Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature
Abstract
:1. Introduction
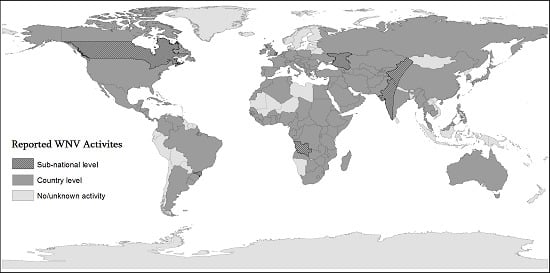
1.1. WNV Epidemical History and Geography

1.2. Ecological Studies
1.3. Recent Reviews
2. Methods
3. Results and Discussion
3.1. Spatial Analysis of Human Case Incidence
| Analysis/Citation | Region/Date | Common Risk Factors (* Location-Dependent) |
|---|---|---|
| Spatial Analysis of human case incidence | ||
| Local Moran’s I [45] | Chicago, 2002 | Less Population density *, higher percent of old and white residents *, poor drainage, mosquito abatement efforts |
| SaTScan, Local Moran’s I [55] | U.S. level, 2002–2008 | Study focused on hot-spots of human case incidence |
| Conditional Autoregressive Model [58] | U.S. level, 2013 | The number of WNV positive mosquito pools |
| Global Moran’s I [64] | U.S. level, 1999–2008 | Temperature and precipitation ranges |
| Ripley’s K test [119] | Chicago, 2005–2006 | Inner suburbs, less densely populated areas *, high percent of white residents *, post world war II housing and a higher median population age, smaller elevation ranges, standing water, more vegetated areas |
| Hot spot analysis [120] | Connecticut, 2000–2005 | Urban/suburban areas |
| Spatial proximity, Moran’s I [121] | Northeast U.S. | Urban/suburban areas, less forested landscapes |
| Global Moran’s I, NDVI [122] | Iowa, 2003–2006 | Less population density * and rural agricultural areas, drier conditions |
| SaTScan [123] | Northern plains, 2003 | Rural areas, irrigated land in rural areas |
| SaTScan, Local Moran’s I [124] | Davis, CA, USA, 2006 | Avian mortality, residential landscape, warm night temperatures |
| Moran’s I [125] | Spatial autocorrelation and contagious diffusion | |
| Spatial-temporal analysis of bird species | ||
| NND Time Model [36] | Twin Cities, 2002 | Densely populated areas * , distance to nearest dead bird and pool location |
| Mapping migration routes [57] | North America | Wintering grounds along coastal plains of Georgia, northern Florida |
| Kriging [62] | Indiana, 2002 | High temperatures in August-September months |
| Bird abundance mapping [69] | British Columbia, 1994–2003 | Dead corvid density |
| Proximity analysis [102] | Texas, 2002 | Proximity of equine cases to human cases in urban populations |
| GLMM [126] | Alberta, Canada 2002–2006 | The grassland natural region, rural/suburban areas |
| Discriminant Analysis, Mahallanobis DS [127] | Virginia, 2011 | Mean precipitation, percent impervious surface with 21–40% canopy density |
| Mahallanobis Distance Statistics [128] | Shorter distance to bird risk areas associates with higher risk | |
| Spatial analysis of horses | ||
| Kriging [61] | Indiana, 2002 | High temperatures in August-September months |
| Sptiotemporal clustering, NDVI analysis[102] | N. Indiana, 2002 | High median estimated NDVI in equine clusters |
| Proximity analysis[129] | Texas, 2002 | Proximity of equine cases to human cases in urban populations |
| LULC analysis, SatScan clustering [130] | France | Rice fields, dry bushes, open water, low elevation salted swamps |
| SaTScan [131] | Hot spot analysis, Cluster identification | |
| SaTScan [132] | Texas | Study focused on areas-of-high-risk |
| Spatial modeling of mosquito pools | ||
| Risk mapping [46] | Mississippi | High road density, low stream density and gentle slopes |
| Mahallanobis Distance Statistics [53] | Tennessee, 2004 | High percentage of black population, low income, high rental occupation, old structures, vacant housing |
| Spatial sensitivity analysis [63] | Colorado, 2003–2007 | Study focused on sub-county scale presentation and how WNV disease occurence influenced by data aggregation |
| Spatio-temporal spread, risk mapping [133] | Australia, 2013 | Predictive risk-zone mapping |
| Real-time GIS Studies for WNV surveliance | ||
| ArboNet, CDC [37] | U.S. | Real-time GIS study for WNV. surveiliance, prevention and control |
| WNV-Multi Agent Geo-Simulation [70] | Quebec, Canada | Short-term decision making related to use of larvicides with climatic scenarios |
| ISPHM-WNV [118] | Quebec, Canada, 2002 | Real-time GIS study for public health surveiliance |
| Real-time GIS-driven Surveilliance [134] | Canada | Real-time GIS driven surveilliance pilot system |
| A nationwide electronic surveilliance [135] | Canada | A nationwide electronic surveilliance |
| Habitat-based Studies | ||
| LULC analysis [2] | Saskatchewan, Canada, 2003–2007 | Study focused on risk mapping |
| Maximum likelihood unsupervised classification LULC change matrix [39] | Urbana Champaign, IL, USA, 1991–2003 | Residential high canopy coverage |
| Generation of DEM, Spatial Hydological Modeling, Eigen vector mapping [40] | Trinidad, 2008–2009 | Terrain elevation |
| Raster-based mosquito abundance model [48] | British Columbia | Study focused on risk prone areas |
| Geospatial models based on LULC [60] | Cook County, IL, USA, 2002–2005 | Warmer temperature and heavy precipitation, forest and middle-range built environment |
| Terrain Analysis, ISODATA [61] | Tuskegee, AL, USA | Smaller elevation range |
| Shortest distance analysis [136] | 17 U.S. States, 2001–2005 | Warmer temperatures, elevated humidity and heavy percipitation |
| NDVI analysis, RS-driven spatial analysis [137] | Morocco | Precipitation |
| Computational neuronetworks [138,139] | Twin Cities, MN, USA, 2002–2006 | Proximity to wetlands |
| RS Studies for early warning systems | ||
| ASTER imagery and high-temporal MODIS [127] | N. Virginia | Elevation and urban built-up conditions negatively correlated with WNV propagation, landsurface temperature positively correlated with viral transmission |
| AMSR-E dervied models [142] | South Dakota | Air temperature and vegetation opacity and surface water fraction |
| Tassled-Cap transformation [143] | Coastal Virginia | Study focused on developing a habitat suitability index |
| AVIRIS [144] | Fresno, Canada | Neglected swimming pools |
| NDWI [145] | Atlanta, GA, USA | Neglected swimming pools |
| Spatial analysis of genetic variation | ||
| Population genetic analysis [111,146,147] | U.S. level | Localized environmental conditions |
| Population genetic analysis [148] | Chicago, 2008 | Seasonal variations in microclimatic conditions at finer scale |
| Spatial uncertainty analysis | ||
| Spatial uncertainty analysis, SaTScan [149] | South Dakota | Lower ability to geocode Indian reservations |
3.2. Spatial-Temporal Analysis of Bird Species
3.3. Spatial Analysis of Horses
3.4. Spatial Modeling of Mosquito Pools
3.5. Real-Time GIS Studies for WNV Surveillance
3.6. Habitat-Based Studies
3.7. Remote Sensing Studies for Early Warning Systems and Vector Control
3.8. Spatial Analysis of Genetic Variation
3.9. Spatial Uncertainty Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nash, D.; Mostashari, F.; Fine, A. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar]
- Epp, T.Y.; Waldner, C.L.; Berke, O. Predicting geographical human risk of West Nile Virus—Saskatchewan, 2003 and 2007. Can. J. Public Health 2009, 100, 344–349. [Google Scholar]
- Rainham, D.G.C. Ecological complexity and West Nile virus: Perspectives on improving public health response. Can. J. Public Health 2004, 96, 37–40. [Google Scholar]
- Smihtburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar]
- Hayes, C. West Nile Fever. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Karabatsos, N. International Catalogue of Arbovirus Including Certain Other Viruses of Vertebrate, 3rd ed.; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Cambell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 353, 767–771. [Google Scholar]
- Cernescu, C.; Nedelcu, N.I.; Trdei, G.; Ruta, S.; Tsai, T.F. Continued transmission of West Nile virus to humans in southeastern Romania, 1997–1998. J. Infect. Dis. 2000, 181, 710–712. [Google Scholar]
- L’vov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Larichev, V.P.; Gaidamovich, S.Y.; Leshschinskaia, E.V.; Vyshemirsky, O.I.; Zhukov, A.N.; Lazorenko, V.V.; Salko, V.N.; et al. Isolation of two strains of West Nile virus during an outbreak in Southern Russia, 1999. Emerg. Infect. Dis. 2000, 6, 373–376. [Google Scholar]
- Hubalek, Z.; Halouzka, J. West Nile fever: A reemerging mosquito borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar]
- Bledso, G.H. The West Nile virus: A lesson in emerging infections. Wilder. Environ. Med. 2004, 15, 113–118. [Google Scholar]
- Peiris, J.S.M.; Amerasinghe, F.P. West Nile Fever. In Handbook of Zoonoses, Section B: Viral, 2nd ed.; Beran, G.W., Steele, J.H., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 139–148. [Google Scholar]
- Berthet, F.; Zeller, D.M.; Rauzier, J.; Digoutte, J.; Deubel, V. Extensive nucleotide changes and deletions within the envelope glycoprotein gene of Euro-African West Nile viruses. J. Genet. Virol. 1997, 78, 2293–2297. [Google Scholar]
- Hubalek, Z.; Halouzka, J. Arthropod-borne viruses of vertebrates in Europe. Acta Sci. Nat. Brno 1996, 30, 1–95. [Google Scholar]
- Blitvich, J.B. Transmission dynamic and changing epidemiology of West Nile virus. Anim. Health Res. Rev. 2008, 9, 71–86. [Google Scholar] [CrossRef]
- Banker, D.D. Preliminary observations on antibody patterns against certain viruses among inhabitants of Bombay city. Indian J. Med. Sci. 1952, 6, 733–746. [Google Scholar]
- Khan, S.A.; Dutta, P.; Khan, A.M.; Chowdhury, P.; Borah, J.; Doloi, P.; Mahanta, J. West Nile virus infection in Assam, North East India. Emerg. Infect. Dis. 2011, 17, 947–948. [Google Scholar]
- Takasaki, T. West Nile fever/encephalitis. Uirusu 2007, 57, 199–205. [Google Scholar] [CrossRef]
- Li, X.L.; Fu, S.H.; Liu, W.B.; Wang, H.Y.; Lu, Z.; Tong, S.X.; Li, Z.X.; Nasci, R.S.; Kosoy, O.; Cui, Y.; et al. West Nile virus infection in Xinxiang, China. Vector Borne Zoonotic Dis. 2013, 13, 131–133. [Google Scholar]
- Yeh, J.H.; Kim, H.J.; Nah, J.J.; Lee, H.; Kim, Y.J.; Moon, J.S.; Cho, I.S.; Choi, I.S.; Song, C.S.; Lee, J.B. Surveillance for West Nile virus in dead wild birds, South Korea, 2005–2008. Emerg. Infect. Dis. 2011, 17, 299–301. [Google Scholar]
- Korea Joongang Daily. Available online: http://koreajoongangdaily.joinsmsn.c...me%7Cnewslist2 (accessed on 14 June 2013).
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- West Nile Virus. Available online: http://www.cdc.gov/ncidod/dvbid/westnile/surv&control.htm (accessed on 14 June 2013).
- Jia, X.Y.; Briese, T.; Jordan, I.; Rambaut, A.; Chi, H.C.; Mackenzie, J.S.; Hall, R.A.; Scherret, J.; Lipkin, W.I. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet 1999, 354, 1971–1972. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef]
- Anderson, J.F.; Andreadis, T.G.; Vossbrinck, C.R.; Tirrell, S.; Wakem, E.M.; French, R.A.; Garmendia, A.E.; van Kruiningen, H.J. Isolation of West Nile virus from mosquitoes, crows, and a Cooper’s hawk in Connecticut. Science 1999, 286, 2331–2333. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Martello, T.; Squarzon, L.; Toppo, S.; Fiorin, F.; Marchiori, G.; Russo, F.; et al. New endemic West Nile virus lineage 1a in northern Italy, July 2012. Euro Surveill. 2012, 17, 20231:1–20231:3. [Google Scholar]
- Connell, J.; McKeown, P.; Garvey, P.; Cotter, S.; Conway, A.; O’Flanagan, D.; O’Herlihy, B.; Morgan, D.; Nicoll, A.; Lloyd, G. Two linked cases of West Nile virus (WNV) acquired by Irish tourists in the Algarve, Portugal. Euroserv. Wkly. 2004, 8. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2517 (accessed on 14 June 2013).
- The Disease Daily. Available online: http://diseasedaily.com/west-nile-mosquito-found-uk (accessed on 14 June 2013).
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar]
- Hubalek, Z. European experience with the West Nile virus ecology and epidemiology: Could it be relevant for the New World? Viral Immunol. 2000, 13, 415–426. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar]
- Nolan, M.S.; Schuermann, J.; Murray, K.O. West Nile virus Infection among Humans, Texas, USA, 2002–2011. Emerg. Infect. Dis. 2013, 19, 137–139. [Google Scholar]
- CDC Telebriefing on West Nile Virus Update. Available online: http://www.cdc.gov/media/releases/2012/t0822_west_nile_update.html (accessed on 14 June 2013).
- Ghosh, D.; Manson, S.M.; McMaster, R.B. Delineating West Nile Virus transmission cycles at various scales: The nearest neighbor distance-time model. Cartogr. Geogr. Inf. Sci. 2010, 37, 149–163. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention. Epidemic/Epizootic West Nile virus in the United States: Revised Guidelines for Surveillance, Prevention and Control. Center for Disease Control and Prevention Division of Vector-Borne Infectious Diseases. Available online: http://www.cdc.gov (accessed on 3 May 2003).
- FDA Consumer. CDC helps in battle against West Nile virus. FDA Consum. 2002, 36, p. 3. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12523281 (accessed on 19 September 2013).
- Jacob, B.G.; Lampman, R.L.; Ward, M.P.; Muturi, E.J.; Morris, J.A.; Caamano, E.X.; Novak, R.J. Geospatial variability in the egg raft distribution and abundance of Culex pipiens and Culex restuans in Urbana-Champaign, Illinois. Int. J. Remote Sens. 2009, 30, 2005–2019. [Google Scholar]
- Jacob, B.G.; Chadee, D.D.; Novak, R.J. Adjusting second moment bias in eigenspace using Bayesian empirical estimators, Dirichlet tessellations and Worldview I data for predicting Culex quinquefasciatus habitats in Trinidad. J. Geogr. Infor. Syst. 2011, 3, 18–49. [Google Scholar]
- Gubler, D.J. The continuing spread of West Nile virus in the western hemisphere. Clin. Infect. Dis. 2007, 45, 1039–1046. [Google Scholar] [CrossRef]
- Melandri, V.C.R.; Mondini, A.; Guimaraes, A.E.; Komar, N.K.; Fernandez-Sesma, A.; Bosch, I.; Nogueira, M.L. West Nile virus Antibodies in Horses and Chickens from the Pantanal Region, 2010. In Proceedings of 22th National Meeting of Virology & 6th Mercosur Meeting of Virology, Sao Paulo, Brazil, 23–26 October 2011; p. 70.
- Reis, V.P.; Farignoli, M.; Carvalho, A.C.; Delsin, D.L.; Souza, W.M.; Machado, A.M.; Figueiredo, L.T.M. West Nile virus in Brazil? Serological Evidences of Human Infection by West Nile virus in Belmonte and Paraiso Counties, Santa Catarina State. In Proceedings of 22th National Meeting of Virology & 6th Mercosur Meeting of Virology, Sao Paulo, Brazil, 23–26 October 2011; p. 174.
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar]
- Ruiz, M.O.; Tedesco, C.; McTighe, T.J.; Austin, C.; Kitron, U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int. J. Health Geogr. 2004, 3. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15099399 (accessed on 15 June 2013).
- Cooke, W.H.; Grala, K.; Wallis, R. Avian GIS models signal human risk for West Nile virus in Mississippi. Int. J. Health Geogr. 2006, 5. [Google Scholar] [CrossRef]
- Gibbs, S.E.J.; Wimberly, M.C.; Madden, M.; Masour, J.; Yabsley, M.J.; Stalknecht, D.E. Factors affecting the geographic distribution of West Nile virus in Georgia, USA: 2002–2004. Vector Borne Zoonotic Dis. 2006, 6, 73–82. [Google Scholar] [CrossRef]
- Tachiiri, K.; Klinkenberg, B.; Mak, S.; Kazmi, J. Predicting outbreaks: A spatial risk assessment of West Nile virus in British Columbia. Int. J. Health Geogr. 2006, 5. [Google Scholar] [CrossRef]
- Sithiprasasna, R.; Lee, W.J.; Ugsang, D.M.; Linthicum, K.J. Identification and characterization of larval and adult anopheline mosquito habitats in the Republic of Korea: Potential use of remotely sensed data to estimate mosquito distributions. Int. J. Health Geogr. 2005, 4. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Li, I. Combining global and local estimates for spatial distribution of mosquito larval habitats. GISci. Remote Sens. 2006, 43, 95–108. [Google Scholar]
- Theophilides, C.N.; Ahearn, S.C.; Grady, S.; Merlino, M. Identifying West Nile virus risk areas: The dynamic continuous-area space-time system. Am. J. Epidemiol. 2003, 157, 843–854. [Google Scholar]
- Gea-Banacloche, J.; Johnson, R.T.; Bagic, A.; Butman, J.A.; Murray, P.R.; Agrawal, A.G. West Nile virus: Pathogenesis and therapeutic options. Ann. Intern. Med. 2004, 140, 545–553. [Google Scholar]
- Ozdenerol, E.; Bialkowska-Jelinska, E.; Taff, G.N. Locating suitable habitats for West Nile virus-infected mosquitoes through association of environmental characteristics with infected mosquito locations: A case study in Shelby County, Tennessee. Int. J. Health Geogr. 2008, 7. [Google Scholar] [CrossRef]
- Influenza Aviária. Available online: http://www.zoonoses.org.br/absoluto/midiazoonoses/arquivos_1258562759/9397_crmv-pr_manual-zoonoses_influenza_aviaria.pdf /imagens/ (accessed on 10 October 2013).
- Sugumaran, R.; Larson, S.R.; DeGroote, J.P. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. Int. J. Health Geogr. 2009, 8. [Google Scholar] [CrossRef]
- Kitron, U.; Jones, C.J.; Bouseman, J.K.; Nelson, J.A.; Baumgartner, D.L. Spatial analysis of the distribution of Ixodes dammini (Acari: Ixodudae) on white-tailed deer in Ogle County, Illinois. J. Med. Entomol. 1992, 29, 259–266. [Google Scholar]
- Rappole, J.H.; Derrickson, S.R.; Hubalek, Z. Migratory Birds and spread of West Nile virus in the western hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Enhancing West Nile virus surveillance, United States. Emerg. Infect. Dis. 2004, 10, 1129–1133. [Google Scholar] [CrossRef]
- Mather, T.N.; Nicholson, M.C.; Donnelly, E.F.; Matyas, B.T. Entomologic Index for human risk of Lyme disease. Am. J. Epidemiol. 1996, 144, 1066–1069. [Google Scholar]
- Jacob, B.G.; Gu, W.; Muturi, E.J.; Caamano, E.X.; Morris, J.M.; Lampman, R.; Novak, R.J. Developing operational algorithms using linear and non-linear least squares estimation in Python® for identification of Culex pipiens and Culex restuans aquatic habitats in a mosquito abatement district (Cook County, Illinois). Geospatial Health 2009, 3, 23–31. [Google Scholar]
- Jacob, B.G.; Burkett-Cadena, N.D.; Luvall, J.C.; Parcak, S.H.; McClure, J.W.; Estep, L.K.; Hill, G.E.; Cupp, E.W.; Novak, R.J. Developing GIS-based Eastern Equine Encephalitis vector-host models in Tuskegee, Alabama. Int. J. Health Geogr. 2010, 9. [Google Scholar] [CrossRef]
- Ward, M.P. Epidemic West Nile virus encephalomyelitis: A temperature-dependent, spatial model of disease dynamics. Prev. Vet. Med. 2005, 71, 253–264. [Google Scholar] [CrossRef]
- Winters, A.M.; Eisen, R.J.; Delorey, M.J.; Fischer, M.; Nasci, R.S.; Zielinski-Gutierrez, E.; Moore, C.G.; Pape, W.; John, E.L. Spatial risk assessments based on vector-borne disease epidemiologic data: Importance of scale for West Nile virus disease in Colorado. Am. J. Trop. Med. Hyg. 2010, 82, 945–953. [Google Scholar] [CrossRef]
- Young, S.G.; Jensen, R.R. Statistical and visual analysis of human West Nile virus infection in the United States, 1999–2008. Appl. Geogr. 2012, 34, 425–431. [Google Scholar] [CrossRef]
- Griffith, D.A. A Comparison of six analytical disease mapping techniques as applied to West Nile virus in the conterminous United States. Int. J. Health Geogr. 2005, 4, 18–26. [Google Scholar] [CrossRef]
- Kitron, U. Risk maps: Transmission and burden of vector-borne diseases. Parasitol. Today 2000, 16, 324–325. [Google Scholar]
- Rogers, D.J.; Randolph, S.E. Studying the global distribution of infectious diseases using GIS and RS. Nat. Rev. Microbiol. 2003, 1, 231–237. [Google Scholar] [CrossRef]
- Hay, S.I.; Omumbo, J.A.; Craig, M.H.; Snow, R.W. Earth observation, geographic information systems and plasmodium falciparum malaria in Sub-Saharan Africa. Adv. Parasitol. 2000, 47, 173–215. [Google Scholar] [CrossRef]
- David, S.T.; Mak, S.; MacDougall, L.; Fyfe, M. A bird’s eye view: Using geographic analysis to evaluate the representativeness of corvid indicators for West Nile virus surveillance. Int. J. Health Geogr. 2007, 6. [Google Scholar] [CrossRef]
- Bouden, M.; Moulin, B.; Gosselin, P. The geosimulation of West Nile virus propagation: A multi-agent and climate sensitive tool for risk management in public health. Int. J. Health Geogr. 2008, 7. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention. CDC and USGS Have Employed GIS and RS to Prepare Interpretive Maps Showing WNV Activity. Available online: http://www.cdc.gov/ncidod/dvbid/westnile/resources/wnvguidelines1999.pdf (accessed on 23 September 2013).
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef]
- Hayes, E.B. Looking the other way: Preventing vector-borne disease among travelers to the United States. Travel Med. Infect. Dis. 2010, 8, 277–284. [Google Scholar] [CrossRef]
- Mann, B.R.; McMullen, A.R.; Guzman, H.; Tesh, R.B.; Barrett, A.D. Dynamic transmission of West Nile virus across the United States-Mexican border. Virology 2013, 436, 75–80. [Google Scholar] [CrossRef]
- Smith, D.W.; Speers, D.J.; Mackenzie, J.S. The viruses of Australia and the risk to tourists. Travel Med. Infect. Dis. 2011, 9, 113–125. [Google Scholar] [CrossRef]
- Brown, E.B.; Adkin, A.; Fooks, A.R.; Stephenson, B.; Medlock, J.M.; Snary, E.L. Assessing the risks of West Nile virus-infected mosquitoes from transatlantic aircraft: Implications for disease emergence in the United Kingdom. Vector Borne Zoonotic Dis. 2012, 12, 310–320. [Google Scholar] [CrossRef]
- Zuckerman, J.N. The traveler and West Nile virus. Travel Med. Infect. Dis. 2003, 1, 149–152. [Google Scholar] [CrossRef]
- Komar, N.; Clark, G.G. West Nile virus activity in Latin America and the Caribbean. Revista Panamericana de Salud Pública 2006, 2, 112–117. [Google Scholar] [CrossRef]
- Komar, N. West Nile viral encephalitis. Rev. Sci. Tech. 2000, 1, 166–176. [Google Scholar]
- Pauli, G. West-Nil-Virus. Bundesgesundheitsblatt Gesundheitsforsching Gesundheitsschutz 2004, 47, 653–660. [Google Scholar]
- Peterson, L.R.; Hayes, E.B. West Nile virus in the Americas. Med. Clin. North Am. 2008, 92, 1307–1322. [Google Scholar] [CrossRef]
- Marfin, A.A.; Gubler, D.J. West Nile encephalitis: An emerging disease in the United States. Clin. Infact. Dis. 2001, 33, 1713–1719. [Google Scholar] [CrossRef]
- Perez, R.M.; Ruiz, M.; Gamez, S.S.; Clavero, M.A. West Nile infection. Enfermedades Infecciosas Microbiología Clínica 2011, 29, 21–26. [Google Scholar] [CrossRef]
- Durand, J.P.; Simon, F.; Tolou, H. West Nile virus: In France again, in humans and horses. La Revue du Praticien 2004, 54, 703–710. [Google Scholar]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef]
- Crook, P.D.; Crowcroft, N.S.; Brown, D.W. West Nile virus and the threat to UK. Commun. Dis. Public Health 2002, 5, 138–143. [Google Scholar]
- McLean, R.G.; Ubico, S.R.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. Curr. Top. Microbiol. Immunol. 2002, 267, 271–308. [Google Scholar] [CrossRef]
- Charrel, R.N.; de Lamballerie, X. West Nile virus, an emerging arbovirus. La Presse Médicale 2004, 33, 1521–1526. [Google Scholar] [CrossRef]
- Murray, K.O.; Mertens, E.; Despres, P. West Nile virus and its emergence in the United States of America. Vet. Res. 2010, 41. [Google Scholar] [CrossRef]
- Takasaki, T. West Nile fever. Nihon Rinsho 2005, 63, 2127–2132. [Google Scholar]
- Beasley, D.W. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy 2011, 3, 269–285. [Google Scholar]
- Lin, S.M.; Koraka, P.; Osterhaus, A.D.; Marina, B.E. West Nile virus: Immunity and pathogenesis. Viruses 2011, 3, 811–828. [Google Scholar] [CrossRef]
- Diamond, M.A. Progress on the development of therapeutics against West Nile virus. Antivir. Res. 2009, 83, 214–227. [Google Scholar] [CrossRef]
- Debiasi, R.L.; Tyler, K.L. West Nile virus memnigoencephalitis. Nat. Clin. Pract. Neurol. 2006, 5, 264–275. [Google Scholar]
- Gould, L.H.; Fikrig, E. West Nile virus: A growing concern? J. Clin. Investig. 2004, 113, 1102–1107. [Google Scholar]
- Garmendia, A.E.; van Kruiningen, H.J.; French, R.A. The West Nile virus: Its recent emergence in North America. Microbes Infect. 2001, 3, 223–229. [Google Scholar]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aaronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- Clements, A.C.A.; Pfeiffer, D.U. Emerging viral zoonoses: Frameworks for spatial and spatiotemporal risk assessment and resource planning. Vet. J. 2009, 182, 21–30. [Google Scholar] [CrossRef]
- Kruska, R.L.; Perry, B.D.; Reid, R.S. Recent Progress in the Development of Decision Support Systems for Improved Animal Health. In Proceedings of the Africa GIS 1995 Meeting, “Integrated Geographic Information Systems Useful for a Sustainable Management of Natural Resources in Africa”, Ivory Coast, CA, USA, 6–9 March 1995.
- McLafferty, S.L. Gis and health care. Annu. Rev. Public Health 2003, 24, 25–42. [Google Scholar]
- Bolling, B.G.; Kennedy, J.H.; Zimmerman, E.G. Seasonal dynamics of four potential West Nile vector species in North-central Texas. J. Vector Ecol. 2005, 30, 186–194. [Google Scholar]
- Ward, M.P.; Ramsay, B.H.; Gallo, K. Rural cases of equine West Nile virus encephalomyelitis and the normalized difference vegetation index. Vector Borne Zoonotic Dis. 2005, 5, 181–188. [Google Scholar] [CrossRef]
- Brownstein, J.S.; Rosen, H.; Purdy, D.; Miller, J.R.; Melino, M.; Mostashari, F.; Fish, D. Spatial analysis of West Nile virus: Rapid risk assessment of an introduced vector-borne zoonosis. Vector Borne Zoonotic Dis. 2002, 2, 157–164. [Google Scholar]
- Ruiz, M.O.; Walker, E.D.; Foster, E.S.; Haramis, L.D.; Kitron, U.D. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int. J. Health Geogr. 2007, 6. Available online: http://www.ij-healthgeographics.com/content/6/1/10 (accessed on 13 September 2013).
- Pfeiffer, D.U.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C.A. Spatial Analysis in Epidemiology; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Peterson, A.T.; Vieglais, D.A.; Andreasen, J.K. Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector Borne Zoonotic Dis. 2003, 3, 27–37. [Google Scholar] [CrossRef]
- Medlock, J.M.; Snow, K.R.; Leach, S. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiol. Infect. 2007, 135, 466–482. [Google Scholar] [CrossRef]
- Clements, A.C.; Pfeiffer, D.U.; Martin, V. Application of knowledge-driven spatial modeling approaches and uncertainty management to a study of Rift Valley fever in Africa. Int. J. Health Geogr. 2006, 5. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1702539/ (accessed on 13 September 2013).
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-induced amplification and epidemic transmission of West Nile virus in Southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef]
- Mongoh, M.N.; Khaitsa, M.L.; Dyer, N.W. Environmnetal and ecological determinants of West Nile virus occurrence in horses in North Dakota, 2002. Epidemiol. Infect. 2007, 135, 57–66. [Google Scholar] [CrossRef]
- Bertoletti, L.; Kitron, U.; Goldberg, T.L. Diversity and evolution of West Nile virus in Illinois and the Unites States, 2002–2005. Virology 2007, 360, 143–149. [Google Scholar] [CrossRef]
- Mostashari, F.; Kulldorff, M.; Hartman, J.J.; Miller, J.R.; Miller, J.R.; Kulasekera, V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg. Infect. Dis. 2003, 9, 641–646. [Google Scholar]
- Gatrell, A.C.; Bailey, T.C. Interactive spatial data analysis in medical geography. Soc. Sci. Med. 1996, 42, 843–855. [Google Scholar] [CrossRef]
- Cuzick, K.; Edwards, R. Spatial clustering for inhomogeneous populations. J. R. Stat. Soc.B 1990, 52, 73–104. [Google Scholar]
- Knox, G. The detection of space-time interactions. Appl. Stat. 1964, 13, 25–29. [Google Scholar] [CrossRef]
- Kulldorf, M.; Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 1995, 14, 799–810. [Google Scholar] [CrossRef]
- Revesz, P.; Wu, S. Spatiotemporal reasoning about epidemiological data. Artif. Intell. Med. 2006, 38, 157–170. [Google Scholar] [CrossRef]
- Gosselin, P.; Lebel, G.; Rivest, S.; Douville-Fradet, M. The integrated system for public health monitoring of West Nile virus (ISHM-WNV): A real-time GIS for surveillance and decision-making. Int. J. Health Geogr. 2005, 4. [Google Scholar] [CrossRef]
- Messina, J.P.; Brown, W.; Amore, G.; Kitron, U.D.; Ruiz, M.O. West Nile virus in the Greater Chicago area: A geographic examination of human illness and risk from 2002 to 2006. URISA J. 2011, 23, 5–22. [Google Scholar]
- Liu, A.; Lee, V.; Galusha, D.; Slade, M.D.; Diuk-Wasser, M.; Andreadis, T.; Scotch, M; Rabinowitz, P.M. Risk factors for human infection with West Nile virus in Connecticut: A multi-year analysis. Int. J. Health Geogr. 2009, 8. [Google Scholar] [CrossRef]
- Brown, H.E.; Childs, J.E.; Diuk-Wasser, M.A.; Fish, D. Ecologic factors associated with West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2008, 14, 1539–1545. [Google Scholar] [CrossRef]
- De Groote, J.P.; Sugumaran, R.; Brend, S.M.; Tucker, B.J.; Bartholomay, L.C. Landscape, demographic, entomological and climatic associations with human disease incidence of West Nile virus in the state of Iowa, USA. Int. J. Health Geogr. 2008, 7. [Google Scholar] [CrossRef]
- Wimberly, M.C.; Hildreth, M.B.; Boyte, S.P.; Lindquist, E.; Kightlinger, L. Ecological niche of the 2003 West Nile virus epidemic in the northern great plains of the United States. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Nielsen, C.F.; Armijos, M.V.; Wheeler, S.; Carpenter, T.E.; Boyce, W.M.; Kelley, K.; Brown, D.; Scott, T.W.; Reisen, W.L. Risk factors associated with human infection during the 2006 West Nile virus outbreak in Davis, a residential community in northern California. Am. J. Trop. Med. Hyg. 2008, 78, pp. 53–62. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2215055/ (accessed on 16 June 2013).
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar]
- Yiannakouilas, N.W.; Schopflocher, D.P.; Svenson, L.W. Modeling geographic variations in West Nile virus. Can. J. Public Health 2006, 97, 374–379. [Google Scholar]
- Liu, H.; Weng, Q.; Gaines, D. Geographic incidence of human West Nile virus in northern Virginia, USA, in relation to incidence in birds and variations in urban environment. Sci. Total Environ. 2011, 409, 4235–4241. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the generalized distance in statistics. Proc. Natl. Inst. Sci. India 1936, 2, 49–55. [Google Scholar]
- Ward, MP.; Scheurmann, J.A. The relationship between equine and human West Nile virus disease occurrence. Vet. Microbiol. 2008, 129, 378–383. [Google Scholar] [CrossRef]
- Leblond, A.; Sandoz, A.; Lefebvre, G.; Zeller, H.; Bicout, D.J. Remote sensing based identification of environmental risk factors associated with West Nile disease in horses in Camargue, France. Prev. Vet. Med. 2007, 79, 20–31. [Google Scholar] [CrossRef]
- SaTScan. Available online: http://www.satscan.org/ (accessed on 23 September 2013).
- Lian, M.; Warner, R.D.; Alexander, J.L.; Dixon, K.R. Using geographic information systems and spatial and space-time scan statistics for a population-based risk analysis of the 2002 equine West Nile epidemic in six contiguous regions of Texas. Int. J. Health Geogr. 2007, 6. [Google Scholar] [CrossRef]
- Hernandez-Jover, M.; Roche, S.; Ward, M.P. The human and animal health impacts of introduction and spread of an exotic strain of West Nile virus in Australia. Prev. Vet. Med. 2013, 109, 186–204. [Google Scholar] [CrossRef]
- Jiangping, S.; Buck, P.; Sockett, P.; Aramini, J.; Pollari, F.A. GIS-driven integrated real-time surveillance pilot system for national West Nile virus dead bird surveillance in Canada. Int. J. Geogr. 2006, 5. [Google Scholar] [CrossRef]
- Center for Food-Borne, Environmental and Zoonotic Infectious Disease, Public Health Agency of Canada (PHAC). Available online: http://www.phac-aspc.gc.ca/efwd-emoha/index-eng.php (accessed on 14 June 2013).
- Soverow, J.E.; Wellenius, G.A.; Fisman, D.N.; Mittleman, M.A. Infectious disease in a warming world: How weather influenced West Nile virus in the United States. (2001–2005). Environ. Health Perspect. 2009, 117, 1049–1055. [Google Scholar]
- Calistri, P.; Ippoliti, C.; Candeloro, L.; Benjelloun, A.; Harrak, M.E.; Bouchra, B.; Danzetta, M.L.; Sabatino, D.D.; Conte, A. Analysis of climatic and environmental variables associated with the occurrence of West Nile virus in Morocco. Prev. Vet. Med. 2013, 110, 549–553. [Google Scholar] [CrossRef]
- Ghosh, D.; Guha, R. Use of genetic algorithm and neural network approaches for risk factor selection: A case study of West Nile virus dynamics in an urban environment. Comput. Environ. Urban Syst. 2010, 34, 189–203. [Google Scholar] [CrossRef]
- Gohosh, D.; Guha, R. Using neural network for mining interpretable relationships of West Nile risk factors. Soc. Sci. Med. 2011, 72, 418–429. [Google Scholar] [CrossRef]
- Liu, H.; Weng, Q.; Gaines, D. Spatio-temporal analysis of the relationship between WNV dissemination and environmental variables in Indianapolis, USA. Int. J. Health Geogr. 2008, 7. [Google Scholar] [CrossRef]
- Liu, H.; Weng, Q. An examination of the effect of landscape pattern, land surface temperature, and socioeconomic conditions on WNV dissemination in Chicago. Environ. Monit. Assess. 2009, 159, 143–161. [Google Scholar] [CrossRef]
- Chuang, T.; Henebry, G.M.; Kimball, J.S.; van Roekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite microwave remote sensing for environmental modeling of mosquito population dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef]
- Cleckner, H.L.; Allen, T.R.; Bellows, S. Remote sensing and modeling of mosquito abundance and habitats in Coastal Virginia, USA. Remote Sens. 2011, 3, 2663–2681. [Google Scholar] [CrossRef]
- Thompson, D.R.; Juarez, M.; Barker, C.M.; Holeman, J.; Lundeen, S.; Mulligan, S.; Painter, T.H.; Pdest, E.; Seidel, F.C.; Ustinov, E. Airborne imaging spectroscopy to monitor urban mosquito microhabitats. Remote Sens. Environ. 2013, 137, 226–233. [Google Scholar] [CrossRef]
- Kim, M.; Holt, J.B.; Eisen, R.; Padgett, K.; Reisen, W.K.; Croft, J. Detection of swimming pools by geographic obkect-based inage analysis to support West Nile virus contro efforts. Photogramm. Eng. Remote Sens. 2011, 77, 1169–1179. [Google Scholar]
- Davis, C.T.; Ebel, G.D.; Lanciotti, R.S.; Brault, A.C.; Guzman, H.; Siirin, M.; Lambert, A.; Parsons, R.E.; Neasley, D.W.; Novak, R.J.; et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004; Evidence for the emergence of a dominant genotyope. Virology 2005, 342, 252–265. [Google Scholar] [CrossRef]
- Snapinn, K.W.; Holmes, E.C.; Young, D.S.; Bernard, K.A.; Kramer, L.D.; Ebel, G.D. Declining growth rate of West Nile virus in North America. Virology 2007, 81, 2531–2534. [Google Scholar] [CrossRef]
- Bertoletti, L.; Kitron, U.D.; Walker, E.D.; Ruiz, M.O.; Brawn, J.D.; Loss, S.R.; Hamer, G.L.; Goldberg, T.L. Fine-scale genetic variation and evolution of West Nile virus in a transmission “hot Spot” in suburban Chicago, USA. Virology 2008, 374, 381–389. [Google Scholar] [CrossRef]
- Wey, C.L.; Griesse, J.; Kightlinger, L.; Wimberly, M.C. Geographic variability in geocoding success for WNV cases in South Dakota. Health Place 2009, 15, 1108–1114. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). West Nile Fever Maps; ECDC: Stockholm, Sweden, 2012. Available online: http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/ (accessed on 1 August 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ozdenerol, E.; Taff, G.N.; Akkus, C. Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature. Int. J. Environ. Res. Public Health 2013, 10, 5399-5432. https://doi.org/10.3390/ijerph10115399
Ozdenerol E, Taff GN, Akkus C. Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature. International Journal of Environmental Research and Public Health. 2013; 10(11):5399-5432. https://doi.org/10.3390/ijerph10115399
Chicago/Turabian StyleOzdenerol, Esra, Gregory N. Taff, and Cem Akkus. 2013. "Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature" International Journal of Environmental Research and Public Health 10, no. 11: 5399-5432. https://doi.org/10.3390/ijerph10115399
APA StyleOzdenerol, E., Taff, G. N., & Akkus, C. (2013). Exploring the Spatio-Temporal Dynamics of Reservoir Hosts, Vectors, and Human Hosts of West Nile Virus: A Review of the Recent Literature. International Journal of Environmental Research and Public Health, 10(11), 5399-5432. https://doi.org/10.3390/ijerph10115399