The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials
Abstract
:1. Introduction
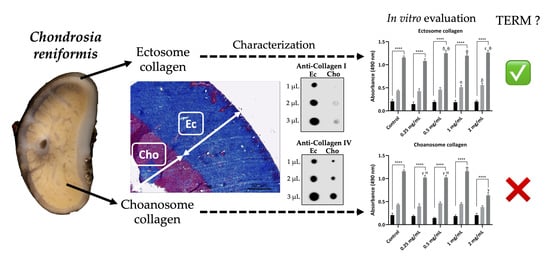
2. Results
2.1. Histological Characterization of C. reniformis
2.2. Collagen Characterization
2.3. Collagen Biological Assessment
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Sample Collection and Storage
4.3. C. reniformis Histological Characterization
4.4. Collagen Isolation
4.5. C. reniformis Collagen Characterization
4.5.1. Fourier Transform Infrared in Attenuated Total Reflection Mode (FTIR-ATR)
4.5.2. Circular Dichroism
4.5.3. Amino Acid Analysis
4.5.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5.5. Dot Blot
4.5.6. Isolation Yield
4.5.7. In Vitro Cytotoxicity Assessment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Czirók, A.; Rongish, B.J.; Little, C.D. Extracellular Matrix Dynamics during Vertebrate Axis Formation. Dev. Biol. 2004, 268, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the Ligand-Binding Sites and Disease-Associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.U.; Montes, G.S. Biology of Collagen-Proteoglycan Interaction. Arch. Histol. Jpn. 1983, 46, 589–629. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Capella, G.L. Foot and Mouth Disease in Human Beings. Lancet 2001, 358, 1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Niu, X.; Sun, L.; She, Z.; Tan, R.; Wang, W. Immune Response of Bovine Sourced Cross-Linked Collagen Sponge for Hemostasis. J. Biomater. Appl. 2018, 32, 920–931. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801639–1801651. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as a Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Alves, A.L.; Fraguas, F.J.; Carvalho, A.C.; Valcárcel, J.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A.; Silva, T.H. Characterization of Codfish Gelatin: A Comparative Study of Fresh and Salted Skins and Different Extraction Methods. Food Hydrocoll. 2022, 124, 107238. [Google Scholar] [CrossRef]
- Gökalp, M.; Wijgerde, T.; Murk, A.; Osinga, R. Design for Large-Scale Maricultures of the Mediterranean Demosponge Chondrosia Reniformis Nardo, 1847 for Collagen Production. Aquaculture 2022, 548, 737702. [Google Scholar] [CrossRef]
- Diogo, G.S.; Marques, C.F.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Cell-Laden Biomimetically Mineralized Shark-Skin-Collagen-Based 3D Printed Hydrogels for the Engineering of Hard Tissues. ACS Biomater. Sci. Eng. 2020, 6, 3664–3672. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Martins, E.; Rocha, M.S.; Silva, T.H.; Reis, R.L. Remarkable Body Architecture of Marine Sponges as Biomimetic Structure for Application in Tissue Engineering. Springer Ser. Biomater. Sci. Eng. 2019, 14, 27–50. [Google Scholar] [CrossRef]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Biomaterial for Topical Drug Delivery. Mater. Sci. Eng. C 2017, 70, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Lazoski, C.; Solé-Cava, A.M.; Boury-Esnault, N.; Klautau, M.; Russo, C.A.M. Cryptic Speciation in a High Gene Flow Scenario in the Oviparous Marine Sponge Chondrosia Reniformis. Mar. Biol. 2001, 139, 421–429. [Google Scholar] [CrossRef]
- Bonasoro, F.; Wilkie, I.C.; Bavestrello, G.; Cerrano, C.; Candia Carnevali, M.D. Dynamic Structure of the Mesohyl in the Sponge Chondrosia Reniformis (Porifera, Demospongiae). Zoomorphology 2001, 121, 109–121. [Google Scholar] [CrossRef]
- Fassini, D.; Parma, L.; Lembo, F.; Candia Carnevali, M.D.; Wilkie, I.C.; Bonasoro, F. The Reaction of the Sponge Chondrosia Reniformis to Mechanical Stimulation Is Mediated by the Outer Epithelium and the Release of Stiffening Factor(S). Zoology 2014, 117, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Barros, A.A.; Aroso, I.M.; Fassini, D.; Silva, T.H.; Reis, R.L.; Duarte, A.R.C. Extraction of Collagen/Gelatin from the Marine Demosponge Chondrosia Reniformis (Nardo, 1847) Using Water Acidified with Carbon Dioxide—Process Optimization. Ind. Eng. Chem. Res. 2016, 55, 6922–6930. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Müller, W.E.G.; Kreuter, J. Microparticles Derived from Marine Sponge Collagen (SCMPs): Preparation, Characterization and Suitability for Dermal Delivery of All-Trans Retinol. Eur. J. Pharm. Biopharm. 2002, 54, 125–133. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia Reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Fassini, D.; Duarte, A.R.C.; Reis, R.L.; Silva, T.H. Bioinspiring Chondrosia Reniformis (Nardo, 1847) Collagen-Based Hydrogel: A New Extraction Method to Obtain a Sticky and Self-Healing Collagenous Material. Mar. Drugs 2017, 15, 380. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural Studies on the Collagen of the Marine Sponge Chondrosia Reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular Characterization of a Nonfibrillar Collagen from the Marine Sponge Chondrosia Reniformis Nardo 1847 and Positive Effects of Soluble Silicates on Its Expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef]
- Garrone, R.; Huc, A.; Junqua, S. Fine Structure and Physicochemical Studies on the Collagen of the Marine Sponge Chondrosia Reniformis Nardo. J. Ultrasruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine Sponge Collagen: Isolation, Characterization and Effects on the Skin Parameters Surface-PH, Moisture and Sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and Carbon Dioxide: Green Solvents for the Extraction of Collagen/Gelatin from Marine Sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef]
- Tassara, E.; Oliveri, C.; Vezzulli, L.; Cerrano, C.; Xiao, L.; Giovine, M.; Pozzolini, M. 2D Collagen Membranes from Marine Demosponge Chondrosia Reniformis (Nardo, 1847) for Skin-Regenerative Medicine Applications: An In Vitro Evaluation. Mar. Drugs 2023, 21, 428. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia Reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Bavestrello, G.; Cerrano, C.; Cattaneo-Vietti, R.; Sara, M.; Calabria, F.; Cortesogno, L. Selective Incorporation of Foreign Material in Chondrosia Reniformis (Porifera, Demospongiae). Ital. J. Zool. 1996, 63, 215–220. [Google Scholar] [CrossRef]
- López De Padilla, C.M.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. 2021, 69, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, S.Y. Purification and Characterization of Type II Collagen from Chick Sternal Cartilage. Food Chem. 2008, 108, 439–445. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of Acid- and Pepsin-Soluble Collagen Extracted from the Skin of Nile Tilapia (Oreochromis Niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Zhao, Y.; Wang, H.; Zhang, H. Effect of Heat Treatment on the Enzymatic Stability of Grass Carp Skin Collagen and Its Ability to Form Fibrils in Vitro. J. Sci. Food Agric. 2015, 95, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Bächinger, H.P. Structure, Stability and Folding of the Collagen Triple Helix. Top. Curr. Chem. 2005, 247, 7–33. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using Circular Dichroism Spectra to Estimate Protein Secondary Structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef]
- Brodsky, B.; Ramshaw, J.A.M. The Collagen Triple-Helix Structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-Hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Leuenberger, B.H. Investigation of Viscosity and Gelation Properties of Different Mammalian and Fish Gelatins. Top. Catal. 1991, 5, 353–361. [Google Scholar] [CrossRef]
- Sipila, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpyla, J.; Myllyharju, J.; Heino, J. Proline Hydroxylation in Collagen Supports Integrin Binding by Two Distinct Mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, Z.; Li, C.; Li, G. Thermal Denaturation of Fish Collagen in Solution: A Calorimetric and Kinetic Analysis. Thermochim. Acta 2014, 581, 32–40. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Bavestrello, G.; Benatti, U.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Favre, A.; Giovine, M.; Lanza, S.; Pronzato, R.; Sara, M. Body Polarity and Mineral Selectivity in the Demosponge Chondrosia Reniformis. Biol. Bull. 1998, 195, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.W. Phylogenesis of Connective Tissue. Morphological Aspects and Biosynthesis of Sponge Intercellular Matrix. Robert Garrone. Q. Rev. Biol. 1979, 54, 468–469. [Google Scholar] [CrossRef]
- Stecco, C.; Hammer, W.; Vleeming, A.; De Caro, R. Functional Atlas of the Human Fascial System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 9780702044304. [Google Scholar]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and Characterization of Fish Scale Collagen of Higher Thermal Stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of Collagen from Skin, Scale and Bone of Carp (Cyprinus Carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Araújo, T.A.T.; de Souza, A.; Santana, A.F.; Braga, A.R.C.; Custódio, M.R.; Simões, F.R.; Araújo, G.M.; Miranda, A.; Alves, F.; Granito, R.N.; et al. Comparison of Different Methods for Spongin-like Collagen Extraction from Marine Sponges (Chondrilla Caribensis and Aplysina Fulva): Physicochemical Properties and in Vitro Biological Analysis. Membranes 2021, 11, 522. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the Marine Sponges Axinella Cannabina and Suberites Carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and Enzymatic Extraction of Collagen from Atlantic Cod (Gadus Morhua) Swim Bladders Envisaging Health-Related Applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.P.; Domingos, M.; Richardson, S.M.; Bella, J. Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma Pulmo. Mar. Drugs 2023, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A.M.; Shah, N.K.; Brodsky, B. Gly-X-Y Tripeptide Frequencies in Collagen: A Context for Host-Guest Triple-Helical Peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Junqua, S.; Lemonnier, M.; Robert, L. Glycoconjugates from “Spongia Officinalis” (Phylum Porifera). Isolation, Fractionation by Affinity Chromatography on Lectins and Partial Characterization. Comp. Biochem. Physiol.—Part B Biochem. 1981, 69, 445–453. [Google Scholar] [CrossRef]
- Tassara, E.; Orel, B.; Ilan, M.; Cavallo, D.; Dodero, A.; Castellano, M.; Vicini, S.; Giovine, M.; Pozzolini, M. Seasonal Molecular Difference in Fibrillar Collagen Extracts Derived from the Marine Sponge Chondrosia Reniformis (Nardo, 1847) and Their Impact on Its Derived Biomaterials. Mar. Drugs 2023, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.K.; Gottardi, D.; Ndagijimana, M.; Betti, M. Glycation and Transglutaminase Mediated Glycosylation of Fish Gelatin Peptides with Glucosamine Enhance Bioactivity. Food Chem. 2014, 142, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Boute, N.; Exposito, J.Y.; Boury-Esnault, N.; Vacelet, J.; Noro, N.; Miyazaki, K.; Yoshizato, K.; Garrone, R. Type IV Collagen in Sponges, the Missing Link in Basement Membrane Ubiquity. Biol. Cell 1996, 88, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The Effect of Depth on the Morphology, Bacterial Clearance, and Respiration of the Mediterranean Sponge Chondrosia Reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Scarfì, S.; Pozzolini, M.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Fenoglio, D.; Altosole, T.; Ilan, M.; Bertolino, M.; et al. Identification, Purification and Molecular Characterization of Chondrosin, a New Protein with Anti-Tumoral Activity from the Marine Sponge Chondrosia Reniformis Nardo 1847. Mar. Drugs 2020, 18, 409. [Google Scholar] [CrossRef]
- Foot, N.C. The Masson Trichrome Staining Methods in Routine Laboratory Use. Biotech. Histochem. 1933, 8, 101–110. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius Staining plus Polarization Microscopy, a Specific Method for Collagen Detection in Tissue Sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]





| C. reniformis Collagen | Isolation Yield (%) |
|---|---|
| Ectosome | 20.0 |
| Choanosome | 20.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, M.S.; Marques, C.F.; Carvalho, A.C.; Martins, E.; Ereskovsky, A.; Reis, R.L.; Silva, T.H. The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials. Mar. Drugs 2024, 22, 55. https://doi.org/10.3390/md22020055
Rocha MS, Marques CF, Carvalho AC, Martins E, Ereskovsky A, Reis RL, Silva TH. The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials. Marine Drugs. 2024; 22(2):55. https://doi.org/10.3390/md22020055
Chicago/Turabian StyleRocha, Miguel S., Catarina F. Marques, Ana C. Carvalho, Eva Martins, Alexander Ereskovsky, Rui L. Reis, and Tiago H. Silva. 2024. "The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials" Marine Drugs 22, no. 2: 55. https://doi.org/10.3390/md22020055
APA StyleRocha, M. S., Marques, C. F., Carvalho, A. C., Martins, E., Ereskovsky, A., Reis, R. L., & Silva, T. H. (2024). The Characterization and Cytotoxic Evaluation of Chondrosia reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials. Marine Drugs, 22(2), 55. https://doi.org/10.3390/md22020055