Trash to Treasure: An Up-to-Date Understanding of the Valorization of Seafood By-Products, Targeting the Major Bioactive Compounds
Abstract
:1. Introduction
2. Fishery By-Products
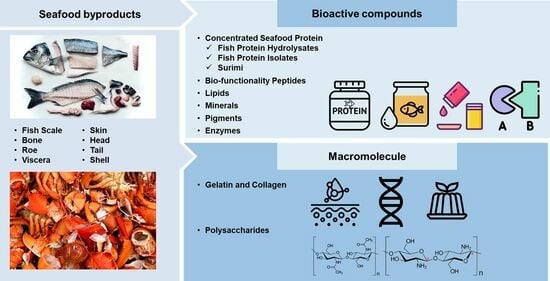
3. Fish By-Products as a Source of Various Bioactive Compounds
3.1. Concentrated Seafood Protein
3.1.1. Fish Protein Hydrolysates
3.1.2. Fish Protein Isolates
3.1.3. Surimi
| Fish Species | By-Products | Extraction Agents (Enzymes) | Properties/Activities | References |
|---|---|---|---|---|
| Channel catfish (Ictalurus punctatus) | Frames and heads | Ficin, neutrase, protamex, papain, novo-proD, thermolysin, alcalase, and bromelain | Protease reaction kinetics showed that ficin was the most efficient to hydrolyze catfish proteins, and the foaming and emulsifying properties of the protein hydrolysates were observed. | [33] |
| Striped catfish (Pangasianodon Hypophthalmus) | Viscera | Enzymatic (pepsin and papain) and chemical process (e.g., NaOH, HCl) | The spray-dried and enzymatically extracted hydrolysate had lower turbidity with increasing pH; the lowest solubility, foaming capacity, and stability were observed at pH 5.0. | [34] |
| Small-spotted catshark (Scyliorhinus canicula) | By-products of muscle | Enzymatic hydrolysis (protamex, esperase, and alcalase) | Protein hydrolysates obtained via enzymatic hydrolysis showed strong antihypertensive and antioxidant properties. | [35] |
| Bluefin leatherjacket (Navodon Septentrionalis) | Heads | Enzymatic hydrolysis (papain) | Protein hydrolysates showed antioxidant properties. | [36] |
| Sardinella (Sardinella aurita) | Viscera and heads | Enzymatic hydrolysis (microbial proteases) | Protein hydrolysates showed antioxidant properties. | [37] |
| Rainbow trout (Oncorhynchus mykiss) | Fins, heads, backbone, and viscera | Enzymatic hydrolysis (alcalase) | Protein hydrolysates showed antioxidant properties. | [38] |
| Red tilapia (Oreochromis spp.) | Viscera | Enzymatic hydrolysis (alcalase) | Peptide fractionation was performed using ultrafiltration, and the <1 kDa fraction (FRTVH-V) expressed the highest iron-binding capacity. | [39] |
| Anchovies (Engraulis encrasicolus) | Viscera | Enzymatic hydrolysis (alcalase, flavourzyme, and protamex) | Protein hydrolysates showed activities in in vitro and in vivo model biological activities by decreasing the severity of oxidative stress. | [40] |
| Bluefin leatherjacket (Navodon Septentrionalis) | Skins | Enzymatic hydrolysis (alcalase, trypsin, papain, neutrase, pepsin, and flavourzyme) | The antioxidant activities of peptides were evaluated with three radical scavenging and lipid peroxidation inhibition assays. | [41] |
| Australian rock lobster (Jasus edwardsii) | Shells | Enzymatic hydrolysis (alcalase) | The protein hydrolysate produced by this study had excellent functionality (solubility 91.7%, water absorption 32%, oil absorption 2.3 mL/g, foaming 51.3%, emulsification 91.3%) and high nutritional quality (34% essential amino acids, 45.4 mg/g arginine, lysine/arginine ratio 0.69) with potential applications for the food industry. | [42] |
| Horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber) | Skins | Enzymatic hydrolysis (trypsin, α-chymotrypsin, and pepsin) | Peptides presented in protein hydrolysates exhibited higher activity against polyunsaturated fatty acid peroxidation than the natural antioxidant α-tocopherol. | [43] |
| Atlantic salmon (Salmo salar) | Backbones and heads | Enzymatic hydrolysis (protex 7 L, promod 671 L, and alcalase 2.4 L) | Chemical, surface activity, and sensory properties were shown. | [44] |
| Serra Spanish mackerel (Scomberomorus Brasiliensis) | Scales crushed and bones | Enzymatic hydrolysis (flavourzyme, and alcalase) | Protein hydrolysate showed better technological performance by stabilizing emulsions and retaining oil, and they could be added to emulsified products, improving their technological and sensory aspects. | [45] |
| Black scabbardfish (Aphanopus carbo) | Frames, heads, skin, trimming, and viscera | Enzymatic hydrolysis (protamex) | The protein hydrolysates presented some antioxidant activity, which increased with increasing degree of hydrolysis. | [46] |
| Anchovy | Fish sauce by-product (FSB) | Enzymatic hydrolysis (proteinase K) | The low-molecular-weight FSB fraction contained potent antioxidative molecules, which were identified as PQLLLLLL and LLLLLLL. | [47] |
| Atlantic holothurian (Cucumaria frondosa) | Internal organs and aqua-pharyngeal bulb | Enzymatic hydrolysis (proteases) | Enzymatic hydrolysates extracted from by-products of the marine invertebrates were demonstrated as active against HSV-1 (Herpes Simplex virus 1). | [48] |
| Skipjack tuna (Katsuwonus pelamis) | Head and bone | In-vitro gastrointestinal (GI) digestion method | Protein hydrolysates can be applied in health care products as antioxidant agents. | [49,50] |
| Chinese sturgeon (Acipenser sinensis) | Whole body | Enzymatic hydrolysis (papain and alcalase 2.4 L) | Hydrolysates can be used as natural antioxidant substitutes in pharmaceuticals and food products. | [51] |
| Monkfish (Lophius piscatorius) | By-products (head and viscera) | Enzymatic hydrolysis (alcalase) | Protein hydrolysates showed antioxidant and antihypertensive activities. | [52] |
| Salmon | Viscera | Enzymatic hydrolysis (papain, alcalase, and autolysis process) | The results showed that the obtained protein-rich hydrolysates from fish industries are a promising alternative for expensive nitrogen sources that are commonly used for fermenting yeasts. | [53] |
| Australian rock lobster | Heads | Chemical process and enzymatic hydrolysis (alcalase) | The results of this study demonstrated the potential value of lobster protein hydrolysates used as a safe emulsifier with significant nutritional value for the food industry. | [54] |
| Eel (Conger myriaster) | Skin | Subcritical water hydrolysis | Strong antioxidant activities. | [55] |
| Yellow corvina (Larimichthys polyactis) | Head and viscera | Subcritical water hydrolysis | Protein hydrolysates showed excellent antioxidant, antidiabetic, and anticancer activity. | [4] |
| Comb Penshell (Atrina pectinata) | Viscera | Subcritical water hydrolysis | Protein hydrolysates showed good antioxidant and antihypertensive activity. | [56] |
3.2. Extraction and Biofunctionality of Peptides from Marine By-Products
| Fish Species | Body Parts | Extraction (Hydrolysis) Agents (Enzymes) | Properties/Activities | References |
|---|---|---|---|---|
| Tilapia | Skin gelatin | Pepsin and pancreatin | ACE-inhibitory activity. | [65] |
| Rainbow trout (Oncorhynchus mykiss) | Skin | Flavourzyme, alcalase, and ultrafiltration method | Anticancer, antioxidant properties present in fractions and non-fraction peptides. | [66] |
| Pacific cod (Gadus macrocephalus) | Skin gelatin | Alcalase, papain, trypsin, neutrase, and pepsin | ACE-inhibitory activity. | [67] |
| Cod (Gadus morhua) | Frames | Trypsin, pepsin, and those chymotrypsin combinations | Antioxidant properties. | [68] |
| Atlantic rock crab (Cancer irroratus) | By-products | Proteolytic enzyme action on processing | Antibacterial activity. | [63] |
| Smooth hound (Mustelus mustelus) | Viscera wastes | Proteases (commercial), endogenous enzymes, and those combinations | ACE-inhibitory, antimicrobial, and antioxidant activity. | [62] |
| Codfish blood and sardine | Cooking-water wastes | Membrane ultrafiltration | The peptide fractions from codfish blood exhibited the highest ABTS+ and ORAC values. Peptide fractions from sardine wastewater were capable of inhibiting Escherichia coli growth. | [69] |
| Threadfin breams (Nemipterus japonicus) | Frames | Plant proteases (bromelain and papain) | Antioxidant properties (2,2 diphenyl-1-picrylhydrazyl [DPPH] radical scavenging activity, ferric-reducing power, and lipid peroxidation inhibition) of hydrolysates increased with an increase in the degree of hydrolysis. | [70] |
| Shortfin scad (Decapterus Macrosoma) | Bones | Alcalase | Obtained peptides showed angiotensin I-converting enzyme (ACE)-inhibitory activity. | [71] |
| Northern shrimp (Pandalus borealis) | By-products | Papain, protamex, trypsin, flavourzyme, and alcalase | Antioxidant and ACE-inhibitory activity. The results of this research suggested that the high-molecular-weight enzymatic hydrolysate derived from shrimp can be used to control oxidative stress and prevent hypertension. | [72] |
| Catfish (Ictalurus punctatus) | Bone frames and heads | Proteases | The emulsifying and foaming properties and stability of selected hydrolysates were evaluated. | [33] |
| Skate (Raja porosa) | Cartilage | Chromatography and ultrafiltration | The result suggested that the isolated peptides have excellent antioxidant properties. | [73] |
| Grass carp (Ctenopharyngodon idella) | Skin | Alcalase | Novel peptides isolated from grass carp skin possess potent antioxidant activities and might be used for food preservation and medicinal purposes. | [74] |
| Lizardfish (Synodus macrops) | Scale gelatin | Trypsin, papain, bromelain, chymotrypsin, and alcalase | ACE-inhibitory peptides derived from scale gelatin have the potential to be used as healthy ACE-inhibiting drug raw materials. | [75] |
| Pacific cod (G. macrocephalus) | Skin gelatin | Pepsin | Extracted peptides showed potent ACE inhibition with IC50 values of 6.9 and 14.5 μM. | [67] |
| Anchovy (Engraulis japonicas) | Cooking-water wastes | Protamex | Purified antimicrobial activity with no hemolytic activity up to a concentration of 512 μg/mL. | [76] |
| Thornback ray (Raja clavata) | Skin gelatin | Alcalase | ACE-inhibitory activity. | [60] |
| Seabass (Lates calcarifer) | Skin gelatin | Alcalase | Peptides prepared from seabass skin showed good antioxidant activity. | [77] |
| Atlantic salmon (Salmo salar) | Trimming | Alcalase 2.4 L, flavourzyme 500 L, Corolase PP, and Promod 144 MG | Bioactive peptides displayed good DPP-IV and ACE inhibitory and antioxidant activity. | [78] |
| Skipjack tuna (Katsuwonus pelamis) | Roe | Flavourzyme | Four peptides among the fifteen extracted peptides showed remarkable ACE-inhibitory activity. | [79] |
| Chinese sturgeon (Acipenser sinensis) | Whole body | Papain and alcalase 2.4 L | The fractions and purified peptides can be used as natural antioxidant substitutes in pharmaceuticals and food products. | [51] |
| Atlantic sea cucumber (Cucumaria frondosa) | Whole body | Alcalase and trypsin | Generated peptides inhibited MPO (a mediator and marker of in vivo oxidative stress) with predicted molecular interactions. | [80] |
| Antarctic krill (Euphausia superba) | By-products | Trypsin | The preparation process of Antarctic krill peptides-zinc chelate was optimized. Chelate showed excellent stability against various pH and gastrointestinal digestion. | [81] |
| Squid (Dosidicus gigas) | By-products | Protease XIV and ultrafiltration (UFI) | Peptide fractions obtained after UFI had higher antioxidant and antimutagenic activities, but the antiproliferative activity did not improve after UFI. | [82] |
| Bigeye tuna | Skin | Subcritical water | Peptides obtained via subcritical water hydrolysis showed high antioxidant and antimicrobial activity. | [83] |
| Skipjack tuna (Katsuwonus pelamis) | Skin | Trypsin, neutrase, papain, pepsin, and alcalase | The antioxidant peptides extracted in this study can act as active ingredients in preventing UVA injury. | [84] |
| Skipjack tuna (Katsuwonus pelamis) | Milts | Trypsin, neutrase, papain, pepsin, and alcalase | Bioactive peptides displayed significant protection to HUVECs against H2O2 damage by increasing antioxidase levels. | [85] |
| Sturgeon (Acipenser ruthenus) | Spermary | Papain | Extracted peptides change the permeability of the microbial cell membranes and may exert antimicrobial activity by inhibiting the metabolic process of the nucleic acids. | [86] |
| Siberian sturgeon (Acipenserbaerii) | Cartilage | Alcalase, papain, trypsin, flavourzyme, and pepsin | The extracted peptides displayed significant cytoprotection on HUVECs against H2O2 injury. | [87] |
| Sea intestine (Urechis unicinctus) | Viscera | Papain, trypsin, and alkaline protease | Extracted peptides exhibited strong antioxidant activity. | [88] |
3.3. Fishery Discards as a Source of Lipids
| Fish Species | Body Parts | Extraction Methods | Yield of Oil/PUFA Content (%) | References |
|---|---|---|---|---|
| Seabass (Dicentrarhus labrax), bluefin tuna (Thunnus thynnus), and gilthead seabream (Sparus aurata) | Liver (bluefin tuna), gills and heads (seabass), guts (seabream) | Raw materials were ground and cooked at 95 °C temperature for 12 min. Then, the materials were pressed with an expeller screw and separated oil, water, and dry matter via centrifugation (at 4200 rpm). | Lipid content: 27 ± 3; PUFA content of tuna by-product: 38 ± 7; tuna; liver: 35 ± 6; cod liver: 34 ± 0.3; sardine oil: 36 ± 3; seabass oil: 30 ± 0.2. | [101] |
| Rohu (Labeo rohita) | Heads | Enzymatic treatment with protamex (1:100 w/w), with microwave (MW), ultrasound (US), and heat pretreatment (HT). | Crude lipid obtained with MW: 60.45–69.75; US: 58.74–68.08; HT: 31.98–39.03. PUFA content with MW: 37.51 ± 0.53, US: 39.28 ± 0.33, HT: 38.31 ± 0.17. | [102] |
| Yellowtail fish (Seriola quinqueradiata) | Viscera | SC-CO2 extraction method and solvent extraction methods. | The yield of oil via SC-CO2 extraction: 11.03–40.87; Solvent extraction: 48.48 to 56.13. Omega 3 PUFA content SC-CO2-extracted oils: 18.97 to 20.14; solvent-extracted oils: 20.37 to 21.38%. | [103] |
| Tuna (Katsuwonus pelamis) | Liver | Enzymatic extraction (EE), wet reduction (WR), SC-CO2 extraction method, and subcritical dimethyl ether (SDE) extraction. | Oils obtained with EE: 85.25 ± 1.29; WR: 56.76 ± 1.57; SC-CO2: 98.45 ± 1.04; and SDE: 98.57 ± 0.60. PUFA content of EE oil: 29.41 ± 0.16; WR: 29.31 ± 0.19; SC-CO2: 32.77 ± 0.19; and SDE: 32.83 ± 0.16. | [104] |
| Horse mackerel (Trachurus mediterraneus), seabream (Pagellus acarne), blue whiting (Micromesistius poutassou), and sardine (Sardina pilchardus). | Discards/by-products | Pre-heated fish discards at 40 °C for 30 min, and then discards were hydraulically pressed (120 bar) and centrifuged to recover the crude oils. | Yield of the oil, HM: 1 to 6.2; SB: 4.7 to 5.8; BW: 1.1 to 3.2; Sar: 2.5 to 18.8. PUFA content, HM: 35 to 43.1; SB: 37.1 to 44.7; BW: 26.3 to 38.9; and Sar: 39.6 to 42.6. | [105] |
| Japanese Spanish mackerel (Scomberomorus niphonius) | Skin, muscle, bone, head, and viscera | SC-CO2 extraction. Temperature: 45 °C; Pressure: 250 bar; Extraction time: 3 h. | Oils obtained—skin: 42.79 ± 1.79; muscle: 24.18 ± 1.09; bone: 29.11 ± 1.81; head: 31.08 ± 2.05; and viscera: 22.70 ± 1.35. PUFA content—skin: 27.54; muscle: 29.15; bone: 18.34; head: 21.88; viscera: 21.88. | [23] |
| Australian rock lobster (Jasus edwardsii) | Liver | SC-CO2 extraction method. Temperature: 50 °C; Pressure: 350 bar; Extraction time: 4 h. | Oil obtained: 24.3% (w/w); PUFA content: 31.3. | [106] |
| Conger eel (Conger myriaster) | Skin | SC-CO2 extraction method. Temperature: 55 °C; Pressure: 300 bar; Extraction time: 2 h. | Crude lipid: 71.9 ± 0.12 PUFA content: Omega 3: 18.62 ± 0.08; Omega 6: 4.16 ± 0.19. | [55] |
| Frigate tuna (Auxis thazard), Eastern little tuna (Euthynnus affinis), and Longtail tuna (Thunnus tonggol). | Viscera, skin, and heads | SC-CO2 extraction method and solvent extraction methods. | Crude oils obtained—viscera: 13.5–16.8; skin 21.8–26.4; and head 30.2–36.2. PUFA content: 24.1–27.9 where docosahexaenoic acid (DHA) was prominent. | [107] |
| Brazilian red-spotted shrimp (Farfantepenaeus paulensis) | Shell, tail, and heads | SC-CO2 extraction method. Temperature: 40–60 °C; Pressure: 200–400 bar. | Methods were reported about 4.9 ± 0.1% of oils obtained and optimized for carotenoid-rich oil extraction. PUFA content: EPA: 3.44 to 11.69; DHA: 2.25 to 12.20. | [108] |
| Northern shrimp (Pandalus borealis) | Shell, tail, and heads | SC-CO2 extraction. Temperature: 40 °C; Pressure: 350 bar. | Crude oils obtained—13.7 PUFA content—EPA: 7.8 ± 0.06; DHA: 8.0 ± 0.07. | [109] |
| Brown seaweeds (Saccharina japonica and Sargassum horneri) | Whole body | SC-CO2 extraction. Temperature: 45 °C; Pressure: 250 bar; Extraction time: 3 h. | Oil content: SJ: 1.09 ± 0.56; SH: 1.41 ± 0.15. PUFA content: SJ: 14.67; SH: 26.7. | [110] |
3.4. Fishery By-Products as a Source of Minerals
3.5. Seafood Wastes as a Source of Pigments
3.6. As a Source of Important Enzymes
| Fish Species | Parts of Body | Extraction Methods | Type of Pigments | Yield | References |
|---|---|---|---|---|---|
| Crabs, shrimp (Penaeus indicus), crayfish, krill, and lobster | Carapace and heads | Enzymatic hydrolysis with Trypsin (2000 U/g), papain (6000 NF Units), and alcalase (0.6 Anson U/g) | Crude carotenoids | Highest yield by alcalase 28.6 μg/g waste; papain (24.8 μg/g); and trypsin (25.3 μg/g). | [136] |
| Freshwater crab (Potamon potamon) and marine crab (Charybdis cruciata) | Shells and meat | Extracted by solvent extraction with acetone and ether | Astaxanthin, zeaxanthin, β-carotene | From the shell and meat of marine crab, astaxanthin was estimated about 65.5 and 67.6 g/100 g of carotenoids. From the shell and meat of marine crab, zeaxanthin was estimated about 0.49 and 5.0 g/100 g of carotenoids. From the shell and meat of marine crab, astaxanthin was estimated about 36.5 and 14.7 g/100 g of carotenoids. Zeaxanthin from shell and meat of freshwater crab about 74.8 and 42.0 g/100 g of carotenoids. The highest β-carotene was obtained from the meat of the freshwater crab, 7.4 g/10 g of carotenoids. | [146] |
| Spiny lobster (Panulirus japonicas) | Carapace | Acetone extraction | Canthaxanthin, astaxanthin, zeaxanthin, β-carotene, and adonixanthin | Total carotenoid yield: 0.1 mg/g carapace; Canthaxanthin: 6g/100 g of carotenoids; Astaxanthin: 65 g/100 g of carotenoids; Zeaxanthin: 1.2 g/100 g of carotenoids; β-carotene: 2g/100 g of carotenoids; Adonixanthin: 1.2 g/100 g of carotenoids. | [147] |
| Shrimp (Peneanus monodon) | Shells | Concurrent SC-CO2-extraction methodology | Astaxanthin-rich oil | A new process design for extraction of astaxanthin has been proposed and the highest yield obtained was 43.09 µg/g of oil. | [14] |
| Jumbo squid (Dosidicus gigas) | Skins | Solvent extraction with acidified methanol | Crude natural pigment | 580 and 690 mg of pigment extract per 100 g of fresh squid skin. | [148] |
| White shrimp (Litopenaeus vannamei) | Hepatopancreas | Alkaline and heat treatment (1.0 M NaOH and pre-incubated at 60 °C) | Carotenoproteins | Carotenoproteins contained—73.58% protein and major carotenoids identified as astaxanthin and β-carotene. | [149] |
| Shrimp (Peneanus monodon) | Shells | Ultrasound-assisted natural deep eutectic solvent | Astaxanthin | Optimized the extraction methodology using response surface methodology, and the highest yield of astaxanthin was obtained at 68.98 ± 1.22 μg ASX/g shrimp waste. | [15] |
| Shrimp (Penaeus vannamei) | By-products | Ultrasonic-assisted ionic liquid extraction | Astaxanthin | Astaxanthin yield: 32.47 µg/g waste. | [150] |
| Red shrimps (Aristeus antennatus) | By-products | Ultrasound and microwave-assisted natural deep eutectic solvent | Astaxanthin | Ultrasound-assisted extraction: 7.85 ± 0.47 mg of astaxanthin/100 g dry sample; Ultrasound-assisted extraction: 26.7 ± 2 mg of astaxanthin/100 g dry sample. | [151] |
| Brown crab | Shell residues | Terpene-based natural deep eutectic solvents | Astaxanthin | The highest yield of astaxanthin was obtained at 9.3 ± 0.8 μg/g dry residue. | [152] |
| Northern shrimp (Pandalus borealis) | By-products | Sunflower oil (SF) and its methyl ester (ME-SF) | Astaxanthin | Yield obtained with SF: 23 mg/kg waste; ME-SF: 34.2 mg/kg waste. | [153] |
| Red microalgae (Porphyridium spp.) | Seaweed | Conventional extraction (maceration and freeze–thaw); Green extraction: (microwave (MW) and ultrasound (US)). | Phycoerythrin | The highest yield by maceration is 15.93 mg/g biomass; freeze–thaw was 16.08 mg/g biomass. Microwave: 23.94 mg/g biomass; ultrasound: 32.63 mg/g biomass. | [154] |
| Fish Species | Body Parts | Group and Name of the Enzymes | Application of Extracted Enzymes | References |
|---|---|---|---|---|
| Pink shrimp (Parapenaeus longirostris) | Gut, viscera and intestine. | Polyphenoloxidase -laccase | This enzyme exhibits very intense activity, and during storage, melanosis may continue to occur due to the oxidation of p-dihydroxyphenols produced mainly by non-specific hydroxylation of aromatic amino acids. | [139] |
| Leather jacket (Aluterus monoceros) | Pyloric caeca | Protease—trypsin | Preparation of protein hydrolysates with higher antioxidant activities. | [155] |
| New Zealand hoki (Macruronus novaezealandiae) and chinook salmon (Oncorhynchus tshawytscha) | Liver and intestine | Digestible lipases | Flavor development in dairy cream with extracted lipases and compared with calf pre-gastric esterase. | [141] |
| Goby (Zosterisessor ophiocephalus) | Viscera | Alkaline protease—crude extract | Deproteinization of shrimp wastes by extracted crude proteases. | [156] |
| Whiteleg shrimp (Litopenaeus vannamei) | Muscle, pleopods, digestive gland, and uropods | Lipase | Potential role in the hydrolysis of triacylglycerides stored as fat in the shrimp body. | [157] |
| Sardinelle (Sardinella aurita) | Viscera | A novel aspartic protease | Proteolytic activity was examined against natural food proteins. | [158] |
| Silver mojarra (Diapterus rhombeus) | Viscera | Alkaline peptidase—trypsin | With high activity and stability at pH from 8.5 to 11, this enzyme has good potential to be used as an additive in commercial detergent formulations. | [159] |
| Crayfish (Pacifastacus leniusculus) | Discards | Trans-glutaminase (TGase) | Extracted crayfish TGase enzyme showed higher activity at low temperatures (4 °C) than pig liver TGase. | [160] |
| Tilapia (Oreochromis mossambicus), bigeye snapper (Priacanthus hamrur), common carp (Cyprinus carpio) and Indian oil sardine (Sardinella longiceps) | Fish muscle tissue | Trans-glutaminase (TGase) | Improvement of the setting and gelling ability of fish mince from Cynoglossus spp. | [144] |
| Antarctic krill (Euphausia superba) | By-products | Trans-glutaminase (TGase) | Extracted enzymes enhanced the mechanical properties of gelatin gels at 4 °C. | [161] |
4. Macromolecules Obtained from Fishery By-Products
4.1. Gelatin and Collagen
4.2. Seafood By-Products as Sustainable Sources of Polysaccharides


| Fish Species | Body Parts | Type of Collagen/Gelatin | Yield | Properties/Finding of the Study | References |
|---|---|---|---|---|---|
| Bullhead shark (Heterodontus japonicas), Ayu (Plecoglossus altivelis), Horse mackerel (Trachurus japonicas), Skipjack tuna (Katsuwonus pelamis), yellow sea bream (Dentex tumifrons), chub mackerel (Scomber japonicas), and Japanese sea-bass (Lateolabrax japonicas) | Fins, bones, and skins | Acid-solubilized collagen (ASC) | (1) Skin collagen, 51.4% (Japanese sea-bass), 49.8% (chub mackerel), and 50.1% (bullhead shark), respectively; (2) bone collagen, 42.3% (skipjack tuna), 40.7% (Japanese sea-bass), 53.6% (ayu), 40.1% (yellow sea bream), and 43.5% (horse mackerel), respectively; (3) fin collagen, 5.2% (Japanese sea-bass acid-soluble collagen) and 36.4% (Japanese sea-bass acid-insoluble collagen). | This report indicates that these fish waste materials have the potential to supplement the skin of land vertebrates as a source of collagen. | [208] |
| Spanish mackerel (Scomberomorous niphonius) | Skin | Acid-solubilized collagen (ASC) | The collagen obtained from the skin is 13.68 ± 0.35%. | Antioxidant activities; emulsifying properties of the extracted collagen varied by average molecular weight. | [209] |
| Jumbo squid (Dosidicus gigas) | Skin and fins | Extraction of acid-solubilized collagen (ASC) and then enzymatic hydrolysis | Collagen was obtained—from fin: 69%; and from skin: 66% (based on dry wt.). | Extracted collagen showed higher levels of polar and hydrophobic amino acids. The collagen hydrolysates produced by subtilisin showed a lower degree of hydrolysis and higher antioxidant activity. | [170] |
| Smooth-hound (Mustelus mustelus) | Skin | Acid-solubilized collagen (ASC) and pepsin-solubilized collagen (PSC) | Collagen obtained—ASC (acid-soluble): 23.07%; and PSC (pepsin-soluble): 35.27% of the sample. | Extracted collagen used for preparing films with chitosan and prepared biofilm showed potential UV barrier properties and antioxidant activity. | [210] |
| Red drum fish (Sciaenops ocellatus) | Scales | Acid-solubilized collagen (ASC) and pepsin-solubilized collagen (PSC) | Collagen obtained—ASC (acid-soluble): 0.61 ± 0.20%; and PSC (pepsin-soluble): 4.32 ± 0.30% of the sample. | Type-I collagen was isolated rapidly via hydrophilic ultrafiltration from the scales of red drum fish (Sciaenops ocellatus) after limited pepsin digestion. | [168] |
| Bigeye tuna (Thunnus obesus) | Bones, scales, and skin | Acid-solubilized collagen (ASC) and pepsin-solubilized collagen (PSC) | Collagen was obtained from skin—ASC (acid-soluble) 13.05 ± 0.6%; and PSC (pepsin-soluble) 16.7 ± 0.7% based on dry wt. Collagen was obtained from scale and bone—PSC (pepsin-soluble) 4.6 ± 0.3% and 2.6 ± 0.3% based on dry wt. | This study concluded physiochemical properties of extracted fish collagen were comparable to mammalian collagen. | [169] |
| Black ruff (Centrolophus niger) | Skin | Acid-solubilized collagen (ASC) | The yield of the extracted collagen varied from 25% to 45% based on the skin weight. | Extraction and characterization of collagen from fish waste and its application in the development of antibacterial active food-packaging film. | [211] |
| Atlantic cod and Atlantic salmon | Scales and skin | Acid-solubilized collagen (ASC) | Yield of collagen—Atlantic salmon: skin 11.95% and fins: 5.76%. Atlantic cod: skin: 3.46% and fins: 4.34% based on wet tissue. | Salmon scales and skin had very high collagen levels, allowing them to be promising sources for high-value collagen production. | [171] |
| Eel fish (Evenchelys macrura) | Skin | Acid-solubilized collagen (ASC) and pepsin-solubilized collagen (PSC) | Collagen yield—ASC: 80% and PSC: 7.1% based on the dry weight of the skin. | The ASC and PSC gels and films also showed equal potency in delivering drugs against bacterial and fungal human pathogens. | [212] |
| Blue whiting (BW, Micromesistius poutassou), Mackerel (M, Scomber scombrus), Red scorpionfish (RS, Scorpaena scrofa), and Pouting (P, Trisoreptus luscus) | Heads and skins | Extraction of gelatin using a sequential combination of 0.05 M NaOH, 0.02 M H2SO4, and 0.05 M citric acid solutions. | Yield of the gelatin (%. w/w fresh skin)—BW: 0.23 ± 0.05; M: 0.69 ± 0.33; RS: 0.28 ± 0.11; P: 0.56 ± 0.25. | Extracted gelatin showed strong antioxidant and antihypertensive activity. | [10] |
| Atlantic mackerel (Scomber scombrus) | Skin | Extraction of gelatin via acid-based and heat treatment | Gelatin yield varied from 29.6 to 31.8%. | The chemical composition, rheological and textural properties, and microstructural characteristics of the extracted gelatins were analyzed and compared with commercial bovine hide gelatin. | [213] |
| Mackerel (Scomber japonicus) | Bone and skin | Collagen hydrolysate using subcritical water hydrolysis | Subcritical water treatment produced low-molecular-weight (<1650 Da) collagen peptides. | The antioxidant activities of collagen hydrolysate obtained via subcritical water hydrolysis were significantly higher than native collagen. | [214] |
| Bigeye tuna (Thunnus obesus) | Bones, scales, and skin | Collagen hydrolysate via catalyst-assisted subcritical water hydrolysis | The average molecular size of the peptides in the obtained collagen hydrolysates varied between 300 and 425 Da. | The collagen hydrolysates obtained in this study showed enormous potential for use in the food and pharmaceutical industries. | [215] |
| Totoaba (Totoaba macdonaldi) | Swim bladder | Pepsin-solubilized collagen (PSC) and collagen hydrolysates via enzymatic hydrolysis (Alcalase and papain) | The yield of collagen was high (68%) and exhibited good thermal stability (32.5 °C). | This study reported that the swim bladder from the farmed totoaba could be an ideal source to produce high-quality type-I collagen and may be considered an alternative to conventional collagen sources. | [216] |
| Seafood | Body Parts | Extraction Methods | Polysaccharide | Yield | Properties/Characteristics | References |
|---|---|---|---|---|---|---|
| Pacific white shrimp (Litopenaeus vannamei) | Heads | Demineralization and deproteinization through HCl and NaOH solutions. The deacetylation process obtained chitosan | Chitin and chitosan | Chitin and chitosan were obtained from shrimp waste processing about 25 ± 2 g/kg and 17 ± 4 g/kg. | Anticoagulant properties and anti-inflammatory activity. | [217] |
| Shrimp | Shrimp waste | Chitin extracted with conventional methods. Chiton extracted using microwave-assisted extraction | Chitin and chitosan | The maximum yield obtained from shrimp waste was about 36.43% (based on dry wt.), and the highest chitosan yield was 90% based on the chitin wt. | Antibacterial, functional, antioxidant, and physicochemical properties. | [218] |
| Shrimp (Metapenaeus monoceros) | Shells | Enzymatic extractions by several microbial and fish alkaline proteases | Chitin and chitosan | Concerning microbial enzyme preparation, high deproteinization (DDP) degrees were obtained with 77 ± 3%. | Antimicrobial, antitumor, and antioxidant activities. | [219] |
| Norway lobster (Nephrops norvegicus) | Thorax, heads, and appendix by-products | Enzymatic extraction (protease from Bacillus lentus) | Chitin | The yield of the chitin extracted from Norway lobster was 24.6 ± 1.02% (based on dry wt.). | Antiproliferative and antimicrobial activities. | [220] |
| Shrimp (Marsupenaeus japonicas) | Shells | Deep eutectic solvent extraction | Chitin | A higher yield was obtained compared with the conventional extraction (16.08%). | Extracted chitins showed excellent potential for preparing biodegradable packaging film. | [198] |
| Lobster | Shells | DES (Deep eutectic solvent) | Chitin | The highest yield of chitin was 23.31% with Choline chloride-lactic acid deep eutectic solvent. | Acid-based deep eutectic solvents have the potential for use as green media for the production of chitin. | [12] |
| Shrimp | Shells | Extraction by ammonium-based ionic liquids | Chitin and chitosan | A chitin extraction of 14% of the original biomass was found after shrimp-shell treatment with ionic liquids. | The experimental results revealed that ionic liquids could be a potential medium for chitin extraction. | [197] |
| Prawn | Shells | Microbial extraction (fermentation) | Chitin and chitosan | The highest yield of chitin was 0.78%, with a higher degree of deacetylation of 72.90%. | A higher degree of deacetylation is valued compared with the commercial chitin. | [221] |
| Shrimp | Shells | Ultrasound-assisted extraction | Chitin and chitosan | Ultrasound reduces the protein content and particle size of chitin. | Chitosan of high deacetylation and medium molecular weight was produced, and the extracted chitosan was applied for beef preservation. | [222] |
| Swimming crab (Portunus trituberculatus) | Shells | Subcritical water pretreatment | Chitosan | The yield and the molecular weight of the chitosan were 12.2% and 1187.2 kDa, respectively. | Chitosan prepared via subcritical water pretreatment was easier to use in preparing oligosaccharides. | [223] |
| Shrimp (Penaeus monodon) | Shells | Subcritical water | Oligochitosan | Subcritical water hydrolysis reduces the molecular weight of the chitosan (3.06 kDa). | Oligochitosan showed potent antioxidant, antimicrobial, and anticancer activities. | [183] |
| Shrimp | Shells | Fermentation by Pseudonocardia antitumoralis | Chitooligosaccharides | The results indicate that the isolate Pseudonocardia antitumoralis 18D36-A1 could convert chitin into chitooligosaccharides. | The extract produced the active fraction D36A1C38, which can inhibit the growth of fungi by 74% at a concentration of 1 mg/mL. | [224] |
| Shrimp | Discards | Co-fermentation in the presence of Bacillus subtilis and Acetobacter sp. | Chitooligosaccharides | Final deproteinization (DP) and demineralization (DM) efficiency and the chitin yield were achieved as 94, 92, and 18%. | The proposed method exhibited excellent stability and high hydrolysis efficiency. | [225] |
| Brown algae (Turbinara ornata) | Seaweed powder | Conventional methods in acid dilution | Fucoidan | 10 different fractions of the crude fucoidan were obtained, and the highest sulfate content was reported as 38.34%. | This study claimed to be the first report to illustrate the potential anti-inflammatory activity of fucoidan extracted from the brown algae T. ornata. | [204] |
| Brown seaweed (Saccharina japonica) | Seaweed powder | Subcritical water extraction (SWE) with different solvents | Fucoidan | The highest yield of crude fucoidan was 8.23 at 140 °C, 50 bar, and 0.1% NaOH solvent. | A high yield of fucoidan was obtained from SWE when compared with the conventional method, and crude fucoidan showed high antioxidant and emulsifying activity properties. | [205] |
| Brown seaweed (Fucus vesiculosus) | Seaweed powder | Microwave-assisted extraction (MAE) | Fucoidan | The highest yield of fucoidan was 15.61%, and its sulfate content was 22.76%. | This method required short extraction times and non-corrosive solvents, resulting in reduced costs for green extraction techniques. | [207] |
5. Conclusions and Implication of This Review Work
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stankus, A. State of World Aquaculture 2020 and Regional Reviews: FAO Webinar Series. In FAO Aquaculture Newsletter; FAO: Rome, Italy, 2021; pp. 17–18. [Google Scholar]
- Roda, M.A.P.; Gilman, E.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P. A Third Assessment of Global Marine Fisheries Discards; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Haque, A.R.; Park, J.-S.; Ho, T.C.; Roy, V.C.; Ali, M.S.; Kiddane, A.T.; Kim, G.-D.; Chun, B.-S. Characterization of oil and amino acids obtained from yellow corvina by-products using subcritical and supercritical fluids. J. Supercrit. Fluids. 2023, 199, 105970. [Google Scholar] [CrossRef]
- Kiddane, A.T.; Roy, V.C.; Kang, M.J.; Patil, M.P.; Chun, B.S.; Kim, G.D. Anticancer and apoptotic activity in neuroblastoma SK-N-SH using phospholipid extract from bone of Scomberomorus niphonius. Chem. Biol. Drug Des. 2022, 102, 424–433. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Xiong, Z.; Xiong, H.; Shahbaz, H.M.; Siddique, F. Valorization of fisheries by-products: Challenges and technical concerns to food industry. Trends Food Sci. Technol. 2020, 99, 34–43. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Shin, Y.-N.; Park, Y.-S.; Shin, Y.-R.; Park, S.-W.; Roy, V.C.; Lee, H.-J.; Kumagai, Y.; Kishimura, H. Exploring Bioactive Compounds in Brown Seaweeds Using Subcritical Water: A Comprehensive Analysis. Mar. Drugs 2023, 21, 328. [Google Scholar] [CrossRef]
- Haq, M.; Ahmed, R.; Cho, Y.-J.; Chun, B.-S. Quality properties and bio-potentiality of edible oils from Atlantic salmon by-products extracted by supercritial carbon dioxide and conventional methods. Waste Biomass Valorization 2017, 8, 1953–1967. [Google Scholar] [CrossRef]
- Sasidharan, A.; Venugopal, V. Proteins and co-products from seafood processing discards: Their recovery, functional properties and applications. Waste Biomass Valorization 2020, 11, 5647–5663. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Ghaly, A.; Ramakrishnan, V.; Brooks, M.; Budge, S.; Dave, D. Fish processing wastes as a potential source of proteins. Amino acids and oils: A critical review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and characterization of chitosan from crab shell (Portunus trituberculatus) by NaOH/urea solution freeze-thaw pretreatment procedure. Int. J. Biol. Macromol. 2020, 147, 931–936. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Roy, V.C.; Ho, T.C.; Lee, H.-J.; Park, J.-S.; Nam, S.Y.; Lee, H.; Getachew, A.T.; Chun, B.-S. Extraction of astaxanthin using ultrasound-assisted natural deep eutectic solvents from shrimp wastes and its application in bioactive films. J. Clean. Prod. 2021, 284, 125417. [Google Scholar] [CrossRef]
- FAO. FAO Yearbook. In Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2016. [Google Scholar]
- Ezquerra-Brauer, J.M.; Aubourg, S.P. Recent trends for the employment of jumbo squid (Dosidicus gigas) by-products as a source of bioactive compounds with nutritional, functional and preservative applications: A review. Int. J. Food Sci. Technol. 2019, 54, 987–998. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Peng, Q.; Nunes, L.M.; Greenfield, B.K.; Dang, F.; Zhong, H. Are Chinese consumers at risk due to exposure to metals in crayfish? A bioaccessibility-adjusted probabilistic risk assessment. Environ. Int. 2016, 88, 261–268. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef]
- Chung, H.; Cadwallader, K.R. Volatile components in blue crab (Callinectes sapidus) meat and processing by-product. J. Food Sci. 1993, 58, 1203–1207. [Google Scholar] [CrossRef]
- Roy, V.C.; Park, J.-S.; Ho, T.C.; Chun, B.-S. Lipid indexes and quality evaluation of omega-3 rich oil from the waste of Japanese spanish mackerel extracted by supercritical CO2. Mar. Drugs 2022, 20, 70. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery wastes as a yet undiscovered treasure from the sea: Biomolecules sources, extraction methods and valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Tang, Z.X.; Ying, R.F.; Shi, L.E. Physicochemical and functional characteristics of proteins treated by a pH-shift process: A review. Int. J. Food Sci. Technol. 2021, 56, 515–529. [Google Scholar] [CrossRef]
- Siddik, M.A.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic hydrolysis of brewers’ spent grain proteins and technofunctional properties of the resulting hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Venugopal, V.; Sasidharan, A. Seafood industry effluents: Environmental hazards, treatment and resource recovery. J. Environ. Chem. Eng. 2021, 9, 104758. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, G.-W.; Yoon, I.S.; Park, S.H.; Park, S.Y.; Kim, J.-S.; Heu, M.S. Preparation and characterization of protein isolate from Yellowfin tuna Thunnus albacares roe by isoelectric solubilization/precipitation process. Fish Aquat. Sci. 2016, 19, 14. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Zhang, P.; Taha, A.; Hu, H.; Pan, S. The role of conformational state of pH-shifted β-conglycinin on the oil/water interfacial properties and emulsifying capacities. Food Hydrocoll. 2020, 108, 105990. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, A.; Shah, H.; Aadil, R.M.; Ali, A.; Shehzad, Q.; Ashraf, W.; Yang, F.; Karim, A.; Khaliq, A. Fish protein and its derivatives: The novel applications, bioactivities, and their functional significance in food products. Food Rev. Int. 2022, 38, 1607–1634. [Google Scholar] [CrossRef]
- Roy, V.C.; Chamika, W.A.S.; Park, J.-S.; Ho, T.C.; Khan, F.; Kim, Y.-M.; Chun, B.-S. Preparation of bio-functional surimi gel incorporation of fish oil and green tea extracts: Physico-chemical activities, in-vitro digestibility, and bacteriostatic properties. Food Control 2021, 130, 108402. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Meng, S. Comparing the kinetics of the hydrolysis of by-product from channel catfish (Ictalurus punctatus) fillet processing by eight proteases. LWT 2019, 111, 809–820. [Google Scholar] [CrossRef]
- Hassan, M.A.; Deepitha, R.; Xavier, K.M.; Gupta, S.; Nayak, B.B.; Balange, A.K. Evaluation of the properties of spray dried visceral protein hydrolysate from Pangasianodon hypophthalmus (Sauvage, 1978) extracted by enzymatic and chemical methods. Waste Biomass Valorization 2019, 10, 2547–2558. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of fish protein hydrolysates from Scyliorhinus canicula discards with antihypertensive and antioxidant activities by enzymatic hydrolysis and mathematical optimization using response surface methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Wang, Y.-M.; Zhang, B.; Deng, S.-G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Gavlighi, H.A.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-product with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Gómez, L.J.; Gómez, N.A.; Zapata, J.E.; López-García, G.; Cilla, A.; Alegría, A. Optimization of the red tilapia (Oreochromis spp.) viscera hydrolysis for obtaining iron-binding peptides and evaluation of in vitro iron bioavailability. Foods 2020, 9, 883. [Google Scholar] [CrossRef]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L. Protein hydrolysates from anchovy (Engraulis encrasicolus) waste: In vitro and in vivo biological activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Hu, F.-Y.; Wang, Y.-M.; Zhang, B.; Deng, S.-G.; Wu, C.-W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Microwave-intensified enzymatic deproteinization of Australian rock lobster shells (Jasus edwardsii) for the efficient recovery of protein hydrolysate as food functional nutrients. Food Bioprocess Technol. 2016, 9, 628–636. [Google Scholar] [CrossRef]
- Sampath Kumar, N.; Nazeer, R.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Aspevik, T.; Totland, C.; Lea, P.; Oterhals, Å. Sensory and surface-active properties of protein hydrolysates based on Atlantic salmon (Salmo salar) by-products. Process Biochem. 2016, 51, 1006–1014. [Google Scholar] [CrossRef]
- Lima, D.A.S.; Santos, M.M.F.; Duvale, R.L.F.; Bezerra, T.K.A.; Araújo, Í.B.d.S.; Madruga, M.S.; da Silva, F.A.P. Technological properties of protein hydrolysate from the cutting byproduct of serra spanish mackerel (Scomberomorus brasiliensis). J. Food Sci. Technol. 2021, 58, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.; Ramos, C.; Coutinho, J.; Bandarra, N.; Nunes, M. Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process Biochem. 2010, 45, 18–24. [Google Scholar] [CrossRef]
- Choksawangkarn, W.; Phiphattananukoon, S.; Jaresitthikunchai, J.; Roytrakul, S. Antioxidative peptides from fish sauce by-product: Isolation and characterization. Agric. Nat. Resour. 2018, 52, 460–466. [Google Scholar] [CrossRef]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.-X.; Zhao, Y.-Q.; Qiu, Y.-T.; Chi, C.-F.; Wang, B. Identification and Active Evaluation of Antioxidant Peptides from Protein Hydrolysates of Skipjack Tuna (Katsuwonus pelamis) Head. Antioxidants 2019, 8, 318. [Google Scholar] [CrossRef]
- Yang, X.-R.; Zhao, Y.-Q.; Qiu, Y.-T.; Chi, C.-F.; Wang, B. Preparation and Characterization of Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Bone Stimulated by in vitro Gastrointestinal Digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef]
- Noman, A.; Wang, Y.; Zhang, C.; Yin, L.; Abed, S.M. Fractionation and purification of antioxidant peptides from Chinese sturgeon (Acipenser sinensis) protein hydrolysates prepared using papain and alcalase 2.4L. Arab. J. Chem. 2022, 15, 104368. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Menduíña, A.; Nogueira, M.; Durán, A.I.; Sanz, N.; Valcarcel, J. Optimal Production of Protein Hydrolysates from Monkfish By-Products: Chemical Features and Associated Biological Activities. Molecules 2020, 25, 4068. [Google Scholar] [CrossRef]
- Lapeña, D.; Vuoristo, K.S.; Kosa, G.; Horn, S.J.; Eijsink, V.G. Comparative assessment of enzymatic hydrolysis for valorization of different protein-rich industrial byproducts. J. Agric. Food Chem. 2018, 66, 9738–9749. [Google Scholar] [CrossRef]
- He, S.; Nguyen, T.T.; Su, P.; Zhang, W. Protein hydrolysates produced from rock lobster (Jasus edwardsii) Head: Emulsifying capacity and food safety. Food Sci. Nutr. 2016, 4, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Roy, V.C.; Kim, S.-Y.; Lee, S.-C.; Chun, B.-S. Extraction of edible oils and amino acids from eel by-products using clean compressed solvents: An approach of complete valorization. Food Chem. 2022, 388, 132949. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Roy, V.C.; Ho, T.C.; Park, J.-S.; Jeong, Y.-R.; Lee, S.-C.; Kim, S.-Y.; Chun, B.-S. Amino Acid Profiles and Biopotentiality of Hydrolysates Obtained from Comb Penshell (Atrina pectinata) Viscera Using Subcritical Water Hydrolysis. Mar. Drugs 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B. Marine Bioactive Peptides—Structure, Function and Application. Mar. Drugs 2023, 21, 275. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish Aquat. Sci. 2019, 22, 1–14. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Peinado, I.; Koutsidis, G.; Ames, J. Production of seafood flavour formulations from enzymatic hydrolysates of fish by-products. LWT-Food Sci. Technol. 2016, 66, 444–452. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M.-É. Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. PharmaNutrition 2013, 1, 149–157. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and angiotensin-I-converting enzyme (ACE)-inhibitory peptides from fish as potential cardioprotective compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, L.; Zhuang, Y. Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food Funct. 2018, 9, 5251–5259. [Google Scholar] [CrossRef]
- Yaghoubzadeh, Z.; Peyravii Ghadikolaii, F.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. Int. J. Pept. Res. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Ghalamara, S.; Silva, S.; Brazinha, C.; Pintado, M. Valorization of fish by-products: Purification of bioactive peptides from codfish blood and sardine cooking wastewaters by membrane processing. Membranes 2020, 10, 44. [Google Scholar] [CrossRef]
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 2016, 23, 24901–24911. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. Optimization of the enzymatic hydrolysis conditions of waste from shortfin scad (Decapterus Macrosoma) for the production of angiotensin I-converting enzyme (ACE) inhibitory peptide using response surface methodology. Int. Food Res. J. 2017, 24, 1735. [Google Scholar]
- Kim, S.-B.; Yoon, N.Y.; Shim, K.-B.; Lim, C.-W. Antioxidant and angiotensin I-converting enzyme inhibitory activities of northern shrimp (Pandalus borealis) by-products hydrolysate by enzymatic hydrolysis. Fish Aquatic Sci. 2016, 19, 29. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wang, G.; Sun, S.; Liu, R.; Hong, B.; Gao, R.; Bai, K. Processing Optimization and Characterization of Angiotensin-Ι-Converting Enzyme Inhibitory Peptides from Lizardfish (Synodus macrops) Scale Gelatin. Mar. Drugs 2018, 16, 228. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Targeted separation of antibacterial peptide from protein hydrolysate of anchovy cooking wastewater by equilibrium dialysis. Food Chem. 2015, 168, 115–123. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Karnjanapratum, S.; O’Callaghan, Y.C.; O’Keeffe, M.B.; FitzGerald, R.J.; O’Brien, N.M.; Benjakul, S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J. Food Biochem. 2017, 41, e12350. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Zhu, W.-Y.; Wang, Y.-M.; Ge, M.-X.; Wu, H.-W.; Zheng, S.-L.; Zheng, H.-Y.; Wang, B. Production, identification, in silico analysis, and cytoprotection on H2O2-induced HUVECs of novel angiotensin-I-converting enzyme inhibitory peptides from Skipjack tuna roes. Front. Nutr. 2023, 10, 1197982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jiménez, G.M.; Burgos-Hernández, A.; Torres-Arreola, W.; López-Saiz, C.M.; Velázquez Contreras, C.A.; Ezquerra-Brauer, J.M. Bioactive peptides from collagen hydrolysates from squid (Dosidicus gigas) by-products fractionated by ultrafiltration. Int. J. Food Sci. Technol. 2019, 54, 1054–1061. [Google Scholar] [CrossRef]
- Ahmed, R.; Chun, B.-S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids 2018, 141, 88–96. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhao, Y.-Q.; Wang, Y.-M.; Yang, X.-R.; Chi, C.-F.; Wang, B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022, 50, 102138. [Google Scholar] [CrossRef]
- Suo, S.-K.; Zheng, S.-L.; Chi, C.-F.; Luo, H.-Y.; Wang, B. Novel angiotensin-converting enzyme inhibitory peptides from tuna byproducts—Milts: Preparation, characterization, molecular docking study, and antioxidant function on H2O2-damaged human umbilical vein endothelial cells. Front. Nutr. 2022, 9, 957778. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Chen, Y.-N.; Cai, J.; Liao, T.; Zu, X.-Y. Identification, Screening and Antibacterial Mechanism Analysis of Novel Antimicrobial Peptides from Sturgeon (Acipenser ruthenus) Spermary. Mar. Drugs 2023, 21, 386. [Google Scholar] [CrossRef]
- Sheng, Y.; Qiu, Y.-T.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Novel Antioxidant Collagen Peptides of Siberian Sturgeon (Acipenserbaerii) Cartilages: The Preparation, Characterization, and Cytoprotection of H2O2-Damaged Human Umbilical Vein Endothelial Cells (HUVECs). Mar. Drugs 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Asakiya, C.; Huang, K.; Zhou, X.; Liu, Q.; He, X. Extraction and Identification of Three New Urechis unicinctus Visceral Peptides and Their Antioxidant Activity. Mar. Drugs 2022, 20, 293. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and stabilization of omega-3 oils: A review. Trends Food Sci. Technol. 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Denomme, J.; Stark, K.D.; Holub, B.J. Directly Quantitated Dietary (n-3) Fatty Acid Intakes of Pregnant Canadian Women Are Lower than Current Dietary Recommendations. J. Nutr. 2005, 135, 206–211. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-obesity effects of DHA and EPA in high fat-induced insulin resistant mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; González-Mohíno, A.; Perez-Palacios, T. Fish oil/lycopene microcapsules as a source of eicosapentaenoic and docosahexaenoic acids: A case study on spreads. J. Sci. Food Agric. 2020, 100, 1875–1886. [Google Scholar] [CrossRef]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Perez-Palacios, T.; Ruiz-Carrascal, J.; Solomando, J.C.; de-la-Haba, F.; Pajuelo, A.; Antequera, T. Recent Developments in the Microencapsulation of Fish Oil and Natural Extracts: Procedure, Quality Evaluation and Food Enrichment. Foods 2022, 11, 3291. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Impact of pretreatment-assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. J. Food Process Eng. 2019, 42, e12990. [Google Scholar] [CrossRef]
- Franklin, E.C.; Haq, M.; Roy, V.C.; Park, J.-S.; Chun, B.-S. Supercritical CO2 extraction and quality comparison of lipids from Yellowtail fish (Seriola quinqueradiata) waste in different conditions. J. Food Process. Preserv. 2020, 44, e14892. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, S.; Hu, W.; Zhang, J.; Ding, Y.; Liu, J. Extraction of Oil from High-Moisture Tuna Livers by Subcritical Dimethyl Ether: A Comparison with Different Extraction Methods. Eur. J. Lipid Sci. Technol. 2019, 121, 1800087. [Google Scholar] [CrossRef]
- Morales-Medina, R.; García-Moreno, P.J.; Pérez-Gálvez, R.; Muñío, M.M.; Guadix, A.; Guadix, E.M. Nutritional indexes, fatty acids profile, and regiodistribution of oil extracted from four discarded species of the Alboran Sea: Seasonal effects. Eur. J. Lipid Sci. Technol. 2016, 118, 1409–1415. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant Enrichment of Polyunsaturated Fatty Acids (PUFAs) in the Lipids Extracted by Supercritical CO2 from the Livers of Australian Rock Lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, Z.I.; Norulaini, N.; Oliveira, A.; Yunus, K.; Chowdury, A.J.; Akanda, J.; Omar, M. Quality of Tuna Fish Oils Extracted from Processing the By-Products of Three Species of Neritic Tuna Using Supercritical Carbon Dioxide. J. Food Process. Preserv. 2015, 39, 432–441. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids. 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Treyvaud Amiguet, V.; Kramp, K.L.; Mao, J.; McRae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological Properties of Fucoxanthin in Oil Recovered from Two Brown Seaweeds Using Supercritical CO2 Extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moemin, A.R. Healthy cookies from cooked fish bones. Food Biosci. 2015, 12, 114–121. [Google Scholar] [CrossRef]
- Kim, S.K.; Jung, W.K. 15—Fish and bone as a calcium source. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; Woodhead Publishing: Sawston, UK, 2007; pp. 328–339. [Google Scholar]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Ideia, P.; Degli Esposti, L.; Miguel, C.C.; Adamiano, A.; Iafisco, M.; Castilho, P.C. Extraction and characterization of hydroxyapatite-based materials from grey triggerfish skin and black scabbardfish bones. Int. J. Appl. Ceram. Technol. 2021, 18, 235–243. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Hapenciuc, C.; Oktar, F.N.; et al. Fish Bone Derived Bi-Phasic Calcium Phosphate Coatings Fabricated by Pulsed Laser Deposition for Biomedical Applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef] [PubMed]
- Kiyochi Junior, H.d.J.; Candido, A.G.; Bonadio, T.G.M.; da Cruz, J.A.; Baesso, M.L.; Weinand, W.R.; Hernandes, L. In vivo evaluation of interactions between biphasic calcium phosphate (BCP)-niobium pentoxide (Nb2O5) nanocomposite and tissues using a rat critical-size calvarial defect model. J. Mater. Sci. 2020, 31, 71. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-Products. Appl. Sci. 2021, 11, 3387. [Google Scholar] [CrossRef]
- Neto, A.S.; Brazete, D.; Ferreira, J.M.F. Cuttlefish Bone-Derived Biphasic Calcium Phosphate Scaffolds Coated with Sol-Gel Derived Bioactive Glass. Materials 2019, 12, 2711. [Google Scholar] [CrossRef] [PubMed]
- Isloor, A.M.; Anil, S.; Venkatesan, J.; Kumar, G.C.M. Calcium phosphate bioceramics with polyvinyl alcohol hydrogels for biomedical applications. Mater. Res. Express 2019, 6, 125404. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Zhu, Q.; Ablikim, Z.; Chen, T.; Cai, Q.; Xia, J.; Jiang, D.; Wang, S. The preparation and characterization of HA/β-TCP biphasic ceramics from fish bones. Ceram. Int. 2017, 43, 12213–12220. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Mechano-tribological properties and in vitro bioactivity of biphasic calcium phosphate coating on Ti-6Al-4V. J. Mech. Behav. Biomed. 2018, 86, 143–157. [Google Scholar] [CrossRef]
- Dou, W.; Chen, H.; Chen, T.; Zhu, Q.; Jiang, D.; Xue, Z.; Wang, S.; Wang, S.; Tang, W. Design and construction of a microporous -containing HA/β-TCP biphasic ceramic as a novel bone graft material. Mater. Res. Express 2020, 7, 025401. [Google Scholar] [CrossRef]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. J. Chem. Eng. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and Biocompatibility Properties of Calcium Phosphate Bioceramics Derived from Salmon Fish Bone Wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, C.; Sun, F.; Wei, Z.; Chen, L.; Chen, W.; Tong, S.; Du, H.; Gao, J.; Ren, J.; et al. Sustainable production of astaxanthin in microorganisms: The past, present, and future. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Subhashini, J.; Mahipal, S.V.K.; Reddy, M.C.; Mallikarjuna Reddy, M.; Rachamallu, A.; Reddanna, P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-Solvent Extraction of Astaxanthin from Lactic Acid Fermented Shrimp Wastes. J. Agric. Food Chem. 2007, 55, 10345–10350. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Row, K.H. Comparison of ionic liquids and deep eutectic solvents as additives for the ultrasonic extraction of astaxanthin from marine plants. J. Ind. Eng. Chem. 2016, 39, 87–92. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, W.; Jin, W. Chapter 17—Microwave-assisted extraction of lipids, carotenoids, and other compounds from marine resources. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Garcia-Vaquero, M., Rajauria, G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 375–394. [Google Scholar]
- Sachindra, N.M.; Mahendrakar, N.S. Effect of Protease Treatment on Oil Extractability of Carotenoids from Shrimp Waste. J. Aquat. Food Prod. Technol. 2011, 20, 22–31. [Google Scholar] [CrossRef]
- Borges, S.; Odila, J.; Voss, G.; Martins, R.; Rosa, A.; Couto, J.A.; Almeida, A.; Pintado, M. Fish By-Products: A Source of Enzymes to Generate Circular Bioactive Hydrolysates. Molecules 2023, 28, 1155. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Rustad, T.; Xu, Y.; Jiang, Q.; Xia, W. Endogenous proteolytic enzymes—A study of their impact on cod (Gadus morhua) muscle proteins and textural properties in a fermented product. Food Chem. 2015, 172, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alvarez, O.; Montero, P.; Gómez-Guillén, C. Evidence of an active laccase-like enzyme in deepwater pink shrimp (Parapenaeus longirostris). Food Chem. 2008, 108, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Kumawat, M.; Babele, P.K. Microbial Enzymes in Food Technology. In Enzymes in Food Technology: Improvements and Innovations; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 1–17. [Google Scholar]
- Kurtovic, I.; Marshall, S.N.; Miller, M.R.; Zhao, X. Flavour development in dairy cream using fish digestive lipases from Chinook salmon (Oncorhynchus tshawytscha) and New Zealand hoki (Macruronus novaezealandiae). Food Chem. 2011, 127, 1562–1568. [Google Scholar] [CrossRef]
- Proespraiwong, P.; Tassanakajon, A.; Rimphanitchayakit, V. Chitinases from the black tiger shrimp Penaeus monodon: Phylogenetics, expression and activities. Comp. Biochem. Physiol. B Biochem. 2010, 156, 86–96. [Google Scholar] [CrossRef]
- Bhagwat, P.K.; Dandge, P.B. Collagen and collagenolytic proteases: A review. Biocatal. Agric. Biotechnol. 2018, 15, 43–55. [Google Scholar] [CrossRef]
- Binsi, P.K.; Shamasundar, B.A. Purification and characterisation of transglutaminase from four fish species: Effect of added transglutaminase on the viscoelastic behaviour of fish mince. Food Chem. 2012, 132, 1922–1929. [Google Scholar] [CrossRef]
- Shahidi, F.; Janak Kamil, Y.V.A. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in crabs from marine and fresh waters of India. LWT-Food Sci. Technol. 2005, 38, 221–225. [Google Scholar] [CrossRef]
- Suhnel, S.; Lagreze, F.; Ferreira, J.F.; Campestrini, L.H.; Maraschin, M. Carotenoid extraction from the gonad of the scallop Nodipecten nodosus (Linnaeus, 1758) (Bivalvia: Pectinidae). Braz. J. Biol. 2009, 69, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Enrique Chan-Higuera, J.s.; Antonio Carbonell-Barrachina, A.; Luis Cárdenas-López, J.; Kačániová, M.; Burgos- Burgos-Hernández, A.; Marina Ezquerra-Brauer, J. Jumbo squid (Dosidicus gigas) skin pigments: Chemical analysis and evaluation of antimicrobial and antimutagenic potential. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 349–353. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S.; Kishimura, H. Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci. 2014, 5, 54–63. [Google Scholar] [CrossRef]
- Gao, J.; You, J.; Kang, J.; Nie, F.; Ji, H.; Liu, S. Recovery of astaxanthin from shrimp (Penaeus vannamei) waste by ultrasonic-assisted extraction using ionic liquid-in-water microemulsions. Food Chem. 2020, 325, 126850. [Google Scholar] [CrossRef]
- Koutsoukos, S.; Tsiaka, T.; Tzani, A.; Zoumpoulakis, P.; Detsi, A. Choline chloride and tartaric acid, a Natural Deep Eutectic Solvent for the efficient extraction of phenolic and carotenoid compounds. J. Clean. Prod. 2019, 241, 118384. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pereira, C.V.; Leonardo, I.C.; Fernández, N.; Gaspar, F.B.; Silva, J.M.; Reis, R.L.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Terpene-Based Natural Deep Eutectic Systems as Efficient Solvents To Recover Astaxanthin from Brown Crab Shell Residues. ACS Sustain. Chem. Eng. 2020, 8, 2246–2259. [Google Scholar] [CrossRef]
- Razi Parjikolaei, B.; Bahij El-Houri, R.; Fretté, X.C.; Christensen, K.V. Influence of green solvent extraction on carotenoid yield from shrimp (Pandalus borealis) processing waste. J. Food Eng. 2015, 155, 22–28. [Google Scholar] [CrossRef]
- Ardiles, P.; Cerezal-Mezquita, P.; Salinas-Fuentes, F.; Órdenes, D.; Renato, G.; Ruiz-Domínguez, M.C. Biochemical Composition and Phycoerythrin Extraction from Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes 2020, 8, 1628. [Google Scholar] [CrossRef]
- Zamani, A.; Benjakul, S. Trypsin from unicorn leatherjacket (Aluterus monoceros) pyloric caeca: Purification and its use for preparation of fish protein hydrolysate with antioxidative activity. J. Sci. Food Agric. 2016, 96, 962–969. [Google Scholar] [CrossRef]
- Sila, A.; Nasri, R.; Bougatef, A.; Nasri, M. Digestive Alkaline Proteases from the Goby (Zosterisessor ophiocephalus): Characterization and Potential Application as Detergent Additive and in the Deproteinization of Shrimp Wastes. J. Aquat. Food Prod. Technol. 2012, 21, 118–133. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; del Toro, M.d.l.Á.N.; García-Carreño, F. Purification and characterization of an intracellular lipase from pleopods of whiteleg shrimp (Litopenaeus vannamei). Comp. Biochem. Physiol. B Biochem. 2011, 158, 99–105. [Google Scholar] [CrossRef]
- Khaled, H.B.; Ghorbel-Bellaaj, O.; Hmidet, N.; Jellouli, K.; Ali, N.E.-H.; Ghorbel, S.; Nasri, M. A novel aspartic protease from the viscera of Sardinelle (Sardinella aurita): Purification and characterisation. Food Chem. 2011, 128, 847–853. [Google Scholar] [CrossRef]
- Silva, J.F.; Espósito, T.S.; Marcuschi, M.; Ribeiro, K.; Cavalli, R.O.; Oliveira, V.; Bezerra, R.S. Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem. 2011, 129, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Sirikharin, R.; Söderhäll, I.; Söderhäll, K. Characterization of a cold-active transglutaminase from a crayfish, Pacifastacus leniusculus. Fish Shellfish Immunol. 2018, 80, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Simpson, B.K. A cold active transglutaminase from Antarctic krill (Euphausia superba): Purification, characterization and application in the modification of cold-set gelatin gel. Food Chem. 2017, 232, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Bruckner, P. Collagen Suprastructures. In Collagen: Primer in Structure, Processing and Assembly; Brinckmann, J., Notbohm, H., Müller, P.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 185–205. [Google Scholar]
- Alfaro, A.d.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Oliveira, V.d.M.; Assis, C.R.D.; Costa, B.d.A.M.; Neri, R.C.d.A.; Monte, F.T.D.; Freitas, H.M.S.d.C.V.; França, R.C.P.; Santos, J.F.; Bezerra, R.d.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Skierka, E.; Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chem. 2007, 105, 1302–1306. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xie, Q.; Hong, B.; Chen, J.; Hua, F.; Bai, K.; He, J.; Yi, R.; Wu, H. Rapid isolation of high purity pepsin-soluble type I collagen from scales of red drum fish (Sciaenops ocellatus). Food Hydrocoll. 2016, 52, 468–477. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Chun, B.-S. Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus). Int. J. Biol. Macromol. 2019, 135, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Acuña, D.A.; Robles-Sanchez, R.M.; Torres-Arreola, W.; Marquez-Rios, E.; Ezquerra-Brauer, J.-M. Collagen from jumbo squid fin: Extracting conditions and influence of the protease system on collagen hydrolysate antioxidant activity. CyTA J. Food 2016, 14, 193–199. [Google Scholar] [CrossRef]
- Dave, D.; Liu, Y.; Clark, L.; Dave, N.; Trenholm, S.; Westcott, J. Availability of marine collagen from Newfoundland fisheries and aquaculture waste resources. Bioresour. Technol. Rep. 2019, 7, 100271. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. J. Biomat. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Ho, T.C.; Lim, J.-S.; Kim, S.-J.; Kim, S.-Y.; Chun, B.-S. In Vitro Biodegradation, Drug Absorption, and Physical Properties of Gelatin–Fucoidan Microspheres Made of Subcritical-Water-Modified Fish Gelatin. Mar Drugs 2023, 21, 287. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain. Chem. Pharm. 2020, 18, 100315. [Google Scholar] [CrossRef]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Saima; Kuddus, M.; Roohi; Ahmad, I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Ali, M.S.; Ho, T.C.; Razack, S.A.; Haq, M.; Roy, V.C.; Park, J.-S.; Kang, H.W.; Chun, B.-S. Oligochitosan recovered from shrimp shells through subcritical water hydrolysis: Molecular size reduction and biological activities. J. Supercrit. Fluids 2023, 196, 105868. [Google Scholar] [CrossRef]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Kadak, A.E. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 1144–1148. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, T.; Chittenden, C. Preparation of chitosan oligomers and characterization: Their antifungal activities and decay resistance. Holzforschung 2012, 66, 119–125. [Google Scholar] [CrossRef]
- Razack, S.A.; Lee, Y.; Shin, H.; Duraiarasan, S.; Chun, B.-S.; Kang, H.W. Cellulose nanofibrils reinforced chitosan-gelatin based hydrogel loaded with nanoemulsion of oregano essential oil for diabetic wound healing assisted by low level laser therapy. Int. J. Biol. Macromol. 2023, 226, 220–239. [Google Scholar] [CrossRef]
- Choi, C.-R.; Kim, E.-K.; Kim, Y.-S.; Je, J.-Y.; An, S.-H.; Lee, J.D.; Wang, J.H.; Ki, S.S.; Jeon, B.-T.; Moon, S.-H.; et al. Chitooligosaccharides decreases plasma lipid levels in healthy men. Int. J. Food Sci. Nutr. 2012, 63, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-J.; Kim, J.-G.; Kim, J.-Y.; Kim, S.C.; Lee, N.H.; Hyun, C.-G. Anti-inflammatory effect of chitosan oligosaccharides in RAW 264.7 cells. Cent. Eur. J. Biol. 2010, 5, 95–102. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, C.; Kong, X.; Liang, Y.; Rong, M.; Liu, W. Chitooligosaccharides and N-acetyl-D-glucosamine stimulate peripheral blood mononuclear cell-mediated antitumor immune responses. Mol. Med. Rep. 2012, 6, 385–390. [Google Scholar] [CrossRef]
- Tan, J.S.; Abbasiliasi, S.; Lee, C.K.; Phapugrangkul, P. Chitin extraction from shrimp wastes by single step fermentation with Lactobacillus acidophilus FTDC3871 using response surface methodology. J. Food Process. Preserv. 2020, 44, e14895. [Google Scholar] [CrossRef]
- Hongkulsup, C.; Khutoryanskiy, V.V.; Niranjan, K. Enzyme assisted extraction of chitin from shrimp shells (Litopenaeus vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Espíndola-Cortés, A.; Moreno-Tovar, R.; Bucio, L.; Gimeno, M.; Ruvalcaba-Sil, J.L.; Shirai, K. Hydroxyapatite crystallization in shrimp cephalothorax wastes during subcritical water treatment for chitin extraction. Carbohydr. Polym. 2017, 172, 332–341. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Effendy, M.; Clausse, D.; Saleh, K.; Guénin, E. Heterogeneous deacetylation reaction of chitin under low-frequency ultrasonic irradiation. Carbohydr. Polym. 2021, 267, 118180. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.-J.; Cho, Y.-J.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of pulsed electric fields on cricket (Acheta domesticus) flour: Extraction yield (protein, fat and chitin) and techno-functional properties. Innov. Food Sci. Emerg. Technol. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Tai, W.; Shan, X.; Hao, J.; Li, G.; Yu, G. Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens. Int. J. Biol. Macromol. 2018, 120, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Nagahawatta, D.P.; Fernando, I.P.S.; Kim, Y.-T.; Kim, J.-S.; Kim, W.-S.; Lee, J.S.; Jeon, Y.-J. A Review on Fucoidan Structure, Extraction Techniques, and Its Role as an Immunomodulatory Agent. Mar. Drugs 2022, 20, 755. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.-J.; Park, Y.-B.; Woo, H.-C.; Chun, B.-S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef] [PubMed]
- Ben Slimane, E.; Sadok, S. Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of drug delivering potential of type-I collagen from eel fish Evenchelys macrura. J. Mater. Sci. Mater. Med. 2012, 23, 1729–1738. [Google Scholar] [CrossRef]
- Khiari, Z.; Rico, D.; Martin-Diana, A.B.; Barry-Ryan, C. Valorization of fish by-products: Rheological, textural and microstructural properties of mackerel skin gelatins. J. Mater. Cycles Waste Manag. 2017, 19, 180–191. [Google Scholar] [CrossRef]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Haq, M.; Chun, B.-S. Characterization of pepsin-solubilized collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Ho, T.C.; Ahmed, R.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Biofunctional properties of bacterial collagenolytic protease-extracted collagen hydrolysates obtained using catalysts-assisted subcritical water hydrolysis. J. Ind. Eng. Chem. 2020, 81, 332–339. [Google Scholar] [CrossRef]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; True, C.; Olivera-Castillo, L.; Fernández-Velasco, D.A.; López, L.M. Swim Bladder of Farmed Totoaba macdonaldi: A Source of Value-Added Collagen. Mar. Drugs 2023, 21, 173. [Google Scholar] [CrossRef]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef]
- Mahdy Samar, M.; El-Kalyoubi, M.H.; Khalaf, M.M.; Abd El-Razik, M.M. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Biol. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Vallejo-Domínguez, D.; Rubio-Rosas, E.; Aguila-Almanza, E.; Hernández-Cocoletzi, H.; Ramos-Cassellis, M.E.; Luna-Guevara, M.L.; Rambabu, K.; Manickam, S.; Siti Halimatul Munawaroh, H.; Loke Show, P. Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason. Sonochem. 2021, 72, 105417. [Google Scholar] [CrossRef]
- Hao, G.; Hu, Y.; Shi, L.; Chen, J.; Cui, A.; Weng, W.; Osako, K. Physicochemical characteristics of chitosan from swimming crab (Portunus trituberculatus) shells prepared by subcritical water pretreatment. Sci. Rep. 2021, 11, 1646. [Google Scholar] [CrossRef]
- Widyastuti, W.; Setiawan, F.; Al Afandy, C.; Irawan, A.; Laila, A.; Juliasih, N.L.; Setiawan, W.A.; Arai, M.; Hendri, J.; Setiawan, A. Antifungal Agent Chitooligosaccharides Derived from Solid-State Fermentation of Shrimp Shell Waste by Pseudonocardia antitumoralis 18D36-A1. Fermentation 2022, 8, 353. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, Z.; Mamtimin, T.; Khan, A.; Peng, L.; Yang, J.; Ali, G.; Zhou, T.; Zhang, Q.; Zhang, J.; et al. Chitooligosaccharides production from shrimp chaff in chitosanase cell surface display system. Carbohydr. Polym. 2022, 277, 118894. [Google Scholar] [CrossRef]



| Groups | Name of the Seafood | By-Products | References | |
|---|---|---|---|---|
| Body Parts | Percentage (w/w) | |||
| Mollusca | Octopus, squid, cuttlefish | Skin, heads, fins, tentacles, and guts | Up to 60 | [17] |
| Clam, oyster, scallop, mussel, etc. | Shells and viscera | 60–80 | [18] | |
| Crustacean | Crayfish | Head and shell | Up to 80 | [19,20] |
| Shrimp | Head, shell, and tail | 40–45 | [14,15] | |
| Lobster | Heads, shells, livers, and eggs | 50–70 | [21] | |
| Crab | Viscera, shell | Up to 85 | [22] | |
| Finfish and other cartilaginous fishes | Trimming | 2–5 | [6,11,23] | |
| Skin | 1–5 | |||
| Scales | 2–4 | |||
| Bones | 10–34 | |||
| Liver and gut | 15–20 | |||
| Head | 15–20 | |||
| Species | Body Part | Extraction of Minerals | Final Products and Activities | References |
|---|---|---|---|---|
| Salmon (Salmo salar) and sea bream (Sparus aurata). | Bones | Alkaline treatment (NaOH) for 24 h; Calcination at 850 °C for 4 h. | Fishbone-derived biphasic calcium phosphate coatings with improved textural, anti-inflammatory, and antimicrobial properties. | [116] |
| Spotted sorubim (Pseudoplatystoma corruscans). | Bones | Cleaned and washed with hot water; Calcination with 900 °C temperature. | Nanocomposite with improved mechanical and physical properties. | [117] |
| Grey triggerfish (Balistes capriscus) and Black scabbardfish (Aphanopus carbo). | Skin and bones | Isolation of hydroxyapatite 400, 600, 800, 1000 °C temperature. | Preparation of natural biphasic materials for targeting bone grafting. | [113] |
| Salmon (Salmo salar), Red scorpionfish (Scorpaena scrofa), and Atlantic horse mackerel (Trachurus trachurus). | Bones | Biogenic calcium phosphate was obtained via alkaline (hydrolysis) treatment and calcination with 750, 900 °C temperatures. | Biphasic carbonated hydroxyapatite (HA)/beta-tricalcium phosphate (TCP) and their application in the biomedical field. | [118] |
| Cuttlefish (Sepia Officinalis). | Bones | Calcination with 700 °C temperature for 120 min. | Biphasic calcium phosphate scaffolds targeting bone tissue engineering applications. | [119] |
| Cuttlefish (Sepia Officinalis). | Bones | Boiling in water, dipped for 1 h; Calcination with 900 °C temperature for 240 min. | Synthesis of biphasic calcium phosphate for hydrogel sample preparation. | [120] |
| Tuna (Thunnus thynnus) and sword fish (Xiphias gladius). | Bones | Boiled and wasted water jet (strong) for 1 h; Calcination with 600, 950 °C temperature 12 h. | Biological hydroxyapatite for biomedical application. | [121] |
| Sardine, salmon (Salmo salar), and sablefish (Anoplopoma Fimbria). | Bones | Boiled for 2 h with deionized water and flowing water wasted; Calcination with 600–1100 °C temperature for 60 min. | HA/β-TCP biphasic calcium phosphate ceramics (BCP) are produced from fish bones. | [122] |
| Nile tilapia (Tilapia nilotica). | Scales | Distilled water wasted and dried up; Calcination with 800 °C temperature. | Obtained hydroxyapatite powder for preparing biphasic calcium phosphate coating. | [123] |
| Salmon (Salmo salar). | Bones | Boiled for 2 h with deionized water and flowing water wasted; Calcination with 600 °C temperature for 60 min. | Construction of HA/β-TCP biphasic ceramic as a novel bone graft material. | [124] |
| Nile tilapia (Tilapia nilotica). | Scales | Washed with 0.1 M HCl several times and dried at 60 °C after washing with distilled water. Afterwards, alkaline treatment with NaOH at 100 °C to obtain the hydroxyapatite. | Nanocrystalline hydroxyapatite and its application for selenium adsorption in aqueous solution. | [125] |
| Salmon (Salmo salar). | Bones | Boiled for 1 h with 1% of NaOH and pure water wasted; Calcination with 800 °C temperature for 180 min. | Preparation of calcium phosphate bioceramics for bone-substitute materials. | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, V.C.; Islam, M.R.; Sadia, S.; Yeasmin, M.; Park, J.-S.; Lee, H.-J.; Chun, B.-S. Trash to Treasure: An Up-to-Date Understanding of the Valorization of Seafood By-Products, Targeting the Major Bioactive Compounds. Mar. Drugs 2023, 21, 485. https://doi.org/10.3390/md21090485
Roy VC, Islam MR, Sadia S, Yeasmin M, Park J-S, Lee H-J, Chun B-S. Trash to Treasure: An Up-to-Date Understanding of the Valorization of Seafood By-Products, Targeting the Major Bioactive Compounds. Marine Drugs. 2023; 21(9):485. https://doi.org/10.3390/md21090485
Chicago/Turabian StyleRoy, Vikash Chandra, Md. Rakibul Islam, Sultana Sadia, Momota Yeasmin, Jin-Seok Park, Hee-Jeong Lee, and Byung-Soo Chun. 2023. "Trash to Treasure: An Up-to-Date Understanding of the Valorization of Seafood By-Products, Targeting the Major Bioactive Compounds" Marine Drugs 21, no. 9: 485. https://doi.org/10.3390/md21090485
APA StyleRoy, V. C., Islam, M. R., Sadia, S., Yeasmin, M., Park, J. -S., Lee, H. -J., & Chun, B. -S. (2023). Trash to Treasure: An Up-to-Date Understanding of the Valorization of Seafood By-Products, Targeting the Major Bioactive Compounds. Marine Drugs, 21(9), 485. https://doi.org/10.3390/md21090485