Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review
Abstract
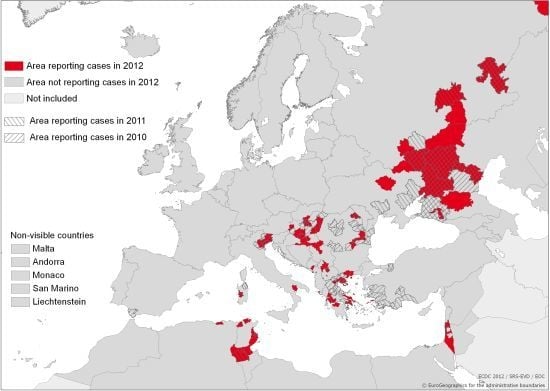
:1. Introduction
| Country | Number of cases | ||
|---|---|---|---|
| 2010 | 2011 | 2012 | |
| Russia | 419 | 135 | 399 |
| Greece | 262 | 100 | 161 |
| Israel | 105 | 33 | 59 |
| Romania | 57 | 10 | 14 |
| Turkey | 47 | 3 | 0 |
| Italy | 6 | 14 | 50 |
| Spain | 2 | 0 | 0 |
| Hungary | 18 | 0 | 10 |
| Ukraine | 0 | 5 | 0 |
| Albania | 0 | 2 | 0 |
| Macedonia | 0 | 4 | 6 |
| Tunisia | 0 | 3 | 32 |
| Serbia | 0 | 0 | 67 |
| Croatia | 0 | 0 | 5 |
| Kosovo | 0 | 0 | 4 |
| Palestinian Territory | 0 | 0 | 2 |
| Total | 1,016 | 309 | 810 |

2. Environmental Drivers
2.1. Climatic Factors
2.1.1. Ambient Temperature
2.1.2. Precipitation
2.1.3. Relative Humidity
2.2. Landscape Features and Land-Use
2.3. Avian Hosts
2.4. Other Drivers
3. Discussion
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Dohm, D.J.; O’Guinn, M.L.; Turell, M.J. Effect of environmental temperature on the ability of Culex Pipiens (Diptera: Culicidae) to transmit West Nile Virus. J. Med. Entomol. 2002, 39, 221–225. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Calder, C.A.; Doran, P.J.; Marra, P.P. West Nile Virus impacts in american crow populations are associated with human land use and climate. Ecol. Res. 2011, 26, 909–916. [Google Scholar] [CrossRef]
- Bakonyi, T.; Hubálek, Z.; Rudolf, I.; Nowotny, N. Novel flavivirus or new lineage of West Nile Virus, central Europe. Emerg. Infect. Dis. 2005, 11, 225–231. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile Fever—A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–658. [Google Scholar] [CrossRef]
- McLean, R.G.; Ubico, S.R.; Docherty, D.E.; Hansen, W.R.; Sileo, L.; McNamara, T.S. West Nile Virus transmission and ecology in birds. Ann. N. Y. Acad. Sci. 2001, 951, 54–57. [Google Scholar]
- Weinberger, M.; Pitlik, S.D.; Gandacu, D.; Lang, R.; Nassar, F.; David, D.B.; Rubinstein, E.; Izthaki, A.; Mishal, J.; Kitzes, R. West Nile Fever outbreak, Israel, 2000: Epidemiologic aspects. Emerg. Infect. Dis. 2001, 7, 686–691. [Google Scholar]
- Dauphin, G.; Zientara, S.; Zeller, H.; Murgue, B. West Nile: Worldwide current situation in animals and humans. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 343–355. [Google Scholar] [CrossRef]
- Gibbs, S.E.J.; Wimberly, M.C.; Madden, M.; Masour, J.; Yabsley, M.J.; Stallknecht, D.E. Factors affecting the geographic distribution of West Nile Virus in Georgia, USA: 2002–2004. Vector Borne Zoonot. 2006, 6, 73–82. [Google Scholar] [CrossRef]
- CDC. Division of Vector-Born Infectious Diseases. West Nile Virus—Transmission. Available online: http://www.cdc.gov/westnile/transmission/index.html (accessed on 11 April 2013).
- Ruiz, M.; Walker, E.; Foster, E.; Haramis, L.; Kitron, U. Association of West Nile Virus illness and urban landscapes in Chicago and Detroit. Int. J. Health Geo. 2007, 6, 10–11. [Google Scholar] [CrossRef]
- Bertolotti, L.; Kitron, U.D.; Walker, E.D.; Ruiz, M.O.; Brawn, J.D.; Loss, S.R.; Hamer, G.L.; Goldberg, T.L. Fine-Scale genetic variation and evolution of West Nile Virus in a Transmission “hot spot” in suburban Chicago, USA. Virology 2008, 374, 381–389. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile Virus. Lancet Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- CDC. FAQ: General Questions about West Nile Virus. Available online: http://www.cdc.gov/westnile/faq/genQuestions.html (accessed on 22 March 2013).
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of temperature on the transmission of West Nile Virus by Culex Tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef]
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of West Nile in Europe and in the Mediterranean Basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar]
- Chaskopoulou, A.; Dovas, C.; Chaintoutis, S.; Bouzalas, I.; Ara, G.; Papanastassopoulou, M. Evidence of enzootic circulation of West Nile Virus (Nea Santa-Greece-2010, Lineage 2), Greece, May to July 2011. Euro Surveill. 2011, 16, 19933. [Google Scholar]
- Monaco, F.; Savini, G.; Calistri, P.; Polci, A.; Pinoni, C.; Bruno, R.; Lelli, R. 2009 West Nile disease epidemic in Italy: First evidence of overwintering in Western Europe? Res. Vet. Sci. 2011, 91, 321–326. [Google Scholar] [CrossRef]
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; del Gobbo, R.; Capobianchi, M.; Menzo, S.; Nicoletti, L.; Magurano, F. Human case of autochthonous West Nile Virus Lineage 2 infection in Italy, September 2011. Euro Surveill. 2011, 16. Available online: http://www.eurosurveillance.org/images/dynamic/EE/V16N43/art20002.pdf (accessed on 9 January 2013).
- Barzon, L.; Pacenti, M.; Cusinato, R.; Cattai, M.; Franchin, E.; Pagni, S.; Martello, T.; Bressan, S.; Squarzon, L.; Cattelan, A. Human cases of West Nile Virus infection in North-Eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011, 16, 33. [Google Scholar]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A. Mosquito, bird and human surveillance of West Nile and Usutu Viruses in Emilia-Romagna Region (Italy) in 2010. PLoS One 2012, 7, e38058. [Google Scholar] [CrossRef]
- Rizzo, C.; Salcuni, P.; Nicoletti, L.; Ciufolini, M.; Russo, F.; Masala, R.; Frongia, O.; Finarelli, A.; Gramegna, M.; Gallo, L. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012, 17, 20. [Google Scholar]
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G. West Nile Virus in Europe: Emergence, epidemiology, diagnosis, treatment, prevention. Clin. Microbiol. Infec. 2013. [Google Scholar] [CrossRef]
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A global perspective on the epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef]
- Randolph, S.E.; Rogers, D.J. The arrival, establishment and spread of exotic diseases: Patterns and predictions. Nat. Rev. Microb. 2010, 8, 361–371. [Google Scholar] [CrossRef]
- Komar, N. West Nile Virus: Epidemiology and ecology in North America. Adv. Virus Res. 2003, 61, 185–234. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Fonseca, D.M.; Ebel, G.D.; Reddy, M.R.; Kramer, L.D. Spatial and temporal variation in vector competence of Culex Pipiens and Cx. Restuans mosquitoes for West Nile Virus. Am. J. Trop. Med. Hyg. 2010, 83, 607–613. [Google Scholar] [CrossRef]
- Cornel, A.J.; Jupp, P.G.; Blackburn, N.K. Environmental temperature on the vector competence of Culex Univittatus (Diptera: Culicidae) for West Nile Virus. J. Med. Entomol. 1993, 30, 449–456. [Google Scholar]
- Paz, S.; Malkinson, D.; Green, M.S.; Tsioni, G.; Papa, A.; Danis, K.; Sirbu, A.; Ceianu, C.; Katalin, K.; Ferenczi, E. Permissive summer temperatures of the 2010 European West Nile Fever upsurge. PloS One 2013, 8, e56398. [Google Scholar] [CrossRef]
- Kinney, R.M.; Huang, C.Y.; Whiteman, M.C.; Bowen, R.A.; Langevin, S.A.; Miller, B.R.; Brault, A.C. Avian virulence and thermostable replication of the North American strain of West Nile Virus. J. Gen. Virol. 2006, 87, 3611–3622. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile Virus by Culex Pipiens mosquitoes. PLoS Pathog 2008, 4, e1000092. [Google Scholar] [CrossRef]
- Andrade, C.C.; Maharaj, P.D.; Reisen, W.K.; Brault, A.C. North American West Nile Virus genotype isolates demonstrate differential replicative capacities in response to temperature. J. Gen. Virol. 2011, 92, 2523–2533. [Google Scholar] [CrossRef]
- Paz, S.; Albersheim, I. Influence of warming tendency on Culex Pipiens population abundance and on the probability of West Nile Fever outbreaks (Israeli Case Study: 2001–2005). EcoHealth 2008, 5, 40–48. [Google Scholar] [CrossRef]
- Meyer, R.; Hardy, J.; Reisen, W. Diel changes in adult mosquito microhabitat temperatures and their relationship to the extrinsic incubation of arboviruses in mosquitoes in Kern County, California. J. Med. Entomol. 1990, 27, 607–614. [Google Scholar]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local impact of temperature and precipitation on West Nile Virus infection in Culex species mosquitoes in Northeast Illinois, USA. Parasites Vector. 2010, 3, 1–16. [Google Scholar] [CrossRef]
- Dohm, D.J.; Turell, M.J. Effect of incubation at overwintering temperatures on the replication of West Nile Virus in New York Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2001, 38, 462–464. [Google Scholar] [CrossRef]
- Jia, Y.; Moudy, R.M.; Dupuis II, A.P.; Ngo, K.A.; Maffei, J.G.; Jerzak, G.V.; Franke, M.A.; Kauffman, E.B.; Kramer, L.D. Characterization of a small plaque variant of West Nile Virus isolated in New York in 2000. Virology 2007, 367, 339–347. [Google Scholar] [CrossRef]
- Kunkel, K.E.; Novak, R.J.; Lampman, R.L.; Gu, W. Modeling the impact of variable climatic factors on the Crossover of Culex Restauns and Culex Pipiens (Diptera: Culicidae), vectors of West Nile Virus in Illinois. Am. J. Trop. Med. Hyg. 2006, 74, 168–173. [Google Scholar]
- Epstein, P.R. West Nile Virus and the climate. J. Urban Health 2001, 78, 367–371. [Google Scholar] [CrossRef]
- Pats, J.A.; Githeko, A.K.; McCarty, J.P.; Hussain, S.; Confalonieri, U.; de Wet, N. Climate Change and fection Disease. In Climate Change and Human Health—Risks and Responses; McMichael, A.J., Campbell-Lendrum, D.H., Corvalán, C.F., Ebi, K.L., Githeko, A.K., Scheraga, J.D., Woodward, A., Eds.; WHO: Geneva, Switzerland, 2003; pp. 103–132. [Google Scholar]
- Paz, S. The West Nile Virus outbreak in Israel (2000) from a new perspective: The regional impact of climate change. Int. J. Environ. Health Res. 2006, 16, 1–13. [Google Scholar] [CrossRef]
- Reisen, W.K. Effect of temperature on Culex Tarsalis (Diptera: Culicidae) from the Coachella and San Joaquin Valleys of California. J. Med. Entomol. 1995, 32, 636–645. [Google Scholar]
- Greer, A.; Ng, V.; Fisman, D. Climate change and infectious diseases in North America: The road ahead. Can. Med. Assoc. J. 2008, 178, 715–722. [Google Scholar]
- Platonov, A.E.; Fedorova, M.V.; Karan, L.S.; Shopenskaya, T.A.; Platonova, O.V.; Zhuravlev, V.I. Epidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (Diptera: Culicidae) bionomics. Parasitol. Res. 2008, 103, 45–53. [Google Scholar] [CrossRef]
- Morin, C.W.; Comrie, A.C. Modeled response of the West Nile Virus Vector Culex Quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int. J. Biometeorol. 2010, 54, 517–529. [Google Scholar] [CrossRef]
- NASA. Climate Change: How do We Know? 2013. Available online: http://climate.Nasa.gov/evidence (accessed on 1 March 2013).
- NOAA. Global Warming—Frequently Asked Questions. 2012. Available online: http://www.Ncdc.Noaa.gov/cmb-faq/globalwarming.Html (accessed on 1 March 2013).
- UNEP. Climate Change—Introduction. 2013. Available online: http://www.Unep.org/climatechange/Introduction/tabid/233/language/fr-FR/Default.Aspx (accessed on 10 March 2013).
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Paz, S.; Xoplaki, E.; Gershunov, A. Scientific Report of the Workshop: Impacts of Mediterranean Climate Change on Human Health. The European Science Foundation (ESF)—MedClivar. 2010. Available online: http://www.medclivar.eu/Science_meeting_reports_FinalReport2488.pdf (accessed on 10 July 2012).
- Richardson, K.; Steffen, W.; Liverman, D. Climate Change: Global Risks, Challenges and Decisions; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Ebi, K.L.; Lindgren, E.; Suk, J.E.; Semenza, J.C. Adaptation to the infectious disease impacts of climate change. Clim. Chang. 2013, 118, 355–365. [Google Scholar] [CrossRef]
- Savage, H.; Ceianu, C.; Nicolescu, G.; Karabatsos, N.; Lanciotti, R.; Vladimirescu, A.; Laiv, L.; Ungureanu, A.; Romanca, C.; Tsai, T. Entomologic and avian investigations of an epidemic of West Nile Fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Am. J. Trop. Med. Hyg. 1999, 61, 600–611. [Google Scholar]
- WMO. Press Release No. 904: 2010 in the Top Three Warmest Years, 2001–2010 Warmest 10-Year Period. Available online: http://www.wmo.int/pages/mediacentre/press_releases/pr_904_en.html (accessed on 4 December 2012).
- Moudy, R.M.; Meola, M.A.; Morin, L.L.L.; Ebel, G.D.; Kramer, L.D. A newly emergent genotype of West Nile Virus is transmitted earlier and more efficiently by Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007, 77, 365–370. [Google Scholar]
- Landesman, W.J.; Allan, B.F.; Langerhans, R.B.; Knight, T.M.; Chase, J.M. Inter-annual associations between precipitation and human incidence of West Nile Virus in the United States. Vector Borne Zoonot. 2007, 7, 337–343. [Google Scholar] [CrossRef]
- Takeda, T.; Whitehouse, C.A.; Brewer, M.; Gettman, A.D.; Mather, T.N. Arbovirus surveillance in Rhode Island: Assessing potential ecologic and climatic correlates. J. Am. Mosq. Control Assoc. 2003, 19, 179–189. [Google Scholar]
- Soverow, J.E.; Wellenius, G.A.; Fisman, D.N.; Mittleman, M.A. Infectious disease in a warming world: How weather influenced West Nile Virus in the United States (2001–2005). Environ. Health Perspect. 2009, 117, 1049–1052. [Google Scholar]
- Shaman, J.; Stieglitz, M.; Stark, C.; Le Blancq, S.; Cane, M. Using a dynamic hydrology model to predict mosquito abundances in flood and swamp water. Emerg. Infect. Dis. 2002, 8, 8–13. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M.; Harrington, L. Flushing effect of rain on container-inhabiting mosquitoes aedes aegypti and Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 28–35. [Google Scholar] [CrossRef]
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-induced amplification and epidemic transmission of West Nile Virus in Southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef]
- Roehr, B. US hit by massive West Nile Virus outbreak centred around Texas. Brit Med J. 2012, 345, e5633. [Google Scholar] [CrossRef]
- Uejio, C.K.; Kemp, A.; Comrie, A.C. Climatic controls on West Nile Virus and Sindbis Virus transmission and outbreaks in South Africa. Vector Borne Zoonot. 2012, 12, 117–125. [Google Scholar] [CrossRef]
- Trawinski, P.; Mackay, D. Meteorologically conditioned time-series predictions of West Nile Virus vector mosquitoes. Vector Borne Zoonot. 2008, 8, 505–522. [Google Scholar] [CrossRef]
- Marra, P.P.; Griffing, S.; Caffrey, C.; Kilpatrick, A.M.; McLEAN, R.; Brand, C.; Saito, E.; Dupuis, A.P.; Kramer, L.; Novak, R. West Nile Virus and wildlife. Bioscience 2004, 54, 393–402. [Google Scholar] [CrossRef]
- Brown, H.E.; Childs, J.E.; Diuk-Wasser, M.A.; Fish, D. Ecologic factors associated with West Nile Virus transmission, northeastern United States. Emerg. Infect. Dis. 2008, 14, 1539–1545. [Google Scholar] [CrossRef]
- Hribar, L.J.; Smith, J.M.; Vlach, J.J.; Verna, T.N. Survey of container-breeding mosquitoes from the Florida Keys, Monroe County, Florida. J. Am. Mosq. Control Assoc. 2001, 17, 245–248. [Google Scholar]
- Reisen, W.K.; Lothrop, H.D.; Wheeler, S.S.; Kennsington, M.; Gutierrez, A.; Fang, Y.; Garcia, S.; Lothrop, B. Persistent West Nile Virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003−2006. J. Med. Entomol. 2008, 45, 494–508. [Google Scholar] [CrossRef]
- Leblond, A.; Sandoz, A.; Lefebvre, G.; Zeller, H.; Bicout, D. Remote sensing based identification of environmental risk factors associated with West Nile Disease in horses in Camargue, France. Prev. Vet. Med. 2007, 79, 20–31. [Google Scholar] [CrossRef]
- Pradier, S.; Leblond, A.; Durand, B. Land cover, landscape structure, and West Nile Virus circulation in southern France. Vector Borne Zoonot. 2008, 8, 253–264. [Google Scholar] [CrossRef]
- Tsai, T.; Popovici, F.; Cernescu, C.; Campbell, G.; Nedelcu, N. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Platonov, A.E.; Shipulin, G.A.; Shipulina, O.Y.; Tyutyunnik, E.N.; Frolochkina, T.I.; Lanciotti, R.S.; Yazyshina, S.; Platonova, O.V.; Obukhov, I.L.; Zhukov, A.N. Outbreak of West Nile Virus infection, Volgograd Region, Russia, 1999. Emerg. Infect. Dis. 2001, 7, 128–132. [Google Scholar] [CrossRef]
- Papa, A.; Danis, K.; Baka, A.; Bakas, A.; Dougas, G.; Lytras, T.; Theocharopoulos, G.; Chrysagis, D.; Vassiliadou, E.; Kamaria, F. Ongoing outbreak of West Nile Virus infections in humans in Greece, July–August 2010. Euro Surveill. 2010, 15. Available online: www.eurosurveillance.org/images/dynamic/EE/V15N34/art19644.pdf (accessed on 15 May 2013).
- Danis, K.; Papa, A.; Theocharopoulos, G.; Dougas, G.; Athanasiou, M.; Detsis, M.; Baka, A.; Lytras, T.; Mellou, K.; Bonovas, S. Outbreak of West Nile Virus infection in Greece, 2010. Emerg. Infect. Dis. 2011, 17. [Google Scholar] [CrossRef]
- Deichmeister, J.M.; Telang, A. Abundance of West Nile Virus mosquito vectors in relation to climate and landscape variables. J. Vector Ecol. 2011, 36, 75–85. [Google Scholar] [CrossRef]
- Savard, J.L.; Clergeau, P.; Mennechez, G. Biodiversity concepts and urban ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- Eisen, L.; Bolling, B.G.; Blair, C.D.; Beaty, B.J.; Moore, C.G. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado front range. J. Med. Entomol. 2008, 45, 800–811. [Google Scholar] [CrossRef]
- Liu, H.; Weng, Q. Environmental factors and risk areas of West Nile Virus in southern California, 2007–2009. Environ. Model. Assess. 2012, 17, 441–452. [Google Scholar] [CrossRef]
- Chuang, T.; Henebry, G.M.; Kimball, J.S.; VanRoekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite microwave remote sensing for environmental modeling of mosquito population dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef]
- Anyamba, A.; Linthicum, K.J.; Tucker, C.J. Climate-disease connections: Rift valley fever in Kenya. Cad. Saúde Pública 2001, 17, S133–S140. [Google Scholar] [CrossRef]
- Gomez-Elipe, A.; Otero, A.; Van Herp, M.; Aguirre-Jaime, A. Forecasting Malaria incidence based on monthly case reports and environmental factors in Karuzi, Burundi, 1997–2003. Malar. J. 2007, 6. [Google Scholar] [CrossRef] [Green Version]
- Bowden, S.E.; Magori, K.; Drake, J.M. Regional differences in the association between land cover and West Nile Virus disease incidence in humans in the United States. Am. J. Trop. Med. Hyg. 2011, 84, 234–238. [Google Scholar] [CrossRef]
- Gates, M.C.; Boston, R.C. Irrigation linked to a greater incidence of human and veterinary West Nile Virus cases in the United States from 2004 to 2006. Prev. Vet. Med. 2009, 89, 134–137. [Google Scholar] [CrossRef]
- García‐Bocanegra, I.; Jaén‐Téllez, J.; Napp, S.; Arenas‐Montes, A.; Fernández‐Morente, M.; Fernández‐Molera, V.; Arenas, A. Monitoring of the West Nile Virus epidemic in Spain between 2010 and 2011. Transbound. Emerg. Dis. 2012, 59, 448–455. [Google Scholar] [CrossRef]
- Rappole, J.H.; Derrickson, S.R.; Hubálek, Z. Migratory birds and spread of West Nile Virus in the western Hemisphere. Emerg. Infect. Dis. 2000, 6, 319–328. [Google Scholar] [CrossRef]
- Weiss, D.; Carr, D.; Kellachan, J.; Tan, C.; Phillips, M.; Bresnitz, E.; Layton, M. Working, West Nile Virus outbreak response. Clinical findings of West Nile Virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg. Infect. Dis. 2001, 7, 654–658. [Google Scholar]
- Reisen, W.; Fang, Y.; Martinez, V. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 2005, 42, 367–375. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Godsey, M.S.; King, R.J.; Guptill, S.C. Avian diversity and West Nile Virus: Testing associations between biodiversity and infectious disease risk. Proc. R. Soc. B. 2006, 273, 109–117. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Calos, S.E. Increased avian diversity is associated with lower incidence of human west nile infection: Observation of the dilution effect. PLoS One 2008, 3, e2488. [Google Scholar] [CrossRef]
- Allan, B.F.; Langerhans, R.B.; Ryberg, W.A.; Landesman, W.J.; Griffin, N.W.; Katz, R.S.; Oberle, B.J.; Schutzenhofer, M.R.; Smyth, K.N.; Maurice, A.D.S. Ecological correlates of risk and incidence of West Nile Virus in the United States. Oecologia 2009, 158, 699–708. [Google Scholar] [CrossRef]
- Bradley, C.A.; Gibbs, S.E.; Altizer, S. Urban land use predicts West Nile Virus exposure in Songbirds. Ecol. Appl. 2008, 18, 1083–1092. [Google Scholar] [CrossRef]
- Maidana, N.A.; Yang, H.M. Spatial spreading of West Nile Virus described by traveling waves. J. Theor. Biol. 2009, 258, 403–417. [Google Scholar] [CrossRef]
- Liu, R.; Shuai, J.; Wu, J.; Zhu, H. Modeling spatial spread of West Nile Virus and impact of directional dispersal of birds. Math. BioSci. Eng. 2006, 3, 145–160. [Google Scholar]
- Rappole, J.; Hubalek, Z. Migratory birds and West Nile Virus. J. Appl. Microbiol. 2003, 94, 47–58. [Google Scholar] [CrossRef]
- May, F.J.; Davis, C.T.; Tesh, R.B.; Barrett, A.D. Phylogeography of West Nile Virus: From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J. Virol. 2011, 85, 2964–2974. [Google Scholar] [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R. Introduction of West Nile Virus in the middle east by migrating white storks. Emerg. Infect. Dis. 2002, 8, 392–397. [Google Scholar] [CrossRef]
- López, G.; Jiménez-Clavero, M.Á.; Tejedor, C.G.; Soriguer, R.; Figuerola, J. Prevalence of West Nile Virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector Borne Zoonot. 2008, 8, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Esteves, A.; Almeida, A.P.G.; Galão, R.P.; Parreira, R.; Piedade, J.; Rodrigues, J.C.; Sousa, C.A.; Novo, M.T. West Nile Virus in southern Portugal, 2004. Vector Borne Zoonot. 2005, 5, 410–413. [Google Scholar] [CrossRef]
- Figuerola, J.; Soriguer, R.; Rojo, G.; Tejedor, C.G.; Jimenez-Clavero, M.A. Seroconversion in wild birds and local circulation of West Nile Virus, Spain. Emerg. Infect. Dis. 2007, 13, 1915–1917. [Google Scholar] [CrossRef]
- Figuerola, J.; Angel Jiménez-Clavero, M.; Rojo, G.; Gomez-Tejedor, C.; Soriguer, R. Prevalence of West Nile Virus neutralizing antibodies in colonial aquatic birds in southern Spain. Avian Pathol. 2007, 36, 209–212. [Google Scholar] [CrossRef]
- Figuerola, J.; Jiménez-Clavero, M.A.; López, G.; Rubio, C.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet. Microbiol. 2008, 132, 39–46. [Google Scholar] [CrossRef]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernandez-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodriguez-Ramos, J.; Perez-Ramirez, E.; Höfle, U. West Nile Virus in golden eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491. [Google Scholar] [CrossRef] [Green Version]
- López, G.; Jiménez-Clavero, M.Á.; Vázquez, A.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A.; Figuerola, J. Incidence of West Nile Virus in birds arriving in wildlife rehabilitation centers in southern Spain. Vector Borne Zoonot. 2011, 11, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Lelli, R.; Calistri, P.; Bruno, R.; Monaco, F.; Savini, G.; Di Sabatino, D.; Corsi, I.; Pascucci, I. West Nile transmission in resident birds in Italy. Transbound. Emerg. Dis. 2012, 59, 421–428. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J.; Juricova, Z.; Šikutová, S.; Rudolf, I.; Honza, M.; Janková, J.; Chytil, J.; Marec, F.; Sitko, J. Serologic survey of birds for West Nile Flavivirus in southern Moravia (Czech Republic). Vector Borne Zoonot 2008, 8, 659–666. [Google Scholar] [CrossRef]
- Hubálek, Z.; Wegner, E.; Halouzka, J.; Tryjanowski, P.; Jerzak, L.; Šikutová, S.; Rudolf, I.; Kruszewicz, A.G.; Jaworski, Z.; Wlodarczyk, R. Serologic survey of potential vertebrate hosts for West Nile Virus in Poland. Viral Immunol. 2008, 21, 247–254. [Google Scholar]
- Malkinson, M.; Banet, C. The Role of Birds in the Ecology of West Nile Virus in Europe and Africa. In Japanese Encephalitis and West Nile Viruses; Springer: Berlin-Heidelberg, Germany, 2002; pp. 309–322. [Google Scholar]
- Jourdain, E.; Gauthier-Clerc, M.; Bicout, D.; Sabatier, P. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg. Infect. Dis. 2007, 13, 365–372. [Google Scholar] [CrossRef]
- Peterson, A.T.; Vieglais, D.A.; Andreasen, J.K. Migratory birds modeled as critical transport agents for West Nile Virus in north America. Vector Borne Zoonot. 2003, 3, 27–37. [Google Scholar] [CrossRef]
- Owen, J.; Moore, F.; Panella, N.; Edwards, E.; Bru, R.; Hughes, M.; Komar, N. Migrating birds as dispersal vehicles for West Nile Virus. EcoHealth 2006, 3, 79–85. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, west nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- Ji-Guang, M.; Mei, X. Progress in studies on the overwintering of the mosquito Culex Tritaeniorhynchus. Southeast Asian J. Trop. Med. Public Health 1996, 27, 810–817. [Google Scholar]
- Komar, N.; Clark, G.G. West Nile Virus activity in Latin America and the Caribbean. Rev. Panam. Salud Publica 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Tsuda, Y.; Komagata, O.; Kasai, S.; Hayashi, T.; Nihei, N.; Saito, K.; Mizutani, M.; Kunida, M.; Yoshida, M.; Kobayashi, M. A mark-release-recapture study on dispersal and flight distance of Culex Pipiens pallens in an urban area of Japan. J. Am. Mosq. Control Assoc. 2008, 24, 339–343. [Google Scholar] [CrossRef]
- Walton, W.E.; Workman, P.D.; Tempelis, C.H. Dispersal, survivorship, and host selection of Culex Erythrothorax (Diptera: Culicidae) associated with a constructed wetland in southern California. J. Med. Entomol. 1999, 36, 30–40. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Suk, J.E.; Semenza, J.C. Future infectious disease threats to Europe. Am. J. Public Health 2011, 101, 2068–2079. [Google Scholar] [CrossRef]
- DeGroote, J.P.; Sugumaran, R. National and regional associations between human West Nile Virus incidence and demographic, landscape, and land use conditions in the coterminous United States. Vector Borne Zoonot. 2012, 12, 657–665. [Google Scholar] [CrossRef]
- Tackett, J.; Charnigo, R.; Caldwell, G. Relating West Nile Virus Case fatality rates to demographic and surveillance variables. Public Health Rep. 2006, 121, 666–673. [Google Scholar]
- Reeves, W.C.; Hardy, J.L.; Reisen, W.K.; Milby, M.M. Potential effect of global warming on mosquito-borne arboviruses. J. Med. Entomol. 1994, 31, 323–332. [Google Scholar]
- Cotton, P.A. Avian migration phenology and global climate change. Proc. Natl. Acad. Sci. USA 2003, 100, 12219–12222. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Mills, A.M. Changes in the timing of spring and autumn migration in north American migrant passerines during a period of global warming. IBIS 2005, 147, 259–269. [Google Scholar] [CrossRef]
- Sparks, T.; Bairlein, F.; Bojarinova, J.; Hüppop, O.; Lehikoinen, E.; Rainio, K.; Sokolov, L.; Walker, D. Examining the Total Arrival Distribution of Migratory Birds. Glob. Chang. Biol. 2005, 11, 22–30. [Google Scholar] [CrossRef]
- Marra, P.P.; Francis, C.M.; Mulvihill, R.S.; Moore, F.R. The Influence of Climate on the Timing and Rate of Spring Bird Migration. Oecologia 2005, 142, 307–315. [Google Scholar] [CrossRef]
- Jenni, L.; Kéry, M. Timing of autumn bird migration under climate change: Advances in long-distance migrants, delays in short-distance migrants. Proc. R. Soc. Lond. B Biol. 2003, 270, 1467–1471. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird migration times, climate change, and changing population sizes. Glob. Chang. Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Bengtsson, L.; Hodges, K.I.; Roeckner, E. Storm tracks and climate change. J. Clim. 2006, 19, 3518–3543. [Google Scholar] [CrossRef]
- Hurrell, J.W.; Kushnir, Y.; Ottersen, G.; Visbeck, M. An overview of the north Atlantic oscillation. Geophys. Monogr. Ser. 2003, 134, 1–36. [Google Scholar] [CrossRef]
- Webster, P.J.; Holland, G.J.; Curry, J.A.; Chang, H. Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 2005, 309, 1844–1846. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E.; Estevez, V.; Ebi, K.L.; Lindgren, E. Mapping climate change vulnerabilities to infectious diseases in Europe. Environ. Health Perspect. 2012, 120, 385–392. [Google Scholar]
- Kilpatrick, A.M. Globalization, land use, and the invasion of West Nile Virus. Science 2011, 334, 323–327. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Paz, S.; Semenza, J.C. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review. Int. J. Environ. Res. Public Health 2013, 10, 3543-3562. https://doi.org/10.3390/ijerph10083543
Paz S, Semenza JC. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review. International Journal of Environmental Research and Public Health. 2013; 10(8):3543-3562. https://doi.org/10.3390/ijerph10083543
Chicago/Turabian StylePaz, Shlomit, and Jan C. Semenza. 2013. "Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review" International Journal of Environmental Research and Public Health 10, no. 8: 3543-3562. https://doi.org/10.3390/ijerph10083543
APA StylePaz, S., & Semenza, J. C. (2013). Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review. International Journal of Environmental Research and Public Health, 10(8), 3543-3562. https://doi.org/10.3390/ijerph10083543