C-15 | |
| 14 | 131.5, C | C-13 | ||
| 15 | 130.2, CH | 7.75 (s) | H-16 | C-13 |
| 16 | 128.8, CH | 7.46, d, (7.6) | H-15, H-17 | |
| 17 | 131.0, CH | 7.49, d, (6.9) | H-16, H-18 | |
| 18 | 128.8, CH | 7.46, d, (7.6) | H-17, H-19 | |
| 19 | 130.2, CH | 7.73 (s) | H-18 |
| Position | δC, mult. | ΔH, mult. (J in Hz) | COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | NH | 8.35–8.37, m | H-6 | C-3, C-5 | H-6, H-7 |
| 2 | 160.7, C | 4-NH, C-6, C-8 | |||
| 3 | 125.3, C | 1-NH, C-10, C-14 | |||
| 4 | NH | 9.79, s | C-2, C-5, C-6 | H-12, H-13 | |
| 5 | 167.6, C | 1-NH, 4-NH, C-6, C-7 | |||
| 6 | 50.3, CH | 4.11, d, (6.8) | 1-NH, H-7 | C-2, C-5, 4-NH, C-7 | 1-NH, H-7 |
| 7 | 19.2, CH3 | 1.33, d, (7.0) | H-6 | C-5, C-6 | 1-NH, H-6 |
| 8 | 114.3, CH | 6.64 (s) | C-2, C-10 | ||
| 9 | 125.7, C | ||||
| 10 | 130.8, CH | 7.44, d, (8.8) | H-14 | C-8, C-12, C-14 | |
| 11 | 114.8, CH | 6.95, d, (8.8) | H-13 | C-3, C-12, C-13 | H-17 |
| 12 | 158.2, C | C-10, C-11, C-13, C-14, C-17 | 4-NH | ||
| 13 | 114.8, CH | 6.95, d, (8.8) | H-11 | C-3, C-11, C-12 | H-17 |
| 14 | 130.8, CH | 7.44, d, (8.8) | H-10 | C-10, C-12 | |
| 15 | 137.3, C | C-17, C-18, C-19 | |||
| 16 | 119.8, CH | 5.43, tt, (6.7, 1.4) | H-17 | C-17, C-18, C-19 | H-17, H-18 |
| 17 | 64.3, CH2 | 4.55, d, (6.7) | H-16, H-18 | C-12, C-15, C-16 | H-11, H-13, H-16 |
| 18 | 18.0, CH3 | 1.71, d, (1.3) | H-17 | C-15, C-16 | H-16 |
| 19 | 25.4, CH3 | 1.74 (s) | C-15, C-16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.-Q.; Zheng, Y.-Y.; Wang, C.-Y.; Liu, Y.; Yao, G.-S. Sclerotioloids A–C: Three New Alkaloids from the Marine-Derived Fungus Aspergillus sclerotiorum ST0501. Mar. Drugs 2023, 21, 219. https://doi.org/10.3390/md21040219
Mao J-Q, Zheng Y-Y, Wang C-Y, Liu Y, Yao G-S. Sclerotioloids A–C: Three New Alkaloids from the Marine-Derived Fungus Aspergillus sclerotiorum ST0501. Marine Drugs. 2023; 21(4):219. https://doi.org/10.3390/md21040219
Chicago/Turabian StyleMao, Jun-Qiu, Yao-Yao Zheng, Chang-Yun Wang, Yang Liu, and Guang-Shan Yao. 2023. "Sclerotioloids A–C: Three New Alkaloids from the Marine-Derived Fungus Aspergillus sclerotiorum ST0501" Marine Drugs 21, no. 4: 219. https://doi.org/10.3390/md21040219
APA StyleMao, J. -Q., Zheng, Y. -Y., Wang, C. -Y., Liu, Y., & Yao, G. -S. (2023). Sclerotioloids A–C: Three New Alkaloids from the Marine-Derived Fungus Aspergillus sclerotiorum ST0501. Marine Drugs, 21(4), 219. https://doi.org/10.3390/md21040219