1. Introduction
Cone snails (genus
Conus) are gastropods that employ venom to immobilize prey, defend against predators, and interact with other living organisms in their environment. Each species produces hundreds of peptides (conotoxins, conopeptides, Ctx) and small, bioactive molecules in their venom gland [
1,
2,
3]. More than 40 years have passed since the characterization of the first conotoxin, αCtx-GI, a 13-amino acid peptide with two disulfide bonds from
Conus geographus venom [
4]. Since then, hundreds of bioactive molecules with pharmacological potential have been discovered, synthesized, and characterized [
5,
6,
7]. The venom of cone snails has proven to be a rich source of bioactive peptides, targeting many different ion channels and receptors. Thus, conotoxins are useful and effective tools to investigate muscular, cardiovascular, and neuronal systems [
8,
9,
10].
Cone snails are organized into three groups according to their preferred prey: piscivores (fish hunters), molluscivores (mollusk hunters), and vermivores (worm hunters). The majority of the peptides identified to date can be attributed to the study of piscivorous
Conus [
5,
11]. However, there are considerably more vermivorous species, which provides an opportunity to discover bioactive molecules with novel pharmacological specificity [
12,
13,
14]. Our laboratories have been systematically exploring bioactive peptides from different lineages of worm-hunting cone snails whenever a sufficient amount of venom becomes available. One of the least investigated lineages is
Kalloconus, a geographically restricted group of
Conus species found only off the coast of West Africa. The senior author (JLBN) had the opportunity to collect
Conus ateralbus, an endemic species in the subgenus
Kalloconus that is unique to the Cape Verde Islands. Prior characterization of one venom component revealed it to be a novel δCtx that inhibits the inactivation of Na
v channels [
15].
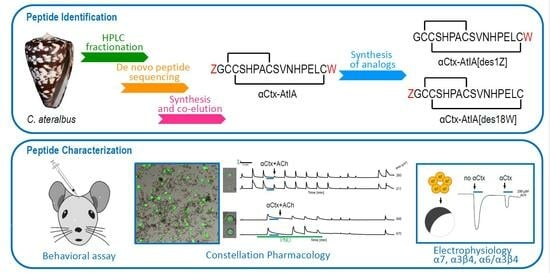
This study focuses on another venom component that was isolated using bioactivity-guided purification and was shown to belong to the αCtx family, a well-characterized group of peptide toxins that target nicotinic acetylcholine receptors (nAChRs). When purified from venom, the amino acid sequence of this peptide was strikingly similar to a previously characterized αCtx from a fish-hunting cone snail,
Conus pergrandis [
16]. A phylogenetic tree of the genus
Conus showing the relationship between
Embrikena and
Kalloconus lineages, which contain
C. pergrandis and
C. ateralbus, respectively, is shown in
Figure 1. Given their divergent biology and phylogenetic distance from one another, it was not expected that these two species would have any highly homologous venom components.
The αCtx are one of the most widely distributed and intensively investigated of Conus venom peptide families. However, no αCtx from the Kalloconus lineage have been investigated previously. In this article, we describe the comprehensive characterization of the αCtx-AtIA peptide from C. ateralbus and conduct a bioactivity comparison with the previously characterized homologous αCtx-PeIA from C. pergrandis.
Compounds targeting nAChRs have demonstrated biomedical potential, such as uncovering new mechanisms for the relief of chronic pain and neuronal disorders, including Alzheimer’s and Parkinson’s disease [
17,
18,
19,
20]. The αCtx are among the families of toxins that have been extensively investigated due to their ability to discriminate nAChR isoforms [
21,
22]. By studying how αCtx interact with nAChRs, we can gain a better understanding of the structure and function of these receptors, leading to new insights into neurological diseases associated with nAChRs.
3. Discussion
Conus ateralbus is a species of cone snail within the
Kalloconus subgenus found in the Cabo Verde archipelago off the coast of West Africa. It is known to feed primarily on marine worms. Thus far, only one conotoxin has been identified from this species: δ-AtVIA, which was shown to be a vertebrate sodium channel blocker [
15]. Here, we report the discovery and characterization of the first αCtx targeting nAChRs from the venom of
C. ateralbus. αCtx-AtIA shares a high sequence identity (16 of 18 amino acids) with αCtx-PeIA and differs only at the N- and C-terminus with the pyroglutamate and tryptophan residues, respectively, and a free carboxyl at the C-terminus. The native peptide was purified from the venom and its sequence was determined by manual de novo sequencing. Two versions of the peptide were chemically synthesized and co-eluted with the native material to resolve the Ile/Leu uncertainty in position 16. Intracranially injected native αCtx-AtIA and its synthetic version evoked similar behavioral responses in 15-day old mice. At 5 nmol, αCtx-AtIA caused seizures followed by death, a phenotype previously observed for αCtx-ImI as well as α-bungarotoxin by McIntosh et al. [
28]. Both peptides were shown to target the α7 subtype of nAChRs. Therefore, we hypothesized that the effect induced by αCtx-AtIA in mice might be mediated via α7 nAChRs.
Because of the heteromeric complexity and diversity of nAChRs, the development of either agonists or antagonists of nicotinic receptors as therapeutic drugs or diagnostic reagents has met with many challenges. Indeed, the nAChRs antagonist that had reached Phase II human clinical trials eventually failed, primarily because the subtype selectivity and affinity of the compound for nicotinic receptors had not been sufficiently defined when the clinical trials were initiated [
29,
30,
31,
32]. In this work, we introduce a novel experimental protocol for the discovery and characterization of new nAChR ligands. By customizing our Constellation Pharmacology platform, we were able to assess the bioactivity of αCtx-AtIA on nAChR-expressing DRG neurons and determine the selectivity profile for different nAChR subtypes expressed in DRGs.
Initially, fractions of the C. ateralbus venom were tested and their effects were monitored on KCl-induced calcium influx as an indicator of biological activity. αCtx-AtIA was found to modulate baseline calcium levels as well as amplifying KCl-induced calcium influx in a small subset of DRG neurons. These phenotypic responses were recapitulated with the synthetic peptide. However, as αCtx-AtIA belongs to the A superfamily of conotoxins that target nAChRs, we sought to characterize its effects on specific nAChRs using dedicated protocols.
In 2013, Smith et al. classified mouse and rat DRG neurons into four categories based on the functional expression of nAChRs: those that do not express nAChRs, those expressing predominantly α7 nAChRs, those expressing predominantly α3β4 and α6β4, and those expressing all three receptor types [
23]. This is consistent with the observed DRG neuronal response to ACh in mice: most neurons do not respond, a subset responds repeatedly and consistently to 1 mM ACh, (α3β4 and α6β4), another subset responds repeatedly and consistently to ACh+PNU, (α7) and a fourth type responds to both ACh and ACh+PNU. A manuscript with a detailed description of the cell-type-specific functional distribution of AChR subtypes in the DRG neurons is currently being prepared by Tun et al. (unpublished data).
To confirm the data collected in DRG neurons, we mainly focused on testing the peptides on these subtypes of nAChRs using electrophysiology. The data collected in DRGs suggested a significant difference in activity between αCtx-AtIA and αCtx-PeIA. When tested in ACh+ neurons expressing α3β4 and α6β4, only αCtx-PeIA produced a substantial block at 10 μM, whereas αCtx-AtIA exhibited inhibitory characteristics at the same concentration on DRGs expressing α7 nAChRs. The same trend was observed when both peptides were tested on heterologously expressed mouse α7, α3β4 and α6/α3β4 nAChRs. αCtx-AtIA blocked mouse α7 with an IC50 = 0.16 µM but did not produce block at 10 µM on α3β4 and was ~six times less potent on α6/α3β4 than αCtx-PeIA.
As the sequences of the two peptides differ only by two residues, we investigated the impact of removing residue Pyr1 or Trp18 on the activity of αCtx-AtIA. Pyroglutamate (Z, Pyr) is a cyclic amino acid identified in many biologically active peptides and proteins [
33] including A-, M- and T-superfamilies of conotoxins [
1]; it was found to affect the stability of the peptides, and in some cases, also their activity. In conotoxins, a pyroglutamate residue was used to improve the stability of χ-MrIA [
34], a small NET inhibitor [
35], without affecting its potency, advancing the peptide to clinical trials for neuropathic pain treatment [
36]. When pyroglutamate was removed from conotoxin μ-SIIIA, a potent Na
v channel blocker, only minor changes in affinity for Na
V1.2 and Na
V1.4 of VGSCs were observed [
37], suggesting that the residue had more of a stabilizing effect rather than contributing to the activity of the peptide. The bulky, hydrophobic tryptophan (W; Trp) residue is known to form noncovalent interactions via π-π, cation-π, and X-H-π, as well as forming hydrogen bonds. It not only stabilizes the structure of the peptide, but mostly contributes to the peptide–protein or protein–protein interaction [
38]. We synthesized both analogs of the peptide and used Constellation Pharmacology to compare their activity to either parent αCtx-AtIA or αCtx-PeIA. As shown in
Figure 10, in the ACh+ DRG neurons, removal of Trp18 led to an analog with an activity similar to that of αCTX-PeIA, while removal of Pyr1 did not change the activity of the analog as compared to the parent peptide. This result suggests an unfavorable interaction between Trp18 of αCtx-AtIA and residues of the α3β4 nAChRs and α6β4. It is possible that the orientation of the bulky C-terminal Trp18 interferes with the efficiency of binding to the receptor by the residues in the second inter-cysteine loop, which was previously shown to be important for the potency and selectivity of αCtx-PeIA on those subtypes [
39]. When conotoxins AtIA[des1Z] and AtIA[des18W] were tested in the ACh/PNU+ DRG neurons (
Figure 11), both peptides exhibited minimal inhibitory effect similar to that of αCtx-PeIA, although the peptide with the N-terminal truncation was more potent than the C-terminal truncation analog.
In summary, based on the extensive sequence homology between αCtx-AtIA and αCtx-PeIA, we anticipated that the subtype selectivity profile of the two peptides would be comparable, if not identical. However, using Constellation Pharmacology, we demonstrated that there were significant differences in the bioactivity of the two peptides. We also characterized analogs of αCtx-AtIA to understand the molecular basis for the differences observed in the selectivity profiles. The Constellation Pharmacology assessment revealed the role of the C-terminal Trp residue in conferring differences in target specificity. The demonstration that a single amino acid can be such a powerful determinant of subtype-selectivity, essentially abolishing affinity for the α3β4 subtype, while concomitantly increasing the affinity for the α7 subtype, may be useful for future drug development programs. Through this work, we have demonstrated the potential broad applicability of Constellation Pharmacology in defining the target selectivity of nicotinic receptor ligands.
4. Materials and Methods
4.1. Phylogenetic Analysis
A dataset composed of concatenated 12S, 16S and cytochrome oxidase I (COI) gene segments from each species was used for phylogenetic analysis. These gene segments were cloned and sequenced as previously described [
14,
40]. Multiple sequence alignment was carried out using MAFFT version 7 [
41]. Maximum likelihood tree reconstruction was performed with IQ-TREE, consisting of best-fit model TIM3+F+I+G4 using ModelFinder [
42] and ultrafast bootstrap approximation [
43]. The tree was visualized using iTOL version 6.4 (
https://itol.embl.de, accessed on 27 February 2024) [
44]. Clades were collapsed and are represented by circles with sizes proportional to the number of species in each clade. The alignment file has been included as
Supplementary Data File S1.
4.2. Venom Preparation
C. ateralbus specimens (length ≥ 40 mm) were collected from shallow waters around Sal Island (Calheta Funda, Cabo Verde) by SCUBA snorkeling. The specimens were frozen at −20 °C. The specimens were carefully dissected to remove the venom gland. The venom was obtained from venom ducts by placing each duct on an ice-cold metal spatula and squeezing the contents into 40% acetonitrile (CH3CN) /water acidified with 0.1% trifluoroacetic acid (TFA). The solution was then lyophilized and stored at −80 °C. Crude venom extract was prepared using 40% (v/v) CH3CN/water acidified with 0.1% (v/v) TFA and a portion of the extract was resuspended in 15 mL of 40% CH3CN and 0.1% TFA and vortexed twice for 1 min each, with a pause of 3 min in between. The samples were homogenized in a Wheaton homogenizer and centrifuged in a Beckman Avanti centrifuge (F650 rotor) for 15 min at 13,650 rpm, at 4 °C. All residual particles were retained by centrifugation.
4.2.1. Peptide Isolation (RPLC Purification)
Reverse-phase high-performance liquid chromatography (RP-HPLC) was carried out using a C18 Vydac, 218TP101522, 50 mm × 22 mm, 10–15 µm particle size; eluted with a flow rate of 7 mL/min and a gradient ranging from 10% to 30% of buffer B in 20 min, 30% to 50% in 25 min, 50% to 100% in 30 min followed by 100% for 15 min. Buffer B was 0.1% (v/v) TFA in 90% aqueous CH3CN, and buffer A was 0.1% (v/v) TFA in water.
All fractionations were purified using a C18 Vydac Monomeric (238EV54, 250 mm × 4.6 mm, 5 µm particle size). The absorbance was monitored at 220 and 280 nm and all fractions were stored at −80 °C.
4.3. Peptide Sequence Determination
The crude HPLC fractions were analyzed using Matrix-Assisted Laser Desorption/Ionization Time of Flight (MALDI-TOF, Applied Biosystems, Foster City, CA, USA) mass spectrometry at the Mass Spectrometry Core, Salk Institute for Biological Studies, La Jolla, CA, USA. To determine the number of cysteines, a DTT reduction and 4-vinylpyridine alkylation was employed on the pure peptide. To 177 µL of the pure peptide (HPLC sub-fraction 22.5), 10 µL of 0.5 M Tris base (pH 7.5) and 52 µL of 50 mM DTT were added, the reaction mixture was gently vortexed, and the solution was incubated at 65 °C for 30 min. Next, 1.6 µL of 4-vinylpyridine was added, and the reaction was mixed and kept in the dark for ~20 min at RT. Finally, the reaction mixture was diluted with at least 2 volumes of the HPLC buffer A and was subjected to LC separation with fraction collection, using the same gradient described for sub-fractionation of the venom. For comparison, a blank run was conducted using the reduction–alkylation solution but without including the peptide.
4.3.1. De Novo Sequencing
An aliquot of the HPLC sub-fraction 22.5 was dried in a SpeedVac, and subsequently reduced and alkylated in vapor using 50% CH3CN, 1% 2-methylaziridine and 2% trimethylphosphine in 100 mM ammonium bicarbonate pH 8.4 (v:v), for 90 min at room temperature (RT). The alkylation vapor was removed, and the sample was dissolved in 10 μL of 0.5% acetic acid. Aliquots were loaded onto a 0.2 × 25 cm Pepswift EasySpray column. The sample was eluted at a flow rate of 1μL/min with a gradient (solvent A: 0.5% acetic acid; solvent B: 90% (v/v) CH3CN in 0.5% acetic acid) of 0% B-45%B in 30 min, and 45–100% B in 5 min, a spray voltage of 2.5kV on an Easy nLC-1000 nanoUHPLC coupled with an Orbitrap Elite mass spectrometer. MS1 scans were acquired at 120,000 resolution (@ 400 m/z). MS2 was acquired for the top 4 precursors that carry at least 2 charges using the following settings: 4 microscans, 2 m/z isolation window, and a target value of 5 × 104 ions. Each precursor was subjected to ETD and HCD fragmentation using the following conditions: 15,000 resolution (@ 400 m/z), ETD using 80 ms ion reaction time, and HCD using 27% normalized collision energy. The measured mass deviates from the theoretical mass by 4.1 ppm and is within the mass error of the instrument. The sequence was obtained by manual de novo sequencing.
4.4. Peptide Synthesis
The synthesis of PeIA has been previously described [
16]. The novel peptides described in this study, At1.1 and At1.2 (the analog with an L16I mutation), AtIA[des1Z], and AtIA[des18W], were synthesized using solid-phase Fmoc peptide chemistry with an AAPPTec Apex 396 automated peptide synthesizer (Louisville, KY, USA). Peptides were constructed either on Fmoc-Trp(Boc)-Wang resin (substitution: 0.29 mmol/g) or Fmoc-Cys(Acm)-Wang resin (substitution: 0.62 mm/g). All standard amino acids were purchased from AAPPTec and the side-chain protection for the following amino acids was as follows: Ser:
O-
tert-butyl, Asn, His, Cys: trityl (Trt) and/or Cys: acetamidomethyl (Acm) (Acm), Glu:
tert-butyl (tBu), Trp: tert-butyloxycarbonyl (Boc). Initially, At1.1 and At1.2 were synthesized with all Cys protected with Trt groups to facilitate random folding after the cleavage of the peptide from the resin. The peptides were synthesized at 50 μmol scale. Coupling activation was achieved with 1 eq of 0.4 M benzotriazol-1-yl- oxytripyrrolidinophosphonium hexafluorophosphate and 2 eq of 2 M
N,
N-diisopropylethyl amine in
N-methyl-2-pyrrolidone as the solvent. For each coupling reaction, a 10-fold excess of amino acid was used, and the reaction was carried out for 60 min. Fmoc deprotection was performed for 20 min with 20% (
v/
v) piperidine in dimethylformamide.
4.4.1. Peptide Cleavage and Purification
The peptides were cleaved from the resin using Reagent K, consisting of trifluoroacetic acid (TFA)/phenol/H2O/thioanisol/ethanedithiol (82.5/5/5/5/2.5 by volume) (Fisher Scientific (Hampton, NH, USA), Millipore Sigma (Burlington, MA, USA), Millipore Sigma and Acros Organics (Geel, Belgium), respectively). Next, the cleavage mixture was filtered and precipitated with 10 mL of cold methyl-tert-butyl ether (MTBE; Fisher Scientific (Hampton, NH, USA)). The crude peptide was then precipitated by centrifugation at 7000× g for 7 min and washed twice with 10 mL cold MTBE. The crude peptide was diluted with 50 mL of 10% CH3CN in buffer A and purified by reverse-phase (RP) HPLC using a semi-preparative C18 HiChrom column (218TP510, 250 × 10 mm, 5 μm particle size) eluted with a linear gradient ranging from 20 to 50% buffer B90 in 30 min with a flow rate 4 mL/min for semi-preparative purification. The following HPLC buffers were used: 0.1% (v/v) TFA in water (buffer A) and 0.1% TFA (v/v) in 90% aqueous CH3CN (Fisher Scientific) (v/v) (buffer B90). The eluent was monitored at 220/280 nm. The purity of the peptides was assessed by analytical C18 Vydac RP-HPLC (218TP54, 250 × 4.6 mm, 5 μm particle size) using the same gradient as described above with a flow rate 1 mL/min. Peptides were quantified by UV absorbance at 280 nm, using an extinction coefficient (ε) value of 5500 M−1·cm−1 For the analogs with all Cys protected with Trt: out of 53 mg of the resin, 2800 nmols of the linear At1.1 and 2000 nmols of At1.2 out of 50 mg (both with 95%) purity were prepared.
4.4.2. Oxidative Folding of At1.1 and At1.2 in the Presence of Reduced and Oxidized Glutathione
Eight hundred nanomoles of linear At1.11 in 5.6 mL of HPLC buffer mixture was added to a solution containing 20 mL of 0.2 M Tris·HCl (pH 7.5) plus 0.2 mM EDTA, 0.5 mL of 10 mM reduced and 4 mL of 10 mM oxidized glutathione, and 10.4 mL of water. The final peptide concentration in the folding mixture was 20 μM. The folding reaction was conducted for 3.5 h and quenched with formic acid to a final concentration of 8%. The quenched reaction mixture was then separated by RP-HPLC using a semi-preparative C18 column and a linear gradient ranging from 20% to 50% of buffer B90 in 30 min with a flow rate of 4 mL/min. The eluent was monitored by absorbance at 220/280 nm. The purity of the folded peptide was assessed by an analytical C18 Vydac RP-HPLC using the gradient described above, with a flow rate 1 mL/min. Pure fully folded At1.11 was quantified by absorbance at 280 nm, as described for the linear peptide. Next, 270 nmols of the major product was isolated in 34% yield with 96% purity. The same method was used to obtain fully folded At1.2 in 33% yield and 96% purity. The identities of At1.1 and At1.2 were confirmed by MALDI MS (calculated monoisotopic MH+1: 1949.90, determined monoisotopic MH+1: 1949.71 for At1.1 and MH+1: 1949.91, determined monoisotopic MH+1: 1949.71 for At1.2, at the University of Utah Mass Spectrometry and Proteomics Core Facility.
4.4.3. Peptide Co-Elution
Solutions of the synthetic peptides At1.1 and At1.2 were prepared in 10% CH
3CN in buffer A. Peptides were analyzed individually on the analytical C18 column using HPLC with a gradient ranging from 20% to 50% buffer B in 30 min, with a flow rate of 1 mL/min. Their elution times were compared to the native material. Since At1.1 had the same retention time as the native material, they were mixed and re-injected on the column to produce a uniform peak (
Figure 4).
4.4.4. Synthesis of the Globular At1.1 (αCtx-AtIA)
To confirm that the active form of the peptide is globular, At1.1 was resynthesized as described above with Cys3 and Cys9 Trt-protected and Cys4 and Cys17 protected with Acm. The cleavage protocol was the same as described earlier. For the purification of the linear peptide, it was applied to a semi-preparative C18 Vydac column (218TP510, 250 × 10 mm, 5 μm particle size) and eluted with a linear gradient ranging from 20 to 50% buffer B90 in 30 min with a flow rate 4 mL/min. The HPLC buffers were 0.1% (v/v) TFA in water (buffer A) and 0.1% TFA (v/v) in 90% aqueous CH3CN for the semi-preparative purification. Out of 24 mg of the resin, 500 nmol of the linear peptide was obtained, with purity ranging from 79 to 87%.
4.4.5. First Disulfide Bond Formation (3Cys-9Cys)
Three hundred nanomoles of linear At1.1 [4,17Cys(Acm)] in 1.5 4.4 mL of HPLC buffers mixture was added to a solution containing 7.5 mL of 0.2 M Tris·HCl (pH 7.5) plus 0.2 mM EDTA, 1.5 mL of 10 mM 1:1 mixture of the reduced and oxidized glutathione, and 4.5 mL of water. The final peptide concentration in the folding mixture was 20 μM. The folding reaction was conducted for 2 h and quenched with formic acid to a final concentration of 8%. The quenched reaction mixture was then separated by RP-HPLC using a semi-preparative C18 column and a linear gradient ranging from 20% to 50% of buffer B90 in 30 min with a flow rate of 4 mL/min. The monocyclic At1.1 was obtained with 63% yield and with 90% purity. The identity of the peptide was confirmed by MALDI MS: calculated monoisotopic MH+1: 2093.81, determined MH+1: 2093.87. Additional masses representing sodium and potassium adducts were also present: M+Na+: 2115.88 and M+K+: 2131.85.
4.4.6. Second Disulfide Bond Formation (4Cys-17Cys)
Removal of the acetamidomethyl groups and the second disulfide bridge formation was accomplished by iodine oxidation. First, 10 mg of iodine (Acros Organics, Antwerp, Belgium) was added to 5 mL of CH3CN and stirred until completely dissolved. Then, 15 mL of nanopure water was added, followed by 0.6 mL of TFA. The monocyclic At1.1 solution of 192 nmol in 475 µL of the HPLC buffer was dripped into 475 µL of the iodine solution (prepared as described above) and allowed to react for 5 min at room temperature. The reaction was quenched by adding 50 µL of 1 M freshly prepared ascorbic acid (0.176 g, 1 mmol; Research Products International, Mount Prospect, IL, USA) solution in water until the reaction mixture became transparent. The reaction was then diluted to 5 mL with buffer A and subsequently purified by RP-HPLC using a semipreparative C18-column and a linear gradient ranging from 20% to 50% of buffer B90 in 30 min with a flow rate of 4 mL/min. The peptide was quantified by UV absorbance at 280 nm, using an extinction coefficient (ε) value of 5500 M−1·cm−1. At1.1 (αCtx-AtIA) was obtained in 25% yield and with 98% purity. The identity of the peptide was confirmed by ESI MS: calculated monoisotopic MH+1: 1949.90, determined monoisotopic MH+1: 1949.71
4.4.7. One-Pot, Two-Step Folding of AtIA[des18W] and AtIA[des1Z]
To 5 mL of the solution of 1000 nmol of crude linear AtIA[des18W] in 90% glacial acetic acid, 5% water, and 5% methanol in a small Erlenmeyer flask equipped with a magnetic stirring bar, a few drops of 40 mM iodine solution in methanol were added until a pale-yellow color appeared. Next, 5 mL of 50 mM HCl was added, followed by 2 mL of 40 mM iodine solution in methanol. The reaction was stirred for 1h and then quenched with 1 M freshly prepared ascorbic acid, diluted with water to 35 mL, and purified by RP-HPLC using C18 column and a gradient ranging from 10% to 40% in 30 min with a 4 mL/min flow. The peptide was quantified by analytical HPLC by comparing the area under the peak of the peptide vs. a reference peptide. The same method was used to synthesize AtIA[des1Z]. AtIA[des18W] was obtained in 42% with 98% purity and AtIA[des1Z] in 13% with 97% as determined by the analytical RP-HPLC. The identities of the peptides were confirmed by ESI MS: AtIA[des18W] calculated monoisotopic MH+1: 1763.65, determined monoisotopic MH+1: 1763.64; AtIA[des1Z] calculated monoisotopic MH+1: 1838.69, determined monoisotopic MH+1: 1838.69.
4.5. Constellation Pharmacology Assay
We used 37–42 days old transgenic mice (Tg(Calca-EGFP)104Gsat) obtained from our collaborator (Ginty Lab, Harvard University) in all experiments. Primary cell cultures from mouse dorsal root ganglia (DRG) were prepared as previously described [
45]. Briefly, lumbar DRG, L1-L6, were removed, trypsinized, and plated on a 24-well poly-D-lysine-coated plate. The plated cells were placed in a 95% O2−5% CO
2 incubator at 37 °C overnight in a minimum essential medium (MEM) supplied with fetal bovine serum (FBS) and supplements. Then, 10–12 h after the primary cell culture preparation, the dissociated cells were loaded with 1mM Fura-2-AM dye for 1 h at 37 °C before the Constellation Pharmacology experiments were performed. In experimental trials, individual responses from ~1000 neurons were simultaneously monitored under 4× objective.
During the venom components screening, aliquots of HPLC venom fractions from C. ateralbus were assayed for activity on DRG neurons that were exposed to two 15 s depolarizing control stimuli (20 mM KCl) that temporarily elevated [Ca2+]i as measured by calcium imaging. The protocol included several control pulses of 20 mM KCl, followed by incubation with a venom fraction. The responses from the venom fractions during and after the incubation of venom were categorized as direct responses (direct calcium influx during venom fraction incubation) and indirect effects (amplification calcium response elicited by 20 mM KCl following the venom fraction incubation) that indicated pharmacological activity.
During the synthetic peptide characterization, for the mouse DRG Constellation Pharmacology experiments, intracellular calcium changes were monitored for response to the application of pharmacological stimuli 100 μM allyl isothiocyanate (AITC), 300 nM capsaicin, 40 mM KCl, 400 μM menthol, and 1 μM RIIIJ, 5 μM PNU-120596 (PNU), 1mM acetylcholine (ACh), 10 μM of αCtx-PeIA, 10 μM synthetic αCtx-AtIA, αCtx-AtIA[des1Z] and αCtx-AtIA[des18W]. Two experimental protocols were used to test the bioactivity effects of the synthetic αCtx-AtIA. Protocols one and two used different cocktails to characterize the bioactivity of conotoxins:
Protocol one used a cocktail containing 1mM ACh and 10 μM of peptide of interest: αCtx-PeIA, αCtx-AtIA, αCtx-AtIA[des1Z], or αCtx-AtIA[des18W].
Protocol two used two different cocktails, one containing 1mM ACh + 5 μM PNU, and other containing 1mM ACh + 5 μM PNU + 10 μM of peptide of interest: αCtx-PeIA, αCtx-AtIA, αCtx-AtIA[des1Z] or αCtx-AtIA[des18W], or 200 nM of ArIB[V11L;V16D].
Both protocols one and two began with 7 min intervals then shifted to 5 min intervals (after the 20 mM KCl application) when neuronal cell classification pharmacological stimuli were applied.
4.5.1. Constellation Pharmacology Data Analysis
A single calcium imaging experiment measured the intracellular calcium concentration (ICC) for 100s–1000s of cells at 2 s intervals over a time course up to 2 h. During the experiment specific modifications were made to the cell culture to test for induced changes in ICC. The ICC values for each cell across the entire experiment were referred to as the calcium response phenotype (CRP) of the cell. These responses were plotted as a time series in
Figure 6,
Figure 7,
Figure 8,
Figure 10, and
Figure 11. Then, 1mM ACh or 1mM ACh+PNU was added at regular intervals to induce a CRP. The CRP is very specific for each cell but was conditional upon a complex set of cellular variables. Cells that showed a repeated strong response to ACh were assumed to be expressing ACh receptors other than α7 (primarily α3β4 and α6β4). These cells were used to test the cellular response to ACh only. Cells that showed no response to ACh alone but showed a repeated strong response to ACh+PNU were assumed to be expressing the α7 nicotinic receptor (CHRNA7) and were used to test the cellular response to stimulation of that receptor. We tested for activity of a compound of interest by incubating the cell culture in the compound between ACh/ACh+PNU pulses and estimating the change in the phenotype of the specified cells. A change in the ICC that occurred while the compound was in the well was referred to as a direct effect (DE). A change that altered the CRP after incubation was referred to as an indirect effect (IDE).
The degree of inhibition induced by pre-incubation with each peptide was estimated by comparing a full model with simple linear model. The block fraction was estimated as the 1-f/l, where f was the predicted magnitude of response in the full model (accounting for the effects of the peptide) and l was the predicted magnitude of response in a null linear trend model. These values were plotted as cumulative distributions in
Figure 6,
Figure 7,
Figure 8,
Figure 10, and
Figure 11.
4.5.2. Estimation of Indirect Effects
Each experiment had a series of ACh- and/or ACh+PNU-induced CRPs. The magnitude of the CRP was measured as the maximum area under the curve for any 15 s interval. The deviation of this response after a test compound incubation was used to estimate the effect of the compound. We estimated this effect on each neuron using a multiple linear regression (the lm function in R) with lm(auc15 ~ linear + compound), where auc15 was the maximum area under the curve for any 15 s interval for each ACh pulse, linear was the trend coded as sequential integers, and compound was an indicator variable for the CRP immediately following incubation with the compound of interest. The Tstat values from the coefficients matrix were taken as estimates of the magnitude and direction of the indirect effects (IDE).
4.5.3. Controlling for Multiple Tests Estimating Significance
Each experiment gave results for 100s–1000s of cells. To control for false positives and set appropriate thresholds for significance, we used Monte Carlo simulations. Each simulation generated random numbers drawn from a normal distribution with mean and standard deviations of the actual data. The Tstats were estimated for all cells and recorded. This was considered a null distribution. The Tstat estimates from 100 Monte Carlo simulations were used to establish the 0.05 experiment-wide threshold for single-cell significance. The counts of cells that exceed this threshold were used to define the contingency tables in
Figure 6,
Figure 7,
Figure 8,
Figure 10, and
Figure 11.
4.6. Biological Activity in Mice
Fraction 22 and all sub-fractions were assayed in Swiss Webster mice. Each sample was resuspended in 12 µL of saline solution (0.9% NaCl) before intracranial injection into mice (15 days old—weight average 8.90 ± 0.84 g). The injection was performed using a 1 mL insulin syringe, and the same volume of normal saline solution was injected into the control mice. The mice were observed for at least 1 h, as previously described [
46]. The same protocol was followed for fraction 22.5 and the synthetic peptide αCtx-AtIA (i.c. injections in 15- to 18-day old mice (weight 9.07 ± 0.74 g)).
4.7. Oocyte Electrophysiology
Frogs were purchased from Xenopus1 (Dexter, MI, USA) and maintained by University of Utah personnel in an AAALAC accredited facility. Oocytes were obtained from frogs anesthetized with 0.4% wt/vol Tricaine-S (Thermo Fisher Scientific, Waltham, MA, USA) and were sacrificed after removal of the ovarian lobes.
Methods for the preparation of cRNA constructs for expression of nAChRs in
X. laevis oocytes have been previously described [
47]. Clones for expression of mouse α3, β4, α6 and α7 subunits were provided by J. Stitzel, University of Colorado, Boulder, CO, USA. An α6/α3 chimera was used to enable expression of nAChRs with the α6 subunit [
27]. Briefly, stage IV–V oocytes were injected with equal ratios of cRNAs encoding nAChR subunits and subjected to two-electrode voltage clamp (TEVC) electrophysiology 1–5 days after injection. The oocyte membranes were clamped at a holding potential of −70 mV, and 200 μM acetylcholine (ACh) was applied at 60 s intervals for a duration of 1s. For the assessment of peptide activity, the oocytes were continuously perfused with frog saline (control solution) and pulsed with ACh until a stable baseline response was observed, then the saline was switched to a solution containing the peptide and the ACh responses were monitored for changes in amplitude. The ACh responses in the presence of peptide were normalized to the average of three responses in control solution. Peptides were applied in this manner for concentrations ≥1 μM. For concentrations >1 μM, the peptides were applied in a static bath for 5 min and normalized to the ACh response after a 5 min bath application of the control solution.
4.7.1. Statistical Analysis for Electrophysiology
All statistical analyses were performed using Prism 8 (GraphPad Software, San Diego, CA, USA). The estimated IC50 values for inhibition of ACh-evoked currents by the peptides were obtained by nonlinear regression using a four-parameter logistic equation and presented with the corresponding 95% CI to evaluate the precision of the IC50 estimate. The error bars represent the SD of the data obtained at each concentration and are provided to assess the variance of the data.