Coccolithophores: Functional Biodiversity, Enzymes and Bioprospecting
Abstract
:1. Introduction
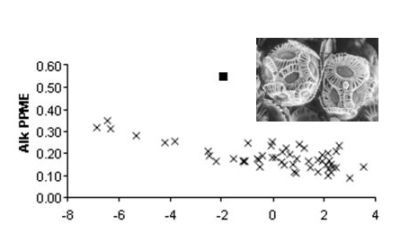
2. Results and Discussion
Enzyme Activity Assays
3. Experimental Section
3.1. Strain Culture and Harvesting
3.2. Enzyme Activity Assays
3.3. Statistical Analysis
4. Conclusions
Acknowledgments
- Samples Availability: Strains with CCMP prefix are available for purchase from The Provasoli-Guillard National Center for Culture of Marine Phytoplankton at Bigelow, USA ( https://ccmp.bigelow.org/). All other Emiliania huxleyi strains are available from the Roscoff Culture Collection ( http://www.sb-roscoff.fr/Phyto/RCC/). Coccolithovirus strain EhV-86 is available from the PML Virus Collection, contact Mike Allen ([email protected]).
References
- Watanabe, Y; Martini, JEJ; Ohmoto, H. Geochemical evidence for terrestrial ecosystems 2.6 billion years ago. Nature 2000, 408, 574–578. [Google Scholar]
- Caporaso, JG; Kuczynski, J; Stombaugh, J; Bittinger, K; Bushman, FD; Costello, EK; Fierer, N; Pena, AG; Goodrich, JK; Gordon, JI; Huttley, GA; Kelley, ST; Knights, D; Koenig, JE; Ley, RE; Lozupone, CA; McDonald, D; Muegge, BD; Pirrung, M; Reeder, J; Sevinsky, JR; Tumbaugh, PJ; Walters, WA; Widmann, J; Yatsunenko, T; Zaneveld, J; Knight, R. Qiime allows analysis of high-throughput community sequencing data. Nat Methods 2010, 7, 335–336. [Google Scholar]
- Hamady, M; Walker, JJ; Harris, JK; Gold, NJ; Knight, R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 2008, 5, 235–237. [Google Scholar]
- Sharon, I; Tzahor, S; Williamson, S; Shmoish, M; Man-Aharonovich, D; Rusch, DB; Yooseph, S; Zeidner, G; Golden, SS; Mackey, SR; Adir, N; Weingart, U; Horn, D; Venter, JC; Mandel-Gutfreund, Y; Beja, O. Viral photosynthetic reaction center genes and transcripts in the marine environment. Isme J 2007, 1, 492–501. [Google Scholar]
- Nealson, KH; Venter, JC. Metagenomics and the global ocean survey: What’s in it for us, and why should we care. Isme J 2007, 1, 185–187. [Google Scholar]
- Holligan, PM; Fernandez, E; Aiken, J; Balch, WM; Boyd, P; Burkill, PH; Finch, M; Groom, SB; Malin, G; Muller, K; Purdie, DA; Robinson, C; Trees, CC; Turner, SM; Vanderwal, P. A biogeochemical study of the coccolithophore, emiliania huxleyi, in the north-atlantic. Glob Biogeochem Cycle 1993, 7, 879–900. [Google Scholar]
- Holligan, PM; Viollier, M; Harbour, DS; Camus, P; Champagnephilippe, M. Satellite and ship studies of coccolithophore production along a continental-shelf edge. Nature 1983, 304, 339–342. [Google Scholar]
- Brown, CW; Yoder, JA. Coccolithophorid blooms in the global ocean. J Geophys Res-Oceans 1994, 99, 7467–7482. [Google Scholar]
- Westbroek, P; Brown, CW; Vanbleijswijk, J; Brownlee, C; Brummer, GJ; Conte, M; Egge, J; Fernandez, E; Jordan, R; Knappertsbusch, M; Stefels, J; Veldhuis, M; Vanderwal, P; Young, J. A model system approach to biological climate forcing—the example of emiliania huxleyi. Glob Planet Change 1993, 8, 27–46. [Google Scholar]
- Wilson, WH; Schroeder, DC; Allen, MJ; Holden, MTG; Parkhill, J; Barrell, BG; Churcher, C; Hamlin, N; Mungall, K; Norbertczak, H; Quail, MA; Price, C; Rabbinowitsch, E; Walker, D; Craigon, M; Roy, D; Ghazal, P. Complete genome sequence and lytic phase transcription profile of a coccolithovirus. Science 2005, 309, 1090–1092. [Google Scholar]
- Suttle, CA. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar]
- Suttle, CA. Marine viruses--major players in the global ecosystem. Nat Rev 2007, 5, 801–812. [Google Scholar]
- Allen, MJ; Martinez-Martinez, J; Schroeder, DC; Somerfield, PJ; Wilson, WH. Use of microarrays to assess viral diversity: From genotype to phenotype. Environ Microbiol 2007, 9, 971–982. [Google Scholar]
- von Dassow, P; Ogata, H; Probert, I; Wincker, P; Da Silva, C; Audic, S; Claverie, JM; de Vargas, C. Transcriptome analysis of functional differentiation between haploid and diploid cells of emiliania huxleyi, a globally significant photosynthetic calcifying cell. Genome Biol 2009, 10. [Google Scholar] [CrossRef] [Green Version]
- Allen, MJ; Forster, T; Schroeder, DC; Hall, M; Roy, D; Ghazal, P; Wilson, WH. Locus-specific gene expression pattern suggests a unique propagation strategy for a giant algal virus. J Virol 2006, 80, 7699–7705. [Google Scholar]
- Guillard, RRL. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, WL, Chanley, MH, Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Clarke, K. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 1993, 18, 117–143. [Google Scholar]
- Clarke, KR; Gorley, RN. Primer v6: User Manual/Tutorial; Primer-E Ltd: Plymouth, UK, 2006. [Google Scholar]
- Anderson, MJ; Gorley, RN; Clarke, KR. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Ltd: Plymouth, UK, 2008. [Google Scholar]
- Iglesias-Rodriguez, MD; Schofield, OM; Batley, J; Medlin, LK; Hayes, PK. Intraspecific genetic diversity in the marine coccolithophore emiliania huxleyi (prymnesiophyceae): The use of microsatellite analysis in marine phytoplankton population studies. J Phycol 2006, 42, 526–536. [Google Scholar]
- Rynearson, TA; Armbrust, EV. DNA fingerprinting reveals extensive genetic diversity in a field population of the centric diatom ditylum brightwellii. Limnol Oceanogr 2000, 45, 1329–1340. [Google Scholar]

| Strain | Source | Date | Strain | Source | Date |
|---|---|---|---|---|---|
| *CCMP2090 | Pacific Ocean—Ecuadorian Coast | 1991 | RCC1812 | Mediterranean Sea | 2008 |
| CCMP12.1 | Atlantic Ocean—Sargasso Sea | 1987 | RCC1818 | Mediterranean Sea | 2008 |
| #CCMP88E | Atlantic Ocean—Sargasso Sea | 1960 | RCC1826 | Mediterranean Sea | 2008 |
| CCMP370 | Atlantic Ocean—North Sea | 1959 | RCC1828 | Mediterranean Sea | 2008 |
| CCMP372 | Atlantic Ocean—Sargasso Sea | 1987 | RCC1830 | Mediterranean Sea | 2008 |
| #CCMP373 | Atlantic Ocean—Sargasso Sea | 1960 | RCC1850 | Mediterranean Sea | 2008 |
| CCMP374 | Atlantic Ocean—Gulf of Maine | 1989 | RCC2054 | Mediterranean Sea | 2008 |
| CCMP376-P | Atlantic Ocean—Gulf of Maine | 1986 | RCC1269 | Atlantic Ocean | 2007 |
| CCMP376-B | Atlantic Ocean—Gulf of Maine | 1986 | RCC1268 | Atlantic Ocean | 2007 |
| CCMP378 | Atlantic Ocean—Gulf of Maine | 1988 | RCC1270 | Atlantic Ocean | 2007 |
| CCMP379 | English Channel | 1957 | RCC1267 | Atlantic Ocean | 2007 |
| CCMP625 | Not known | 2006 | RCC912 | Pacific Ocean—Marquises islands | 2004 |
| *CCMP1516 | Pacific Ocean—Ecuadorian Coast | 1991 | RCC948 | Pacific Ocean—South East Pacific | 2004 |
| CCMP2758-P | Pacific Ocean—Gulf of Alaska | 2006 | RCC958 | Pacific Ocean—Marquises Islands | 2004 |
| CCMP2758-B | Pacific Ocean—Gulf of Alaska | 2006 | RCC962 | Pacific Ocean—Marquises Islands | 2004 |
| RCC1263 | Atlantic Ocean—Ireland | 2007 | RCC1261 | Mediterranean Sea—Spanish coast | 1999 |
| RCC1271 | Atlantic Ocean—Ireland | 2007 | RCC1246 | Mediterranean Sea—Spanish coast | 1999 |
| RCC1250 | Mediterranean Sea—Alboran Sea | 1999 | RCC1257 | Atlantic Ocean—Icelandic coast | 1991 |
| RCC1221 | Mediterranean Sea—Alboran Sea | 1999 | RCC1256 | Atlantic Ocean—Icelandic coast | 1991 |
| RCC1254 | Mediterranean Sea—Alboran Sea | 1999 | PLY92A | English Channel | 1957 |
| RCC1208 | Mediterranean Sea—Alboran Sea | 1999 | RCC1222 | Baltic Sea—Swedish coast | 1998 |
| RCC1248 | Atlantic Ocean—Portugal | 1999 | BLOOM2195 | English Channel | 1999 |
| RCC1251 | Atlantic Ocean—Portugal | 1999 | RCC1258 | Atlantic Ocean—Ireland | 1998 |
| RCC1710 | Japan | 2007 | CH24/90 | Indian Ocean—NZ Coast | 1992 |
| RCC1217 | Pacific Ocean—Tasman Sea | 1998 | 5-9-25B | North Atlantic | 1990 |
| RCC1216 | Pacific Ocean—Tasman Sea | 1998 | RCC1243 | Northern Spain | 2002 |
| Strain | Acid PPME | Strain | Acid PPME | ||
|---|---|---|---|---|---|
| Activity | St Dev | Activity | St Dev | ||
| CCMP2090 | 0.17961 | 0.01539 | RCC1812 | 0.51630 | 0.10917 |
| CCMP2090inf | 0.19424 | 0.00928 | RCC1818 | 0.17238 | 0.00087 |
| CCMP1516 | 0.16531 | 0.00290 | RCC1826 | 0.20055 | 0.00458 |
| CCMP 12-1 | 0.17563 | 0.00844 | RCC1828 | 0.46822 | 0.00322 |
| CCMP88E | 0.13792 | 0.00339 | RCC1830 | 0.31137 | 0.04407 |
| CCMP373 | 0.78789 | 0.04376 | RCC1850 | 0.15542 | 0.00791 |
| CCMP370 | 0.19339 | 0.00631 | RCC2054 | 0.18864 | 0.00347 |
| CCMP372 | 0.13417 | 0.01233 | RCC1269 | 0.74327 | 0.15210 |
| CCMP374 | 0.16857 | 0.00709 | RCC1268 | 0.27304 | 0.05692 |
| CCMP376-P | 0.37632 | 0.02432 | RCC1270 | 0.32470 | 0.04458 |
| CCMP376-B | 0.23170 | 0.00434 | RCC1267 | 0.16693 | 0.00359 |
| CCMP378 | 0.22527 | 0.00329 | RCC912 | 0.17509 | 0.00945 |
| CCMP379 | 0.21367 | 0.00578 | RCC948 | 0.23107 | 0.00760 |
| CCMP625 | 0.32951 | 0.06536 | RCC958 | 0.32239 | 0.08688 |
| CCMP2758-P | 0.38968 | 0.09136 | RCC962 | 0.14034 | 0.00297 |
| CCMP2758-B | 0.21094 | 0.00124 | RCC1261 | 0.18559 | 0.00133 |
| RCC1263 | 0.34975 | 0.01739 | RCC1246 | 0.30096 | 0.01448 |
| RCC1271 | 0.30134 | 0.00911 | RCC1257 | 0.21443 | 0.00780 |
| RCC1250 | 0.20014 | 0.01012 | RCC1256 | 0.20445 | 0.00779 |
| RCC1221 | 0.45293 | 0.01948 | PLY92A | 0.21706 | 0.00958 |
| RCC1254 | 0.14088 | 0.00192 | RCC1222 | 0.20877 | 0.00179 |
| RCC1208 | 0.16183 | 0.00392 | BLOOM2195 | 0.12373 | 0.05572 |
| RCC1248 | 0.21912 | 0.02420 | RCC1258 | 0.19028 | 0.00276 |
| RCC1251 | 0.27738 | 0.00216 | CH24/90 | 0.28767 | 0.02433 |
| RCC1710 | 0.30138 | 0.08715 | 5-9-25B | 0.20566 | 0.00246 |
| RCC1217 | 0.09583 | 0.00125 | RCC1243 | 0.17680 | 0.00478 |
| RCC1216 | 0.28645 | 0.01523 | |||
| Strain | Alkali PPME | Strain | Alkali PPME | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.19366 | 0.00951 | RCC1812 | 0.31577 | 0.02209 |
| CCMP2090inf | 0.55219 | 0.01506 | RCC1818 | 0.13591 | 0.00566 |
| CCMP1516 | 0.16258 | 0.01049 | RCC1826 | 0.14854 | 0.00502 |
| CCMP12-1 | 0.23399 | 0.05954 | RCC1828 | 0.34873 | 0.00139 |
| CCMP88E | 0.14955 | 0.00922 | RCC1830 | 0.16449 | 0.00537 |
| CCMP373 | 0.25032 | 0.01367 | RCC1850 | 0.11324 | 0.00895 |
| CCMP370 | 0.08650 | 0.00507 | RCC2054 | 0.15019 | 0.03608 |
| CCMP372 | 0.14826 | 0.04771 | RCC1269 | 0.31049 | 0.01740 |
| CCMP374 | 0.12796 | 0.00980 | RCC1268 | 0.11152 | 0.00623 |
| CCMP376-P | 0.17664 | 0.00666 | RCC1270 | 0.17496 | 0.01980 |
| CCMP376-B | 0.11524 | 0.00550 | RCC1267 | 0.18210 | 0.00519 |
| CCMP378 | 0.16864 | 0.04599 | RCC912 | 0.20918 | 0.03438 |
| CCMP379 | 0.20780 | 0.00538 | RCC948 | 0.18221 | 0.00421 |
| CCMP 625 | 0.19062 | 0.01509 | RCC958 | 0.16516 | 0.01366 |
| CCMP2758-P | 0.20995 | 0.00784 | RCC962 | 0.10016 | 0.00814 |
| CCMP2758-B | 0.24732 | 0.00857 | RCC1261 | 0.16676 | 0.00926 |
| RCC1263 | 0.25610 | 0.01770 | RCC1246 | 0.17552 | 0.00798 |
| RCC1271 | 0.16352 | 0.01319 | RCC1257 | 0.25125 | 0.02641 |
| RCC1250 | 0.16956 | 0.01280 | RCC1256 | 0.16433 | 0.00759 |
| RCC1221 | 0.28018 | 0.00894 | PLY92A | 0.22421 | 0.00354 |
| RCC1254 | 0.11557 | 0.00349 | RCC1222 | 0.16387 | 0.00372 |
| RCC1208 | 0.13661 | 0.01011 | BLOOM2195 | 0.20202 | 0.03559 |
| RCC1248 | 0.13519 | 0.02426 | RCC1258 | 0.23966 | 0.04469 |
| RCC1251 | 0.16360 | 0.01027 | CH24/90 | 0.23484 | 0.00194 |
| RCC1710 | 0.14002 | 0.00485 | 5-9-25B | 0.22395 | 0.00937 |
| RCC1217 | 0.13866 | 0.01339 | RCC1243 | 0.18878 | 0.00606 |
| RCC1216 | 0.19610 | 0.00894 | |||
| Strain | Acid PPDE | Strain | Acid PPDE | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.71535 | 0.03780 | RCC1812 | 1.78429 | 0.26475 |
| CCMP2090inf | 0.81663 | 0.01473 | RCC1818 | 0.71560 | 0.02312 |
| CCMP1516-P | 0.74463 | 0.05355 | RCC1826 | 0.85526 | 0.02156 |
| CCMP12-1 | 0.34747 | 0.04737 | RCC1828 | 1.71480 | 0.08981 |
| CCMP88E | 0.55860 | 0.02616 | RCC1830 | 0.88175 | 0.02302 |
| CCMP373 | 1.26092 | 0.08947 | RCC1850 | 0.51413 | 0.05150 |
| CCMP370 | 0.34545 | 0.03623 | RCC2054 | 0.77038 | 0.01277 |
| CCMP372 | 0.50588 | 0.03153 | RCC1269 | 1.53905 | 0.11675 |
| CCMP374 | 0.65942 | 0.03761 | RCC1268 | 0.66338 | 0.03980 |
| CCMP376-P | 1.10460 | 0.10275 | RCC1270 | 1.17517 | 0.12956 |
| CCMP376-B | 0.64738 | 0.06432 | RCC1267 | 0.77207 | 0.03226 |
| CCMP378 | 1.42860 | 0.00885 | RCC912 | 0.53782 | 0.02768 |
| CCMP379 | 0.81666 | 0.01853 | RCC948 | 1.03075 | 0.08816 |
| CCMP625 | 0.90846 | 0.14299 | RCC958 | 1.14167 | 0.10030 |
| CCMP2758-P | 1.15915 | 0.13235 | RCC962 | 0.64502 | 0.01470 |
| CCMP2758-B | 1.15550 | 0.08619 | RCC1261 | 0.48601 | 0.02034 |
| RCC1263 | 1.35473 | 0.11517 | RCC1246 | 0.94996 | 0.07626 |
| RCC1271 | 1.02994 | 0.06474 | RCC1257 | 0.74399 | 0.04046 |
| RCC1250 | 0.75125 | 0.09794 | RCC1256 | 0.69247 | 0.04739 |
| RCC1221 | 1.55556 | 0.26597 | PLY92A | 0.94201 | 0.10085 |
| RCC1254 | 0.66117 | 0.01171 | RCC1222 | 0.80130 | 0.01172 |
| RCC1208 | 0.55242 | 0.06229 | BLOOM2195 | 0.59083 | 0.01506 |
| RCC1248 | 0.59579 | 0.04379 | RCC1258 | 0.97228 | 0.05713 |
| RCC1251 | 0.97180 | 0.09077 | CH24/90 | 0.97041 | 0.02280 |
| RCC1710 | 0.97134 | 0.05270 | 5-9-25B | 0.89314 | 0.05387 |
| RCC1217 | 0.28298 | 0.03174 | RCC1243 | 0.64056 | 0.01086 |
| RCC1216 | 0.91697 | 0.12004 | |||
| Strain | Alkali PPDE | Strain | Alkali PPDE | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 1.01498 | 0.03284 | RCC1812 | 2.09800 | 0.16065 |
| CCMP2090inf | 2.09356 | 0.09370 | RCC1818 | 0.81822 | 0.00583 |
| CCMP1516 | 1.06632 | 0.16400 | RCC1826 | 0.83726 | 0.01113 |
| CCMP12-1 | 0.54110 | 0.01648 | RCC1828 | 2.34065 | 0.35407 |
| CCMP88E | 0.73552 | 0.06820 | RCC1830 | 1.34868 | 0.19322 |
| CCMP373 | 1.57758 | 0.07356 | RCC1850 | 0.74402 | 0.00866 |
| CCMP370 | 0.56268 | 0.02989 | RCC2054 | 0.79718 | 0.00362 |
| CCMP372 | 0.77211 | 0.03632 | RCC1269 | 2.03660 | 0.28402 |
| CCMP374 | 0.88076 | 0.00574 | RCC1268 | 0.85921 | 0.00252 |
| CCMP376-P | 1.43431 | 0.03418 | RCC1270 | 1.26434 | 0.07075 |
| CCMP376-B | 0.65800 | 0.05355 | RCC1267 | 0.99680 | 0.09117 |
| CCMP378 | 1.15499 | 0.05097 | RCC912 | 0.87723 | 0.08615 |
| CCMP379 | 1.52023 | 0.17445 | RCC948 | 1.11699 | 0.02386 |
| CCMP625 | 1.68249 | 0.02327 | RCC958 | 1.25341 | 0.04583 |
| CCMP2758-P | 1.35426 | 0.17741 | RCC962 | 0.84704 | 0.01028 |
| CCMP2758-B | 0.91871 | 0.09512 | RCC1261 | 0.80675 | 0.03748 |
| RCC1263 | 1.92140 | 0.04877 | RCC1246 | 1.12463 | 0.13606 |
| RCC1271 | 1.40183 | 0.16875 | RCC1257 | 1.24291 | 0.05902 |
| RCC1250 | 1.03431 | 0.00522 | RCC1256 | 1.01402 | 0.05034 |
| RCC1221 | 2.13127 | 0.11071 | PLY92A | 1.42313 | 0.00162 |
| RCC1254 | 0.86972 | 0.00557 | RCC1222 | 1.18854 | 0.01239 |
| RCC1208 | 0.74311 | 0.02046 | BLOOM2195 | 0.93662 | 0.04273 |
| RCC1248 | 1.02170 | 0.02901 | RCC1258 | 1.40924 | 0.03235 |
| RCC1251 | 1.61663 | 0.04860 | CH24/90 | 1.53915 | 0.09025 |
| RCC1710 | 1.26281 | 0.16583 | 5-9-25B | 1.40952 | 0.03855 |
| RCC1217 | 0.43044 | 0.01153 | RCC1243 | 0.65717 | 0.02023 |
| RCC1216 | 1.34065 | 0.05008 | |||
| Strain | DH-IPA | Strain | DH-IPA | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.01859 | 0.00091 | RCC1812 | 0.15706 | 0.01907 |
| CCMP2090inf | 0.04983 | 0.00080 | RCC1818 | 0.02373 | 0.00577 |
| CCMP1516 | 0.02230 | 0.00586 | RCC1826 | 0.04896 | 0.00194 |
| CCMP12-1 | 0.02799 | 0.00502 | RCC1828 | 0.07718 | 0.02393 |
| CCMP88E | 0.02996 | 0.00247 | RCC1830 | 0.04540 | 0.01346 |
| CCMP373 | 0.10472 | 0.03671 | RCC1850 | 0.03843 | 0.00227 |
| CCMP370 | 0.01530 | 0.00907 | RCC2054 | 0.03811 | 0.00333 |
| CCMP372 | 0.04377 | 0.00297 | RCC1269 | 0.14375 | 0.01265 |
| CCMP374 | 0.02062 | 0.00308 | RCC1268 | 0.06893 | 0.01351 |
| CCMP376-P | 0.07574 | 0.01807 | RCC1270 | 0.03873 | 0.00103 |
| CCMP376-B | 0.02102 | 0.00300 | RCC1267 | 0.06616 | 0.00180 |
| CCMP378 | 0.05063 | 0.00438 | RCC912 | 0.02083 | 0.00207 |
| CCMP379 | 0.04085 | 0.01177 | RCC948 | 0.01846 | 0.00474 |
| CCMP625 | 0.07719 | 0.00417 | RCC958 | 0.10264 | 0.00864 |
| CCMP2758 | 0.04864 | 0.00951 | RCC962 | 0.01629 | 0.00182 |
| CCMP2758-B | 0.05074 | 0.00287 | RCC1261 | 0.02589 | 0.02280 |
| RCC1263 | 0.06434 | 0.00928 | RCC1246 | 0.04088 | 0.00037 |
| RCC1271 | 0.04228 | 0.00997 | RCC1257 | 0.04499 | 0.00471 |
| RCC1250 | 0.02493 | 0.00371 | RCC1256 | 0.03144 | 0.01738 |
| RCC1221 | 0.06522 | 0.01793 | PLY92A | 0.03740 | 0.00804 |
| RCC1254 | 0.02331 | 0.00261 | RCC1222 | 0.03121 | 0.00718 |
| RCC1208 | 0.01528 | 0.00846 | BLOOM2195 | 0.02737 | 0.00617 |
| RCC1248 | 0.02063 | 0.00227 | RCC1258 | 0.02449 | 0.00184 |
| RCC1251 | 0.03713 | 0.01565 | CH24/90 | 0.03571 | 0.00404 |
| RCC1710 | 0.03576 | 0.01327 | 5-9-25B | 0.03008 | 0.00745 |
| RCC1217 | 0.01462 | 0.00462 | RCC1243 | 0.05970 | 0.00298 |
| RCC1216 | 0.02415 | 0.00335 | |||
| Strain | DH-DLT | Strain | DH-DLT | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.02210 | 0.00242 | RCC1812 | 0.17880 | 0.01746 |
| CCMP2090inf | 0.05144 | 0.00607 | RCC1818 | 0.09427 | 0.03561 |
| CCMP1516 | 0.01792 | 0.00440 | RCC1826 | 0.06735 | 0.01943 |
| CCMP12-1 | 0.02436 | 0.00624 | RCC1828 | 0.24829 | 0.03027 |
| CCMP88E | 0.02772 | 0.00255 | RCC1830 | 0.15705 | 0.00788 |
| CCMP373 | 0.14775 | 0.02680 | RCC1850 | 0.03672 | 0.00079 |
| CCMP370 | 0.07007 | 0.00911 | RCC2054 | 0.09311 | 0.00333 |
| CCMP372 | 0.04402 | 0.00278 | RCC1269 | 0.16521 | 0.01267 |
| CCMP374 | 0.03151 | 0.00534 | RCC1268 | 0.08357 | 0.01715 |
| CCMP376-P | 0.09640 | 0.00586 | RCC1270 | 0.08399 | 0.00768 |
| CCMP376-B | 0.06679 | 0.00179 | RCC1267 | 0.07144 | 0.00497 |
| CCMP378 | 0.03853 | 0.00314 | RCC912 | 0.01955 | 0.00395 |
| CCMP379 | 0.03778 | 0.00819 | RCC948 | 0.09411 | 0.01239 |
| CCMP625 | 0.14808 | 0.01738 | RCC958 | 0.13437 | 0.01311 |
| CCMP2758-P | 0.23046 | 0.01668 | RCC962 | 0.02285 | 0.00151 |
| CCMP2758-B | 0.12875 | 0.00213 | RCC1261 | 0.06040 | 0.00638 |
| RCC1263 | 0.19303 | 0.01293 | RCC1246 | 0.03275 | 0.00741 |
| RCC1271 | 0.10255 | 0.02299 | RCC1257 | 0.11920 | 0.00962 |
| RCC1250 | 0.02776 | 0.00481 | RCC1256 | 0.03870 | 0.01396 |
| RCC1221 | 0.23650 | 0.02854 | PLY92A | 0.04026 | 0.00832 |
| RCC1254 | 0.03170 | 0.00137 | RCC1222 | 0.03048 | 0.00777 |
| RCC1208 | 0.07265 | 0.00819 | BLOOM2195 | 0.02652 | 0.00642 |
| RCC1248 | 0.01594 | 0.00079 | RCC1258 | 0.02770 | 0.00434 |
| RCC1251 | 0.13390 | 0.02492 | CH24/90 | 0.03547 | 0.00404 |
| RCC1710 | 0.13055 | 0.01102 | 5-9-25B | 0.02561 | 0.00521 |
| RCC1217 | 0.04174 | 0.00496 | RCC1243 | 0.07169 | 0.00182 |
| RCC1216 | 0.02934 | 0.00622 | |||
| Strain | DH-SS | Strain | DH-SS | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.01303 | 0.00208 | RCC1812 | 0.09719 | 0.00000 |
| CCMP2090inf | 0.03563 | 0.00764 | RCC1818 | 0.01991 | 0.00494 |
| CCMP1516-P | 0.01135 | 0.00367 | RCC1826 | 0.04740 | 0.00654 |
| CCMP12-1 | 0.01994 | 0.00437 | RCC1828 | 0.06835 | 0.02432 |
| CCMP88E | 0.02584 | 0.00235 | RCC1830 | 0.04044 | 0.00549 |
| CCMP373 | 0.02546 | 0.02951 | RCC1850 | 0.03343 | 0.00090 |
| CCMP370 | 0.01377 | 0.01196 | RCC2054 | 0.03984 | 0.00770 |
| CCMP372 | 0.03945 | 0.00372 | RCC1269 | 0.07398 | 0.01069 |
| CCMP374 | 0.01947 | 0.00256 | RCC1268 | 0.02026 | 0.00615 |
| CCMP376-P | 0.02860 | 0.02781 | RCC1270 | 0.03130 | 0.01443 |
| CCMP376-B | 0.01931 | 0.00043 | RCC1267 | 0.05337 | 0.00960 |
| CCMP378 | 0.03019 | 0.00419 | RCC912 | 0.01583 | 0.00179 |
| CCMP379-B | 0.03268 | 0.00117 | RCC948 | 0.01756 | 0.00844 |
| CCMP625 | 0.07641 | 0.00984 | RCC958 | 0.06765 | 0.00570 |
| CCMP2758-P | 0.05166 | 0.01193 | RCC962 | 0.01314 | 0.00038 |
| CCMP2758-B | 0.04932 | 0.00321 | RCC1261 | 0.01409 | 0.00426 |
| RCC1263 | 0.06626 | 0.01356 | RCC1246 | 0.02333 | 0.00499 |
| RCC1271 | 0.05092 | 0.01477 | RCC1257 | 0.03934 | 0.00326 |
| RCC1250 | 0.01273 | 0.00883 | RCC1256 | 0.02063 | 0.01396 |
| RCC1221 | 0.08224 | 0.01251 | PLY92A | 0.01921 | 0.00614 |
| RCC1254 | 0.02360 | 0.00107 | RCC1222 | 0.01734 | 0.00767 |
| RCC1208 | 0.00941 | 0.00374 | BLOOM2195 | 0.02130 | 0.00730 |
| RCC1248 | 0.01078 | 0.00090 | RCC1258 | 0.01566 | 0.00276 |
| RCC1251 | 0.03377 | 0.01087 | CH24/90 | 0.02691 | 0.00377 |
| RCC1710 | 0.03576 | 0.01023 | 5-9-25B | 0.01382 | 0.00153 |
| RCC1217 | 0.01356 | 0.00316 | RCC1243 | 0.04835 | 0.00912 |
| RCC1216 | 0.01664 | 0.00326 | |||
| Strain | CBXY-C4 | Strain | CBXY-C4 | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.91263 | 0.05811 | RCC1812 | 3.47836 | 0.22679 |
| CCMP2090inf | 2.01264 | 0.03272 | RCC1818 | 1.28586 | 0.06262 |
| CCMP1516 | 4.72720 | 0.08391 | RCC1826 | 1.46971 | 0.01877 |
| CCMP12-1 | 0.56503 | 0.01607 | RCC1828 | 3.75741 | 0.04434 |
| CCMP88E | 1.19434 | 0.02193 | RCC1830 | 2.19248 | 0.08135 |
| CCMP373 | 3.30686 | 0.02323 | RCC1850 | 0.60506 | 0.00868 |
| CCMP370 | 0.57237 | 0.00195 | RCC2054 | 1.31421 | 0.07909 |
| CCMP372 | 0.52416 | 0.01124 | RCC1269 | 3.14825 | 0.06077 |
| CCMP374 | 0.85715 | 0.05896 | RCC1268 | 1.26878 | 0.07059 |
| CCMP376-P | 2.37180 | 0.12357 | RCC1270 | 1.83042 | 0.02733 |
| CCMP376-B | 1.23213 | 0.11012 | RCC1267 | 1.57030 | 0.13579 |
| CCMP378 | 2.12765 | 0.05996 | RCC912 | 0.44616 | 0.01026 |
| CCMP379 | 1.22888 | 0.07401 | RCC948 | 1.98188 | 0.05798 |
| CCMP625 | 2.27299 | 0.11844 | RCC958 | 1.92105 | 0.04765 |
| CCMP2758-P | 2.31912 | 0.15994 | RCC962 | 1.44293 | 0.03853 |
| CCMP2758-B | 1.87580 | 0.10302 | RCC1261 | 0.76397 | 0.01772 |
| RCC1263 | 2.61899 | 0.06146 | RCC1246 | 0.84784 | 0.01731 |
| RCC1271 | 2.07483 | 0.06317 | RCC1257 | 1.20087 | 0.02386 |
| RCC1250 | 0.90858 | 0.07761 | RCC1256 | 0.46525 | 0.02618 |
| RCC1221 | 3.24845 | 0.07028 | PLY92A | 1.92798 | 0.06885 |
| RCC1254 | 2.69194 | 0.05944 | RCC1222 | 0.99775 | 0.15048 |
| RCC1208 | 0.80126 | 0.01865 | BLOOM2195 | 0.69953 | 0.01288 |
| RCC1248 | 0.71100 | 0.02739 | RCC1258 | 0.93082 | 0.01764 |
| RCC1251 | 2.05211 | 0.07192 | CH24/90 | 1.48482 | 0.02644 |
| RCC1710 | 1.80632 | 0.09925 | 5-9-25B | 0.74347 | 0.02828 |
| RCC1217 | 0.41034 | 0.01356 | RCC1243 | 2.96084 | 0.19796 |
| RCC1216 | 2.09708 | 0.02705 | |||
| Strain | CBXY-C16 | Strain | CBXY-C16 | ||
|---|---|---|---|---|---|
| Activity | Std Dev | Activity | Std Dev | ||
| CCMP2090 | 0.83590 | 0.03418 | RCC1812 | 2.90956 | 0.17719 |
| CCMP2090inf | 1.93762 | 0.16983 | RCC1818 | 1.14399 | 0.00598 |
| CCMP1516 | 1.71990 | 0.03231 | RCC1826 | 1.35472 | 0.11735 |
| CCMP12-1 | 0.53526 | 0.05624 | RCC1828 | 3.75648 | 0.18971 |
| CCMP88E | 0.50175 | 0.04024 | RCC1830 | 1.85034 | 0.00488 |
| CCMP373 | 2.54264 | 0.07732 | RCC1850 | 0.59257 | 0.09620 |
| CCMP370 | 0.55533 | 0.02635 | RCC2054 | 1.30630 | 0.01170 |
| CCMP372 | 0.48642 | 0.00696 | RCC1269 | 2.80862 | 0.20754 |
| CCMP374 | 0.82781 | 0.07642 | RCC1268 | 1.04188 | 0.07728 |
| CCMP376-P | 1.91921 | 0.09312 | RCC1270 | 1.68226 | 0.12782 |
| CCMP376-B | 1.19587 | 0.03814 | RCC1267 | 1.49031 | 0.12209 |
| CCMP378 | 1.79742 | 0.11825 | RCC912 | 0.43431 | 0.01801 |
| CCMP379 | 1.21532 | 0.12853 | RCC948 | 1.93536 | 0.04057 |
| CCMP625 | 1.93508 | 0.07774 | RCC958 | 1.86366 | 0.31931 |
| CCMP2758-P | 2.12730 | 0.12864 | RCC962 | 1.36145 | 0.09054 |
| CCMP2758-B | 1.70422 | 0.04218 | RCC1261 | 0.70427 | 0.04096 |
| RCC1263 | 2.72156 | 0.42697 | RCC1246 | 0.77306 | 0.05014 |
| RCC1271 | 2.04049 | 0.06138 | RCC1257 | 1.09724 | 0.14293 |
| RCC1250 | 0.92810 | 0.06444 | RCC1256 | 0.47866 | 0.04627 |
| RCC1221 | 2.92656 | 0.25378 | PLY92A | 0.61792 | 0.13032 |
| RCC1254 | 2.49711 | 0.08247 | RCC1222 | 0.94993 | 0.04210 |
| RCC1208 | 0.71650 | 0.04868 | BLOOM2195 | 0.60580 | 0.06661 |
| RCC1248 | 0.63364 | 0.03153 | RCC1258 | 0.81610 | 0.04466 |
| RCC1251 | 2.14337 | 0.07085 | CH24/90 | 1.35955 | 0.01822 |
| RCC1710 | 1.69602 | 0.05115 | 5-9-25B | 0.69739 | 0.01877 |
| RCC1217 | 0.38351 | 0.01883 | RCC1243 | 2.46560 | 0.24177 |
| RCC1216 | 0.77526 | 0.06996 | |||
| Strain | PC1 | Strain | PC1 |
|---|---|---|---|
| RCC1812 | −6.85 | RCC1217 | 0.66 |
| RCC1828 | −6.44 | RCC2054 | 0.78 |
| RCC1269 | −6.33 | RCC1254 | 0.84 |
| RCC1221 | −5.33 | RCC1268 | 0.91 |
| CCMP373 | −4.21 | RCC1246 | 0.92 |
| RCC1263 | −3.81 | RCC1258 | 1.03 |
| CCMP2758 | −2.53 | 5-9-25B | 1.22 |
| CCMP625 | −2.47 | N44-20D | 1.39 |
| RCC958 | −2.21 | RCC1818 | 1.46 |
| CCMP2090inf | −1.96 | RCC1250 | 1.76 |
| CCMP376 | −1.52 | CCMP376-B | 1.77 |
| RCC1271 | −1.15 | CCMP2090 | 1.93 |
| RCC1830 | −1.10 | RCC1256 | 1.94 |
| RCC1251 | −1.08 | CCMP372 | 2.07 |
| CCMP2758-B | −0.96 | RCC962 | 2.15 |
| RCC1270 | −0.60 | BLOOM2195 | 2.15 |
| CCMP378 | −0.55 | CCMP374 | 2.18 |
| RCC1710 | −0.50 | RCC1261 | 2.21 |
| RCC1243 | −0.40 | CCMP88E | 2.26 |
| CH24/90 | −0.07 | RCC1850 | 2.26 |
| RCC1257 | −0.02 | RCC1248 | 2.35 |
| RCC1267 | −0.01 | RCC1208 | 2.46 |
| RCC948 | 0.10 | RCC912 | 2.47 |
| CCMP1516 | 0.34 | CCMP12.1 | 2.59 |
| CCMP379 | 0.37 | CCMP370 | 3.00 |
| RCC1826 | 0.45 | RCC1216 | 3.54 |
| PLY92A | 0.53 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reid, E.L.; Worthy, C.A.; Probert, I.; Ali, S.T.; Love, J.; Napier, J.; Littlechild, J.A.; Somerfield, P.J.; Allen, M.J. Coccolithophores: Functional Biodiversity, Enzymes and Bioprospecting. Mar. Drugs 2011, 9, 586-602. https://doi.org/10.3390/md9040586
Reid EL, Worthy CA, Probert I, Ali ST, Love J, Napier J, Littlechild JA, Somerfield PJ, Allen MJ. Coccolithophores: Functional Biodiversity, Enzymes and Bioprospecting. Marine Drugs. 2011; 9(4):586-602. https://doi.org/10.3390/md9040586
Chicago/Turabian StyleReid, Emma L., Charlotte A. Worthy, Ian Probert, Sohail T. Ali, John Love, Johnathan Napier, Jenny A. Littlechild, Paul J. Somerfield, and Michael J. Allen. 2011. "Coccolithophores: Functional Biodiversity, Enzymes and Bioprospecting" Marine Drugs 9, no. 4: 586-602. https://doi.org/10.3390/md9040586
APA StyleReid, E. L., Worthy, C. A., Probert, I., Ali, S. T., Love, J., Napier, J., Littlechild, J. A., Somerfield, P. J., & Allen, M. J. (2011). Coccolithophores: Functional Biodiversity, Enzymes and Bioprospecting. Marine Drugs, 9(4), 586-602. https://doi.org/10.3390/md9040586