Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
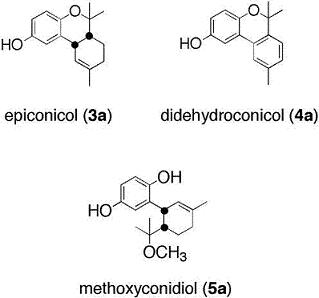
2.1. Chemistry
2.2. Biological activities
2.2.1. Antibacterial assays
2.2.2. Antiproliferative activities
3. Experimental Section
3.1. Chemistry
Procedures for the preparation of compounds 4a–5a and 4b
Methoxyconidiol (5a)
Didehydroconicol (4a)
O-methyldidehydroconicol (4b)
3.2. Biological activities
3.2.1. Assays on sea urchin eggs
3.2.1.1. Extraction of methoxyconidiol from treated sea urchin eggs
3.2.1.1.1. Preparation of cytoplasmic extracts
3.2.1.1.2. Chemical extraction of methoxyconidiol and HPLC analysis
3.2.1.2. Cell culture and cytotoxicity assay
4. Conclusions
Acknowledgements
References and Notes
- Fadli, M; Aracil, JM; Jeanty, G; Banaigs, B; Francisco, C. Novel meroterpenoids from Cystoseira mediterranea: Use of the crown-gall bioassay as a primary screen for lipophilic antineoplastic agents. J Nat Prod 1991, 54, 261–264. [Google Scholar]
- Cueto, M; MacMillan, JB; Jensen, PR; Fenical, W. Tropolactones A–D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry 2006, 67, 1826–1831. [Google Scholar]
- Sheu, JH; Su, JH; Sung, PJ; Wang, GH; Dai, CF. Novel meroditerpenoid-related metabolites from the formosan soft coral Nephthea chabrolii. J Nat Prod 2004, 67, 2048–2052. [Google Scholar]
- Mitome, H; Nagasawa, T; Miyaoka, H; Yamada, Y; Van Soest, RWM. Dactyloquinones C, D and E novel sesquiterpenoid quinones, from the Okinawan marine sponge Dactylospongia elegans. Tetrahedron Lett 2002, 58, 1693–1996. [Google Scholar]
- Garrido, L; Zubia, E; Ortega, MJ; Salva, J. New meroterpenoids from the ascidian Aplidium conicum. J Nat Prod 2002, 65, 1328–1331. [Google Scholar]
- Shen, YC; Chen, CY; Kuo, YH. New sesquiterpene hydroquinones from a taiwanese marine sponge Hippospongia metachromia. J Nat Prod 2001, 64, 801–803. [Google Scholar]
- Aiello, A; Fattorusso, E; Luciano, P; Menna, M; Esposito, G; Iuvone, T; Pala, D. Conicaquinones A and B, two novel cytotoxic terpene quinones from the Mediterranean ascidian Aplidium conicum. Eur J Org Chem 2003, 5, 898–900. [Google Scholar]
- de Menezes, JE; Machado, FEA; Lemos, TLG; Silveira, ER; Braz Filho, R; Pessoa, ODL. Sesquiterpenes and a Phenylpropanoid from Cordia trichotoma. Zeis Natur 2004, 59, 19–22. [Google Scholar]
- Marion, F; Williams, DE; Patrick, BO; Hollander, I; Mallon, R; Kim, SC; Roll, DM; Feldberg, L; Van Soest, R; Andersen, R. Liphagal, a selective inhibitor of PI3 kinase α isolated from the sponge Aka coralliphaga: Structure elucidation and biomimetic synthesis. J Org Lett 2006, 8, 321–324. [Google Scholar]
- Williams, DE; Telliez, JB; Liu, J; Tahir, A; van Soest, R; Andersen, RJ. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the Indonesian marine sponge Acanthodendrilla sp. J Nat Prod 2004, 67, 2127–2129. [Google Scholar]
- Simon-Levert, A; Arrault, A; Bontemps-Subielos, N; Canal, C; Banaigs, B. Meroterpenes from the ascidian Aplidium aff densum. J Nat Prod 2005, 68, 1412–1415. [Google Scholar]
- Simon-Levert, A; Aze, A; Bontemps-Subielos, N; Banaigs, B; Genevière, AM. Antimitotic activity of methoxyconidiol, a meroterpene isolated from an ascidian. Chem Biol Inter 2007, 168, 106–116. [Google Scholar]
- Cruz-Almanza, R; Pérez-Flores, F; Lemini, C. Synthesis of 2H-1-benzopyrans from aryllithium and α,β-unsaturated aldehydes. Heterocycles 1994, 37, 759–774. [Google Scholar]
- Benslimane, AF; Pouchus, YF; Le Boterf, J; Verbist, JF. Substances cytotoxiques et antibacteriennes de l’ascidie Aplidium antillense. J Nat Prod 1988, 51, 582–583. [Google Scholar]
- Benslimane, AF; Pouchus, YF; Verbist, JF; Petit, JY; Brion, JD; Welin, L. Antiinflammatory mechanism of cordiachromene A action. J Clin Pharmacol 1995, 35, 298–301. [Google Scholar]
- National Commitee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobicaly Approved Standards. In NCCLS Document M7-A4, 4th ed; Vilanova, PA, USA, 1997.
- Caroll, AR; Bowden, BF; Coll, JC. Studies of Australian Ascidians III. A new THC derivatice from the ascidian Synocium castellatum. Aust J Chem 1993, 46, 1079–1083. [Google Scholar]
- Collas, P; Poccia, D. Methods for studying in vitro assembly of male pronuclei using oocyte extracts from marine invertebrates: Sea urchins and surf clams. Methods Cell Biol 1998, 53, 417–452. [Google Scholar]
- Semenova, MN; Kiselyov, A; Semenov, VV. Sea urchin embryo as a model organism for the rapid functional screening of tubulin modulators. BioTechnniques 2006, 40, 765–774. [Google Scholar]
- Gupta, RS. Species-specific differences in toxicity of antimitotic agents toward cultured mammalian cells. J Natl Cancer Inst 1985, 74, 159–164. [Google Scholar]
- Barthomeuf, C; Debiton, E; Mshvildadze, V; Kemertelidze, E; Balansard, G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med 2002, 68, 672–675. [Google Scholar]




| Compounds | IC50 (μM) | |
|---|---|---|
| P. lividus | S. granularis | |
| 3a | >25.0 | 9.8 ± 1.3 |
| 3b | >25.0 | >25.0 |
| 4a | 11.3 ± 0.8 | >25.0 |
| 4b | >25.0 | >25.0 |
| 5a | 4.3 ± 0.1 | 0.8 ± 0.0 |
| 5b | >25.0 | >25.0 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simon-Levert, A.; Menniti, C.; Soulère, L.; Genevière, A.-M.; Barthomeuf, C.; Banaigs, B.; Witczak, A. Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity. Mar. Drugs 2010, 8, 347-358. https://doi.org/10.3390/md8020347
Simon-Levert A, Menniti C, Soulère L, Genevière A-M, Barthomeuf C, Banaigs B, Witczak A. Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity. Marine Drugs. 2010; 8(2):347-358. https://doi.org/10.3390/md8020347
Chicago/Turabian StyleSimon-Levert, Annabel, Christophe Menniti, Laurent Soulère, Anne-Marie Genevière, Chantal Barthomeuf, Bernard Banaigs, and Anne Witczak. 2010. "Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity" Marine Drugs 8, no. 2: 347-358. https://doi.org/10.3390/md8020347
APA StyleSimon-Levert, A., Menniti, C., Soulère, L., Genevière, A. -M., Barthomeuf, C., Banaigs, B., & Witczak, A. (2010). Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity. Marine Drugs, 8(2), 347-358. https://doi.org/10.3390/md8020347