Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance
Abstract
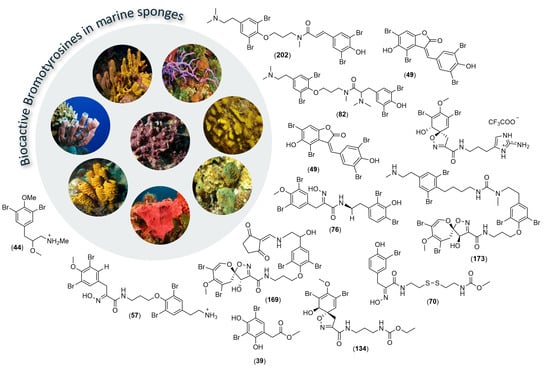
:1. Introduction
2. Bromotyrosine Chemodiversity
2.1. Low-Molecular-Weight Metabolites
2.1.1. Simple Bromotyrosines
- Subereamides, tyrokeradines, and derivatives
- Ma’edamines C–F
- Other simple bromotyrosines
2.1.2. Oximes
- Aplysamines, aplyzanzines, and purpuramines
- Psammaplins C–D and O–P
- Psammaplins and tyrokeradines
2.1.3. Hemibastadin Derivatives
2.2. High-Molecular-Weight Metabolites
2.2.1. Anomoians A–F and Ianthelliformisamine C
2.2.2. Spirocyclohexadienylisoxazolines
Mono-spirocyclohexadienylisoxazolines
- (+/−)-Purealidin R, lacunosins A–B, subereamollines A–B, and purpuroceratic acids A–B
- Araplysillins, ianthesin E, purealidins, purpurealidins, and pseudoceratinamides
- Ceratinadins A–D and (−)-aerophobin-2
- (+/−)-Purealin and desaminopurealin
Bis-spirocyclohexadienylisoxazolines
2.2.3. Spirooxepinisoxazolines
- Psammaplysin derivatives
- Ceratinadins E–F
2.2.4. Oximes
- Tyrokeradines A–D
- Araplysillin VI and purealins C–D
- Psammaplin A and its derivatives
- Pseudoceroximes A–E and pseudoceramines A–D
2.2.5. Bastadin Derivatives
2.2.6. Other Derivatives
3. Biological Activities
3.1. Antibacterial Activities
3.2. Antifungal Activities
3.3. Cytotoxic Activities
3.4. Antiparasitic Activities
3.5. Other Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- König, G.M.; Wright, A.D. Agelorins A and B, and 11-Epi-Fistularin-3, Three New Antibacterial Fistularin-3 Derivatives from the Tropical Marine Sponge Agelas oroides. Heterocycles 1993, 36, 1351–1358. [Google Scholar]
- Weiss, B.; Ebel, R.; Elbrächter, M.; Kirchner, M.; Proksch, P. Defense Metabolites from the Marine Sponge Verongia aerophoba. Biochem. Syst. Ecol. 1996, 24, 1–12. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Stonik, V.A.; Alcolado, P.; Elyakov, Y.B. Comparative Study of the Halogenated Tyrosine Derivatives from Demospongiae (Porifera). Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 68, 481–484. [Google Scholar] [CrossRef]
- Peng, J.; Hamann, M.T. Chapter 2. The Marine Bromotyrosine Derivatives. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2005; Volume 61, pp. 59–262. [Google Scholar]
- Carney, J.R.; Rinehart, K.L. Biosynthesis of Brominated Tyrosine Metabolites by Aplysina fistularis. J. Nat. Prod. 1995, 58, 971–985. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.L.; Chan, Y.S.; Yin, C.M.; Dan, X.L.; Wang, H.X.; Fang, E.F.; Lam, S.K.; Ngai, P.H.K. Antifungal and Antiviral Products of Marine Organisms. Appl. Microbiol. Biotechnol. 2014, 98, 3475–3494. [Google Scholar] [CrossRef] [PubMed]
- Gómez Archila, L.G.; Zapata, W.; Galeano, E.; Martínez, A.; Díaz, F.J.; Rugeles, M.T. Bromotyrosine Derivatives from Marine Sponges Inhibit the HIV-1 Replication in vitro. Vitae Medellín 2014, 21, 114–125. [Google Scholar] [CrossRef]
- Wittine, K.; Saftić, L.; Peršurić, Ž.; Kraljević Pavelić, S. Novel Antiretroviral Structures from Marine Organisms. Molecules 2019, 24, 3486. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs 2019, 17, 115. [Google Scholar] [CrossRef]
- Jing, Q.; Hu, X.; Ma, Y.; Mu, J.; Liu, W.; Xu, F.; Li, Z.; Bai, J.; Hua, H.; Li, D. Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification. Mar. Drugs 2019, 17, 384. [Google Scholar] [CrossRef]
- Sabir, M.; Tan, Y.Y.; Aris, A.; Mani, A.R. The Role of Endogenous Bromotyrosine in Health and Disease. Free Radic. Res. 2019, 53, 1019–1034. [Google Scholar] [CrossRef]
- Geahchan, S.; Ehrlich, H.; Rahman, A. A Short Overview: Marine Resources as Potential Interventions for the Omicron SARS-CoV-2 Variant. COVID 2022, 2, 501–512. [Google Scholar] [CrossRef]
- Geahchan, S.; Ehrlich, H.; Rahman, M.A. The Anti-Viral Applications of Marine Resources for COVID-19 Treatment: An Overview. Mar. Drugs 2021, 19, 409. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W. The Importance of Traditional Chinese Medicine in the Intervention and Treatment of HIV While Considering Its Safety and Efficacy. Curr. HIV Res. 2023, 21, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Uemura, D. Bioactive Marine Metabolites from Okinawan Waters. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products (Part O); Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 57–100. [Google Scholar]
- Reverter, M.; Perez, T.; Ereskovsky, A.V.; Banaigs, B. Secondary Metabolome Variability and Inducible Chemical Defenses in the Mediterranean Sponge Aplysina cavernicola. J. Chem. Ecol. 2016, 42, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, F.; Lindel, T. Synthesis of Oximinotyrosine-Derived Marine Natural Products. Synthesis 2010, 2010, 181–204. [Google Scholar]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A–C, Antibacterial Bromotyrosine-Derived Metabolites from the Marine Sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Tilvi, S.; Rodrigues, C.; Naik, C.G.; Parameswaran, P.S.; Wahidhulla, S. New Bromotyrosine Alkaloids from the Marine Sponge Psammaplysilla purpurea. Tetrahedron 2004, 60, 10207–10215. [Google Scholar] [CrossRef]
- Tian, L.-W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.A.; Wellington, C.; Hooper, J.N.A.; Quinn, R.J. ApoE Secretion Modulating Bromotyrosine Derivative from the Australian Marine Sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 2014, 24, 3537–3540. [Google Scholar] [CrossRef]
- Tian, L.-W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.; Wellington, C.; Hooper, J.N.A.; Quinn, R.J. Aplysinellamides A-C, Bromotyrosine-Derived Metabolites from an Australian Aplysinella sp. Marine Sponge. J. Nat. Prod. 2014, 77, 1210–1214. [Google Scholar] [CrossRef]
- Miguel-Gordo, M.; Gegunde, S.; Calabro, K.; Jennings, L.K.; Alfonso, A.; Genta-Jouve, G.; Vacelet, J.; Botana, L.M.; Thomas, O.P. Bromotryptamine and Bromotyramine Derivatives from the Tropical Southwestern Pacific Sponge Narrabeena nigra. Mar. Drugs 2019, 17, 319. [Google Scholar] [CrossRef]
- Salim, A.A.; Khalil, Z.G.; Capon, R.J. Structural and Stereochemical Investigations into Bromotyrosine-Derived Metabolites from Southern Australian Marine Sponges, Pseudoceratina spp. Tetrahedron 2012, 68, 9802–9807. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Fechner, G.A.; Boyle, A.; Simpson, M.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Cheung, T.; Chen, H. Aplysamine 6, an Alkaloidal Inhibitor of Isoprenylcysteine Carboxyl Methyltransferase from the Sponge Pseudoceratina sp. J. Nat. Prod. 2008, 71, 1066–1067. [Google Scholar] [CrossRef]
- Ragini, K.; Fromont, J.; Piggott, A.M.; Karuso, P. Enantiodivergence in the Biosynthesis of Bromotyrosine Alkaloids from Sponges? J. Nat. Prod. 2017, 80, 215–219. [Google Scholar] [CrossRef]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial Bromotyrosine Derivatives from the Australian Marine Sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef]
- Yin, S.; Davis, R.A.; Shelper, T.; Sykes, M.L.; Avery, V.M.; Elofsson, M.; Sundin, C.; Quinn, R.J. Pseudoceramines A–D, New Antibacterial Bromotyrosine Alkaloids from the Marine Sponge Pseudoceratina sp. Org. Biomol. Chem. 2011, 9, 6755–6760. [Google Scholar] [CrossRef]
- Kurimoto, S.; Seino, S.; Fromont, J.; Kobayashi, J.; Kubota, T. Ma’edamines C and D, New Bromotyrosine Alkaloids Possessing a Unique Tetrasubstituted Pyridinium Moiety from an Okinawan Marine Sponge Suberea sp. Org. Lett. 2019, 21, 8824–8826. [Google Scholar] [CrossRef]
- Teruya, T.; Iwasaki, A.; Suenaga, K. 20-N-Methylpurpuramine E: New Bromotyrosine-Derived Metabolite from Okinawan Marine Sponge Pseudoceratina purpurea. Bull. Chem. Soc. Jpn. 2008, 81, 1026–1027. [Google Scholar] [CrossRef]
- Kon, Y.; Kubota, T.; Shibazaki, A.; Gonoi, T.; Kobayashi, J. Ceratinadins A-C, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Bioorg. Med. Chem. Lett. 2010, 20, 4569–4572. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, S.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Ōmura, S.; Kobayashi, J.; Kubota, T. Ceratinadins E and F, New Bromotyrosine Alkaloids from an Okinawan Marine Sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Tyrokeradines A and B, New Bromotyrosine Alkaloids with an Imidazolyl-Quinolinone Moiety from a Verongida Sponge. Bioorg. Med. Chem. Lett. 2009, 19, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.F.; de Oliveira, J.H.; Galetti, F.C.; de Souza, A.O.; Silva, C.L.; Hajdu, E.; Peixinho, S.; Berlinck, R.G. Antimycobacterial Brominated Metabolites from Two Species of Marine Sponges. Planta Med. 2006, 72, 437–441. [Google Scholar] [CrossRef]
- Tintillier, F.; Moriou, C.; Petek, S.; Fauchon, M.; Hellio, C.; Saulnier, D.; Ekins, M.; Hooper, J.N.A.; Al-Mourabit, A.; Debitus, C. Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina sp. Mar. Drugs 2020, 18, 272. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Toullec, J.; Besson, M.; Soulet, S.; Schmitt, N.; Petek, S.; Lecchini, D.; Debitus, C.; Al-Mourabit, A. Bioactive Bromotyrosine-Derived Alkaloids from the Polynesian Sponge Suberea ianthelliformis. Mar. Drugs 2018, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watase, S.; Mukai, H.; Fromont, J.; Kobayashi, J. Tyrokeradines C-F, New Bromotyrosine Alkaloids from the Verongid Sponges. Chem. Pharm. Bull. 2012, 60, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Han, S.; Lee, H.-S.; Kang, J.S.; Yun, J.; Sim, C.J.; Shin, H.J.; Lee, J.S. Cytotoxic Psammaplysin Analogues from a Suberea sp. Marine Sponge and the Role of the Spirooxepinisoxazoline in Their Activity. J. Nat. Prod. 2013, 76, 1731–1736. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kobayashi, H.; van Soest, R.W.M.; Fusetani, N. Novel Bromotyrosine Derivatives That Inhibit Growth of the Fish Pathogenic Bacterium Aeromonas hydrophila, from a Marine Sponge Hexadella sp. J. Org. Chem. 2005, 70, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Chen, Y.-C.; El-Shazly, M.; Du, Y.-C.; Su, C.-W.; Tsao, C.-W.; Liu, L.-L.; Chou, Y.; Chang, W.-B.; Su, Y.-D. Towards the Small and the Beautiful: A Small Dibromotyrosine Derivative from Pseudoceratina sp. Sponge Exhibits Potent Apoptotic Effect through Targeting IKK/NFκB Signaling Pathway. Mar. Drugs 2013, 11, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi, L.; Schmitz, F.J. Brominated Tyrosine Metabolites from an Unidentified Sponge. J. Org. Chem. 1987, 52, 3584–3586. [Google Scholar] [CrossRef]
- Oluwabusola, E.T.; Tabudravu, J.N.; Al Maqbali, K.S.; Annang, F.; Pérez-Moreno, G.; Reyes, F.; Jaspars, M. Antiparasitic Activity of Bromotyrosine Alkaloids and New Analogues Isolated from the Fijian Marine Sponge Aplysinella rhax. Chem. Biodivers. 2020, 17, e2000335. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, S.; Okamoto, A.; Seino, S.; Fromont, J.; Kobayashi, J.; Kubota, T. Ma’edamines E and F, Rare Bromotyrosine Alkaloids Possessing a 1,2,3,5-Tetrasubstituted Pyridinium Moiety from an Okinawan Marine Sponge Suberea sp. Tetrahedron Lett. 2022, 103, 153985. [Google Scholar] [CrossRef]
- Ichiba, T.; Scheuer, P.J.; Kelly-Borges, M. Three Bromotyrosine Derivatives, One Terminating in an Unprecedented Diketocyclopentenylidene Enamine. J. Org. Chem. 1993, 58, 4149–4150. [Google Scholar] [CrossRef]
- Tran, T.D.; Pham, N.B.; Fechner, G.; Hooper, J.N.A.; Quinn, R.J. Bromotyrosine Alkaloids from the Australian Marine Sponge Pseudoceratina verrucosa. J. Nat. Prod. 2013, 76, 516–523. [Google Scholar] [CrossRef]
- Sirimangkalakitti, N.; Yokoya, M.; Chamni, S.; Chanvorachote, P.; Plubrukrn, A.; Saito, N.; Suwanborirux, K. Synthesis and Absolute Configuration of Acanthodendrilline, a New Cytotoxic Bromotyrosine Alkaloid from the Thai Marine Sponge Acanthodendrilla sp. Chem. Pharm. Bull. 2016, 64, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Hellio, C.; Pavia, H.; Stensen, W.; Stensvåg, K.; Svendsen, J.-S.; Haug, T.; Svenson, J. Antifouling Compounds from the Sub-Arctic Ascidian Synoicum pulmonaria: Synoxazolidinones A and C, Pulmonarins A and B, and Synthetic Analogues. J. Nat. Prod. 2014, 77, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.E.; Roth, G.P.; Hoffman, J.K.; Divlianska, D.B.; Pechter, D.; Sennett, S.H.; Guzmán, E.A.; Linley, P.; McCarthy, P.J.; Pitts, T.P. Isolation, Synthesis, and Biological Activity of Aphrocallistin, an Adenine-Substituted Bromotyramine Metabolite from the Hexactinellida Sponge Aphrocallistes beatrix. J. Nat. Prod. 2009, 72, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Göthel, Q.; Sirirak, T.; Köck, M. Bromotyrosine-Derived Alkaloids from the Caribbean Sponge Aplysina lacunosa. Beilstein J. Org. Chem. 2015, 11, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shoer, M.I.; Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Habib, A.-A.M. Bioactive Brominated Metabolites from the Red Sea Sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.M.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Müller, W.E.G.; Proksch, P. From Anti-Fouling to Biofilm Inhibition: New Cytotoxic Secondary Metabolites from Two Indonesian Agelas Sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida. Mar. Drugs 2011, 9, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, Y.; Ge, H.; Jiao, W.-H.; Zhang, Z.; Lin, H.-W. Pseudoceroximes A–E and Pseudocerolides A–E–Bromotyrosine Derivatives from a Pseudoceratina sp. Marine Sponge Collected in the South China Sea. Eur. J. Org. Chem. 2020, 2020, 2583–2591. [Google Scholar] [CrossRef]
- Gotsbacher, M.P.; Karuso, P. New Antimicrobial Bromotyrosine Analogues from the Sponge Pseudoceratina purpurea and Its Predator Tylodina corticalis. Mar. Drugs 2015, 13, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Parrish, S.M.; Yoshida, W.Y.; Yip, M.L.R.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-Derived Metabolites from an Indonesian Marine Sponge in the Family Aplysinellidae (Order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Tilvi, S.; D’Souza, L. Identifying the Related Compounds Using Electrospray Ionization Tandem Mass Spectrometry: Bromotyrosine Alkaloids from Marine Sponge Psammaplysilla purpurea. Eur. J. Mass Spectrom. Chichester Engl. 2012, 18, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Crews, P. Novel Marine Sponge Derived Amino Acids 13. Additional Psammaplin Derivatives from Psammaplysilla purpurea. Tetrahedron 1991, 47, 2097–2102. [Google Scholar] [CrossRef]
- Park, Y.; Liu, Y.; Hong, J.; Lee, C.-O.; Cho, H.; Kim, D.-K.; Im, K.S.; Jung, J.H. New Bromotyrosine Derivatives from an Association of Two Sponges, Jaspis wondoensis and Poecillastra wondoensis. J. Nat. Prod. 2003, 66, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.B.; Lee, Y.M.; Dang, H.T.; Hong, J.; Lee, C.-O.; Jung, J.H. Cytotoxic Bromotyrosine Derivatives from a Two-Sponge Association of Jaspis sp. and Poecillastra sp. Bioorg. Med. Chem. Lett. 2008, 18, 6414–6418. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hwang, J.-H.; Kanamoto, A.; Nagai, H.; Takagi, M.; Shin-ya, K. JBIR-44, a New Bromotyrosine Compound from a Marine Sponge Psammaplysilla purpurea. J. Antibiot. 2009, 62, 393–395. [Google Scholar] [CrossRef]
- Kernan, M.R.; Cambie, R.C.; Bergquist, P.R. Chemistry of Sponges, VIII. Anomoian A, a Bromotyrosine Derivative from Anomoiantbella popeae. J. Nat. Prod. 1990, 53, 720–723. [Google Scholar] [CrossRef]
- Tarazona, G.; Santamaría, G.; Cruz, P.G.; Fernández, R.; Pérez, M.; Martínez-Leal, J.F.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Cytotoxic Anomoian B and Aplyzanzine B, New Bromotyrosine Alkaloids from Indonesian Sponges. ACS Omega 2017, 2, 3494–3501. [Google Scholar] [CrossRef]
- Kobayashi, J.; Honma, K.; Sasaki, T.; Tsuda, M. Purealidins J-R, New Bromotyrosine Alkaloids from the Okinawan Marine Sponge Psammaplysilla purea. Chem. Pharm. Bull. 1995, 43, 403–407. [Google Scholar] [CrossRef]
- Salib, M.N.; Jamison, M.T.; Molinski, T.F. Bromo-Spiroisoxazoline Alkaloids, Including an Isoserine Peptide, from the Caribbean Marine Sponge Aplysina lacunosa. J. Nat. Prod. 2020, 83, 1532–1540. [Google Scholar] [CrossRef]
- Kijjoa, A.; Bessa, J.; Wattanadilok, R.; Sawangwong, P.; Nascimento, M.S.J.; Pedro, M.; Silva, A.M.S.; Eaton, G.; van Soest, R.; Herz, W. Dibromotyrosine Derivatives, a Maleimide, Aplysamine-2 and Other Constituents of the Marine Sponge Pseudoceratina purpurea. Z. Für Naturforschung B 2005, 60, 904–908. [Google Scholar] [CrossRef]
- Ma, K.; Yang, Y.; Deng, Z.; de Voogd, N.J.; Proksch, P.; Lin, W. Two New Bromotyrosine Derivatives from the Marine Sponge Pseudoceratina sp. Chem. Biodivers. 2008, 5, 1313–1320. [Google Scholar] [CrossRef]
- Kalaitzis, J.A.; Leone, P.D.A.; Hooper, J.N.A.; Quinn, R.J. Ianthesine E, a New Bromotyrosine-Derived Metabolite from the Great Barrier Reef Sponge Pseudoceratina sp. Nat. Prod. Res. 2008, 22, 1257–1263. [Google Scholar] [CrossRef]
- Mani, L.; Jullian, V.; Mourkazel, B.; Valentin, A.; Dubois, J.; Cresteil, T.; Folcher, E.; Hooper, J.N.A.; Erpenbeck, D.; Aalbersberg, W. New Antiplasmodial Bromotyrosine Derivatives from Suberea ianthelliformis Lendenfeld, 1888. Chem. Biodivers. 2012, 9, 1436–1451. [Google Scholar] [CrossRef]
- Motti, C.A.; Freckelton, M.L.; Tapiolas, D.M.; Willis, R.H. FTICR-MS and LC-UV/MS-SPE-NMR Applications for the Rapid Dereplication of a Crude Extract from the Sponge Ianthella flabelliformis. J. Nat. Prod. 2009, 72, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ankudey, F.J.; Kiprof, P.; Stromquist, E.R.; Chang, L.C. New Bioactive Bromotyrosine-Derived Alkaloid from a Marine Sponge Aplysinella sp. Planta Med. 2008, 74, 555–559. [Google Scholar] [CrossRef]
- Hernández-Guerrero, C.J.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Cytotoxic Dibromotyrosine-Derived Metabolites from the Sponge Aplysina Gerardogreeni. Bioorg. Med. Chem. 2007, 15, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Nicacio, K.J.; Ióca, L.P.; Fróes, A.M.; Leomil, L.; Appolinario, L.R.; Thompson, C.C.; Thompson, F.L.; Ferreira, A.G.; Williams, D.E.; Andersen, R.J. Cultures of the Marine Bacterium Pseudovibrio denitrificans Ab134 Produce Bromotyrosine-Derived Alkaloids Previously Only Isolated from Marine Sponges. J. Nat. Prod. 2017, 80, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kunze, K.; Niemann, H.; Ueberlein, S.; Schulze, R.; Ehrlich, H.; Brunner, E.; Proksch, P.; Pée, K.-H.V. Brominated Skeletal Components of the Marine Demosponges, Aplysina cavernicola and Ianthella basta: Analytical and Biochemical Investigations. Mar. Drugs 2013, 11, 1271–1287. [Google Scholar] [CrossRef]
- Thompson, J.E.; Barrow, K.D.; Faulkner, D.J. Localization of Two Brominated Metabolites, Aerothionin and Homoaerothionin, in Spherulous Cells of the Marine Sponge Aplysina fistularis (=Verongia Thiona). Acta Zool. 1983, 64, 199–210. [Google Scholar] [CrossRef]
- Maru, N.; Koyama, T.; Ohno, O.; Yamada, K.; Uemura, D. Sunabedine, a Novel Toxic Bromotyrosine-Derivative Alkaloid from Okinawan Sponge, Order Verongida. Heterocycles 2010, 82, 371–375. [Google Scholar] [CrossRef]
- Feng, Y.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Camp, D.; Quinn, R.J. Pseudoceratinazole A: A Novel Bromotyrosine Alkaloid from the Australian Sponge Pseudoceratina sp. Tetrahedron Lett. 2010, 51, 4847–4850. [Google Scholar] [CrossRef]
- Moriou, C.; Lacroix, D.; Petek, S.; El-Demerdash, A.; Trepos, R.; Leu, T.M.; Florean, C.; Diederich, M.; Hellio, C.; Debitus, C. Bioactive Bromotyrosine Derivatives from the Pacific Marine Sponge Suberea clavata (Pulitzer-Finali, 1982). Mar. Drugs 2021, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y.; Groweiss, A.; Carmely, S.; Kinamoni, Z.; Czarkie, D.; Rotem, M. Recent Research in Marine Natural Products from the Red Sea. Pure Appl. Chem. 1982, 54, 1995–2010. [Google Scholar] [CrossRef]
- Roll, D.M.; Chang, C.W.J.; Scheuer, P.J.; Gray, G.A.; Shoolery, J.N.; Matsumoto, G.K.; Van Duyne, G.D.; Clardy, J. Structure of the Psammaplysins. J. Am. Chem. Soc. 1985, 107, 2916–2920. [Google Scholar] [CrossRef]
- Mándi, A.; Mudianta, I.W.; Kurtán, T.; Garson, M.J. Absolute Configuration and Conformational Study of Psammaplysins A and B from the Balinese Marine Sponge Aplysinella strongylata. J. Nat. Prod. 2015, 78, 2051–2056. [Google Scholar] [CrossRef]
- Liu, S.; Fu, X.; Schmitz, F.J.; Kelly-Borges, M. Psammaplysin F, a New Bromotyrosine Derivative from a Sponge, Aplysinella sp. J. Nat. Prod. 1997, 60, 614–615. [Google Scholar] [CrossRef]
- Xu, M.; Andrews, K.T.; Birrell, G.W.; Tran, T.L.; Camp, D.; Davis, R.A.; Quinn, R.J. Psammaplysin H, a New Antimalarial Bromotyrosine Alkaloid from a Marine Sponge of the Genus Pseudoceratina. Bioorg. Med. Chem. Lett. 2011, 21, 846–848. [Google Scholar] [CrossRef]
- Wright, A.D.; Schupp, P.J.; Schrör, J.-P.; Engemann, A.; Rohde, S.; Kelman, D.; de Voogd, N.; Carroll, A.; Motti, C.A. Twilight Zone Sponges from Guam Yield Theonellin Isocyanate and Psammaplysins I and J. J. Nat. Prod. 2012, 75, 502–506. [Google Scholar] [CrossRef]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin Derivatives from the Balinese Marine Sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic Psammaplysin Analogues from the Verongid Red Sea Sponge Aplysinella Species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, H.-S.; Seo, Y.; Rho, J.-R.; Cho, K.W.; Paul, V.J. New Bromotyrosine Metabolites from the Sponge Aplysinella rhax. Tetrahedron 2000, 56, 9071–9077. [Google Scholar] [CrossRef]
- Thoms, C.; Schupp, P.J. Activated Chemical Defense in Marine Sponges—A Case Study on Aplysinella rhax. J. Chem. Ecol. 2008, 34, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Marmann, A.; Lin, W.; Proksch, P. Sponge Derived Bromotyrosines: Structural Diversity through Natural Combinatorial Chemistry. Nat. Prod. Commun. 2015, 10, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Calcul, L.; Inman, W.D.; Morris, A.A.; Tenney, K.; Ratnam, J.; McKerrow, J.H.; Valeriote, F.A.; Crews, P. Additional Insights on the Bastadins: Isolation of Analogues from the Sponge Ianthella Cf. reticulata and Exploration of the Oxime Configurations. J. Nat. Prod. 2010, 73, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lousberg, R.J.J.C.; Weiss, U.; Salemink, C.A.; Arnone, A.; Merlini, L.; Nasini, G. The Structure of Cercosporin, a Naturally Occurring Quinone. J. Chem. Soc. Chem. Commun. 1971, 22, 1463. [Google Scholar] [CrossRef]
- Van Wyk, A.W.W.; Zuck, K.M.; McKee, T.C. Lithothamnin A, the First Bastadin-like Metabolite from the Red Alga Lithothamnion fragilissimum. J. Nat. Prod. 2011, 74, 1275–1280. [Google Scholar] [CrossRef]
- Gartshore, C.J.; Salib, M.N.; Renshaw, A.A.; Molinski, T.F. Isolation of Bastadin-6-O-Sulfate and Expedient Purifications of Bastadins-4, -5 and -6 from Extracts of Ianthella basta. Fitoterapia 2018, 126, 16–21. [Google Scholar] [CrossRef]
- Campos, P.-E.; Wolfender, J.-L.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Amphimedonoic Acid and Psammaplysene E, Novel Brominated Alkaloids from Amphimedon sp. Tetrahedron Lett. 2017, 58, 3901–3904. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Quinn, R.J. Psammaplysenes C and D, Cytotoxic Alkaloids from Psammoclemma sp. J. Nat. Prod. 2007, 70, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; Soleiman Dehkordi, E.; Mehdinezhad, N.; Ashour, M.L.; Mohammadi Pour, P. Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef]
- Jang, J.-H.; van Soest, R.W.M.; Fusetani, N.; Matsunaga, S. Pseudoceratins A and B, Antifungal Bicyclic Bromotyrosine-Derived Metabolites from the Marine Sponge Pseudoceratina purpurea. J. Org. Chem. 2007, 72, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sorres, J.; Martin, M.-T.; Petek, S.; Levaique, H.; Cresteil, T.; Ramos, S.; Thoison, O.; Debitus, C.; Al-Mourabit, A. Pipestelides A–C: Cyclodepsipeptides from the Pacific Marine Sponge Pipestela candelabra. J. Nat. Prod. 2012, 75, 759–763. [Google Scholar] [CrossRef] [PubMed]
































| Compound | Biological Activity | Ref. |
|---|---|---|
| (−)-aerophobin-2 (130) | Active against B. subtilis (IC50 = 2.1 µM) a | [23] |
| aplysamine-8 (58) | Active against E. coli (MIC = 125 µM) and against S. aureus (MIC = 31 µM) | [53] |
| ceratinines J–M (52–55) | Low activity against methicillin-resistant S. aureus (MIC > 20 µM) b | [52] |
| 11-N-cyano-11-N-methylmoloka’iamine (22) | Moderate activity against the fish pathogen bacteria A. hydrophila (ZI 8.0 mm at 100 µg) | [38] |
| ianthelliformisamine A (7) | Selective activity on P. aeruginosa, (MIC = 35 µM) and 77% inhibition of S. aureus at 175 µM | [18] |
| ianthelliformisamine B (8) | Minor inhibition of P. aeruginosa (80% at 87.5 µM) | [18] |
| ianthelliformisamine C (79) | Activity against P. aeruginosa (MIC = 17.5 µM) and S. aureus (MIC = 8.75 µM) | [18] |
| 13-ketohemifistularin-3 (143) | Low activity against methicillin-resistant S. aureus (MIC > 20 µM) b | [52] |
| kuchinoenamine (29) | Moderate activity against pathogen fish bacteria A. hydrophila (IZ 8.0 mm at 100 µg) | [38] |
| 11-N-methylmoloka’iamine (30) | Moderate activity against pathogen fish bacteria A. hydrophila (ZI 7.5 mm at 100 µg) | [38] |
| pseudoceramine B (210) | Active against Y. pseudotuberculosis (IC50 = 40 µM) | [27] |
| pseudoceratin A (231) | Moderate activity against E. coli (IZ 7 mm at 10 µg/disk), B. subtilis (IZ 7.0 mm at 10 µg/disk), and S. aureus (ZI 6.5 mm at 10 µg/disk) | [95] |
| pseudoceratin B (232) | Moderate activity against E. coli (IZ 8 mm at 10 µg/disk), B. subtilis (IZ 8.0 mm at 10 µg/disk), and S. aureus (IZ 7.0 mm at 10 µg/disk) | [95] |
| pseudocerolide B (48) | Low activity against methicillin-resistant S. aureus (MIC > 20 µM) b | [52] |
| pseudocerolide C (49) | Active against methicillin-resistant S. aureus (MIC = 7.1 µM) b | [52] |
| pseudocerolide D (50) | Moderate activity against methicillin-resistant S. aureus (MIC = 12.8 µM) b | [52] |
| pseudocerolide E (51) | Moderate activity against methicillin-resistant S. aureus (MIC = 11.2 µM) b | [52] |
| pseudoceroxime A (204) | Active against methicillin-resistant S. aureus (MIC = 6.6 µM) b | [52] |
| pseudoceroxime B (205) | Active against methicillin-resistant S. aureus (MIC = 5.2 µM) b | [52] |
| pseudoceroxime C (206) | Low activity against methicillin-resistant S. aureus (MIC = 17.2 µM) b | [52] |
| pseudoceroxime D (207) | Low activity against methicillin-resistant S. aureus (MIC = 1.5 µM) b | [52] |
| pseudoceroxime E (208) | Low activity against methicillin-resistant S. aureus (MIC = 10.6 µM) b | [52] |
| (+/−)-purealin (131) | Active against B. subtilis (IC50 = 2.3 et 3.8 µM) and against S. aureus (IC50 = 3.8 and 0.83 µM) | [23] |
| (−)-purealin B (123) | Active against B. subtilis (IC50 = 3.4 and 3.8 µM) a | [23] |
| purpurealidin B (105) | Activity against E. coli (IC50 > 12 µM), S. aureus (IC50 = 10 µM), and V. cholerae (IC50 = 25 µM); low activity against S. flexineri (IC50 = 100 µM) | [19] |
| subereamolline A (100) | IZ 3 mm against S. aureus c | [49] |
| subereaphenol B (39) | IZ 5 mm against S. aureus c | [49] |
| suberein-1 (146) | Active against V. aesturianus (MIC = 0.01 µM) and R. littoralis (MIC = 1 µM) d | [76] |
| suberein-2 (147) | Active against V. aesturianus (MIC = 0.01 µM) and E. coli (MIC = 0.01 µM) d | [76] |
| tyrokeradine B (190) | Low inhibitory activity against M. luteus and S. aureus (MIC = 25 µM) | [32] |
| Compound | Biological Activity | Ref. |
|---|---|---|
| ceratinadin A (126) | Active against C. neoformans (MIC = 4 µM) and C. albicans (MIC = 2 µM) a | [30] |
| ceratinadin B (127) | Active against C. neoformans (MIC = 8 µM) and C. albicans (MIC = 4 µM) a | [30] |
| ceratinines J–M (52–55) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| 13-ketohemifistularin-3 (143) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| pseudoceratin A (231) | Growth inhibition of a mutant of S. cerevisiae (IZ 6.5 mm at 10 µg/disk); good activity against C. albicans (IZ 8 mm at 10 µg/disk | [95] |
| pseudoceratin B (232) | Growth inhibition of a mutant of S. cerevisiae (IZ 6.5 mm at 10 µg/disk; good activity against C. albicans (IZ 6.5 mm at 10 µg/disk) | [95] |
| pseudocerolide B (48) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| pseudocerolide C (49) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| pseudocerolide D (50) | Low activity against C. albicans (MIC = 16.0 µM) b | [52] |
| pseudocerolide E (51) | Low activity against C. albicans (MIC = 19.2 µM) b | [52] |
| pseudoceroxime A (204) | Active against C. albicans (MIC = 11.9 µM) b | [52] |
| pseudoceroxime B (205) | Active against C. albicans (MIC = 13.0 µM) b | [52] |
| pseudoceroxime C (206) | Low activity against C. albicans (MIC = 19.8 µM) b | [52] |
| pseudoceroxime D (207) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| pseudoceroxime E (208) | Low activity against C. albicans (MIC > 20 µM) b | [52] |
| Compound | Biological Activity | Ref. |
|---|---|---|
| acanthodendrilline (32) | Activity against lung cancer cells H292 (IC50 = 58.5 µM) without cytotoxicity on healthy cells HaCaT (IC50 > 400 µM) | [45] |
| anomoian B (81) | Significant activity against lung A549 (IC50 = 5.1 µM), colorectal HT-29 (IC50 = 3.2 µM), and breast MDA-MB231 (IC50 = 5.3 µM) cancer cells | [61] |
| anomoian C (82) | Active against human squamous cell carcinoma KB cancer cells (28% inhibition at 10 µM and 15% inhibition at 1 µM) | [35] |
| anomoian D (83) | Active against human squamous cell carcinoma KB cancer cells (29% inhibition at 10 µM and 17% inhibition at 1 µM) | [35] |
| anomoian E (84) | Active against human squamous cell carcinoma KB cancer cells (82% inhibition at 10 µM and 6% inhibition at 1 µM) | [35] |
| anomoian F (85) | Active against human squamous cell carcinoma KB cancer cells (100% inhibition at 10 µM and 20% inhibition at 1 µM) | [35] |
| aplysamine-6 (78) | IC50 = 14 µM against isoprenylcysteine carboxyl methyltransferase Icmt | [64] |
| aplysinine B (38) | Moderate activity against breast cancer cells (MCF-7, IC50 = 25.8 µM), human fibroblasts FS4LTM (IC50 = 77.5 µM), and squamous cell carcinoma KB31 (IC50 = 32.2 µM) | [48] |
| aplyzanzine B (230) | Significant activity against lung A549 (IC50 = 6.1 µM), colorectal HT-29 (IC50 = 1.6 µM), and breast MDA-MB231 (IC50 = 7.8 µM) cancer cells | [61] |
| agelanesin A (41) | IC50 = 9.55 µM against the mouse lymphoma cell L5178Y | [50] |
| agelanesin C (42) | IC50 = 16.76 µM against the mouse lymphoma cell L5178Y | [50] |
| araplysillin VII (118) | Cytotoxic against cancerous and healthy cell lines | [54] |
| araplysillin IX (120) | Cytotoxic against cancerous and healthy cell lines | [54] |
| araplysillin-N20-formamide (112) | IC50 = 3.8 µM against breast cancer cells MCF-7 | [54] |
| cyclobispsammaplin A (203) | Active against cancer cells of the lung A549 (ED50 = 1.95 µM), ovary SK-OV-3 (ED50 = 1.21 µM), skin SKMEL-2 (ED50 = 1.14 µM), nervous system XF-498 (ED50 = 2.88 µM), and colon HCT-15 (ED50 = 3.82 µM) | [58] |
| 19-hydroxypsammaplysin X (186) | Active against cancer cells of the colon HCT-15 (GI50 = 3.5 µM), prostate PC-3 (GI50 = 2.1 µM), kidney ACHN (GI50 = 2.5 µM), breast MDA-MB-231 (GI50 = 0.8 µM), stomach NUGC-3 (GI50 = 4.0 µM), and lung NCI-H23 (GI50 = 3.5 µM) | [37] |
| 19-hydroxypsammaplysin E (183) | Active against colorectal cancer cell line HCT-15 (IC50 = 3.8 µM), prostate cancer cell line PC-3 (IC50 = 1.4 µM), renal cell carcinoma cell line ACHN (IC50 = 2.3 µM), breast cancer cell line MDA-MB-231 (IC50 = 0.51 µM), gastric cancer cell line NUGC-3 (IC50 = 2.3 µM), and non-small cell lung cancer cell line NCI-H23 (IC50 = 3.6 µM) | [84] |
| 19-hydroxypsammaplysin Z (182) | Active against the triple-negative breast cancer MDA-MB-231 (IC50 = 13.2 µM), cervical carcinoma HeLa (IC50 = 17.6 µM), and colorectal carcinoma HCT 116 (IC50 = 7.0 µM) | [84] |
| 20-N-methylpurpuramine E (63) | Low cytotoxicity on cervical cancer cells HeLa S3 (IC50 = 4.3 µM) | [29] |
| JBIR-44 (76) | Activity against HeLa cervical cancer cells (IC50 = 3.7 µM) | [59] |
| pipestelide A (233) | 100% inhibition against oral carcinoma KB cells at 10 and 1 µM (IC50 = 0.10 µM) | [96] |
| psammaplysin X (184) | Active against cancer cells of the colon HCT-15 (GI50 = 3.3 µM), prostate PC-3, (GI50 = 2.3 µM), kidney ACHN (GI50 = 3.3 µM), breast MDA-MB-231 (GI50 = 1.2 µM), stomach NUGC-3 (GI50 = 3.5 µM), and lung NCI-H23 (GI50 = 6.4 µM) | [37] |
| pseudoceralidinone A (31) | Active against prostate cancer cells PC3 (IC50 = 4.9 µM) | [44] |
| pseudoceroxime B (205) | Active at 40 µM on human glioma cells U87MG (IC50 = 17.7 µM) and U251 (IC50 = 14.1 µM) | [52] |
| pseudoceroxime D (207) | Active at 40 µM on human glioma cells U87MG (IC50 = 25.3 µM) and U251 (IC50 = 20.5 µM) | [52] |
| psammaplysene D (224) | Active against human squamous cell carcinoma KB cancer cells (100% inhibition at 10 µM and 95% inhibition at 1 µM), and 90% inhibition of DNA methyltransferase 1 DNMT1 enzyme | [93] |
| psammaplysene F (226) | Active against human squamous cell carcinoma KB cancer cells (73% inhibition at 10 µM and 20% inhibition at 1 µM), and 90% inhibition of DNA methyltransferase 1 DNMT1 enzyme | [35] |
| psammaplysene G (227) | Active against human squamous cell carcinoma KB cancer cells (75% inhibition at 10 µM and 17% inhibition at 1 µM), and 90% inhibition of DNA methyltransferase 1 DNMT1 enzyme | [35] |
| psammaplysin A (154) | Active against the triple-negative breast cancer MDA-MB-231 (IC50 = 3.90 µM), cervical carcinoma HeLa (IC50 = 8.50 µM), colorectal carcinoma HCT 116 (IC50 = 5.1 µM), colorectal cancer cell line HCT-15 (IC50 = 3.9 µM), prostate cancer cell line PC-3 (IC50 = 6.9 µM), renal cell carcinoma cell line ACHN (IC50 = 5.1 µM), breast cancer cell line MDA-MB-231 (IC50 = 4.3 µM), gastric cancer cell line NUGC-3 (IC50 = 3.8 µM), and non-small cell lung cancer cell line NCI-H23 (IC50 = 12.4 µM) | [78,84] |
| psammaplysin E (183) | Active against colorectal cancer cell line HCT-15 (IC50 = 3.8 µM), prostate cancer cell line PC-3 (IC50 = 3.7 µM), renal cell carcinoma cell line ACHN (IC50 = 10.3 µM), breast cancer cell line MDA-MB-231 (IC50 = 3.9 µM), gastric cancer cell line NUGC-3 (IC50 = 4.0 µM), and non-small cell lung cancer cell line NCI-H23 (IC50 = 7.0 µM) | [84] |
| psammaplysin F (156) | Active against embryonic kidney cell line HEK293 (IC50 = 10.9 µM) and hepatocellular carcinoma cell line HEpG2 (IC50 = 3.7 µM) | [80] |
| psammaplysin G (155) | Active against embryonic kidney cell line HEK293 (IC50 = 18.7 µM) and hepatocellular carcinoma cell line HEpG2 (IC50 = 17.4 µM) | [26,80] |
| psammaplin X (184) | Active against colorectal cancer cell line HCT-15 (IC50 = 3.3 µM), prostate cancer cell line PC-3 (IC50 = 2.3 µM), renal cell carcinoma cell line ACHN (IC50 = 3.3 µM), breast cancer cell line MDA-MB-231 (IC50 = 1.2 µM), gastric cancer cell line NUGC-3 (IC50 = 3.5 µM), and non-small cell lung cancer cell line NCI-H23 (IC50 = 6.4 µM) | [84] |
| psammaplysin Z (181) | Active against the triple-negative breast cancer MDA-MB-231 (IC50 = 19.4 µM), cervical carcinoma HeLa (IC50 = 22.2 µM), and colorectal carcinoma HCT 116 (IC50 = 8.2 µM) | [84] |
| purealidin T (109) | Low activity (IC50 >10 µM) against colon HCT-8, liver Bel-7402, stomach BGC-823, lung A549, and ovarian A2780 cancer cells | [65] |
| purealidin U (110) | Low activity (IC50 > 10 µM) against colon HCT-8, liver Bel-7402, stomach BGC-823, lung A549, and ovarian A2780 cancer cells | [65] |
| purpuramine M (59) | Growth inhibition of ovarian cancer cells (A2780S, IC50 = 20 µM), its resistant variant (A2780SCP5, IC50 = 40 µM), and glioma U251MG (IC50 = 50 µM) | [54] |
| purpuramine N (60) | Low inhibition (IC50 > 50 µM) against the growth of ovarian cancer cells A2780S, its resistant variant A2780SCP5, and glioma U251MG | [54] |
| sunabedine (144) | IC50 = 39 µM against mouse cancer cells B16 | [74] |
| Compound | Biological Activity | Ref. |
|---|---|---|
| Araplysillin-N20-formamide (112) | Activity against chloroquine-resistant and -sensitive P. falciparum strains: FcB-1 (IC50 = 3.6 µM) and 3D7 (IC50 = 7 µM) with an IS of 1.4 between FcB-1 and Vero cells | [67] |
| ceratinadin E (187) | Active against drug-resistant P. falciparum strain (IC50 = 1.05 µM) and drug-sensitive P. falciparum strain (IC50 = 0.77 µM) | [30] |
| fistularin-3 (139) | Very weak activity against L. panamensis (8% inhibition), P. falciparum (11% inhibition), and T. cruzi (6% inhibition) | [51] |
| 11-hydroxyaerothionin (138) | Very weak activity against P. falciparum (8% inhibition) and L. panamensis intracellular amastigotes (13% inhibition) | [51] |
| 19-hydroxypsammaplysin E (183) | Active against 3D7 strains of P. falciparum (IC50 = 6.4 µM) | [83] |
| psammaplin A (196) | Moderate activity against Tulahuen C4 strains of T. cruzi (IC50 = 30 µM) and 3D7 strains of P. falciparum (IC50 = 60 µM) | [41] |
| psammaplin D (70) | Moderate activity against Tulahuen C4 strains of T. cruzi (IC50 = 43 µM) and 3D7 strains of P. falciparum (IC50 = 67 µM) | [41] |
| psammaplysin F (156) | Active against P. falciparum chloroquine-resistant strain Dd2 (IC50 = 1.4 µM) and 3D7 strains of P. falciparum (IC50 = 0.87 µM) | [26] |
| psammaplysin G (155) | 98% inhibition of P. falciparum chloroquine-resistant strain (Dd2) at 40 µM; IC50 of 5.23 µM against P. falciparum chloroquine-sensitive strain (3D7) | [26,81] |
| psammaplysin H (157) | IC50 = 0.41 µM against the chloroquine-sensitive P. falciparum strain (3D7). | [26,81] |
| purpurealidin B (105) | Weakly active against L. panamensis (2% inhibition) and P. falciparum (23% inhibition) | [51] |
| purealidin R (96) | Weakly active against T. cruzi (2% inhibition) and P. falciparum (7% inhibition) | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira Montenegro, P.; Pham, G.N.; Abdoul-Latif, F.M.; Taffin-de-Givenchy, E.; Mehiri, M. Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance. Mar. Drugs 2024, 22, 132. https://doi.org/10.3390/md22030132
Ferreira Montenegro P, Pham GN, Abdoul-Latif FM, Taffin-de-Givenchy E, Mehiri M. Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance. Marine Drugs. 2024; 22(3):132. https://doi.org/10.3390/md22030132
Chicago/Turabian StyleFerreira Montenegro, Paula, Giang Nam Pham, Fatouma Mohamed Abdoul-Latif, Elisabeth Taffin-de-Givenchy, and Mohamed Mehiri. 2024. "Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance" Marine Drugs 22, no. 3: 132. https://doi.org/10.3390/md22030132
APA StyleFerreira Montenegro, P., Pham, G. N., Abdoul-Latif, F. M., Taffin-de-Givenchy, E., & Mehiri, M. (2024). Marine Bromotyrosine Derivatives in Spotlight: Bringing Discoveries and Biological Significance. Marine Drugs, 22(3), 132. https://doi.org/10.3390/md22030132