Gonadal Disorder in the Thinlip Grey Mullet (Liza ramada, Risso 1827) as a Biomarker of Environmental Stress in Surface Waters
Abstract
:1. Introduction
2. Experimental Section
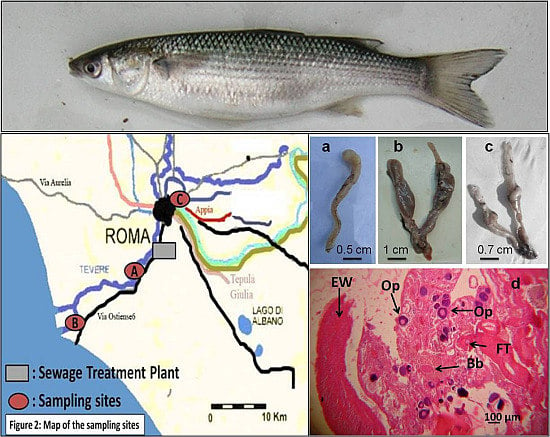
2.1. Description of the Study Area
2.2. Sample Collection
2.3. Sample Observation
| Title | Term | Diagnostic Description |
|---|---|---|
| Gross morphology level | Degeneration of gonadal tissue (Castration according to Hecker et al. [7]) | Removal of the gonads or their destruction as by external influence resulting in a non fertile organism |
| Segmented gonads | Gonads are segmented into discrete subunits with obvious gonadal tissue separated by thin pieces of connective or non gonadal tissue | |
| Histology level | Intersex (Mixed gonadal tissue) | Testicular and ovarian tissues occur in the same individual; phenotypic sex is unclear |
| Intersex (Testicular oocytes) | Oocytes present in the testes regardless of maturation stage |
2.4. Statistics
3. Results
3.1. Physical and Chemical Analyses of the Water at the Sampling Sites
| Sampling Site | Sampling Season | T °C | pH | O2 % |
|---|---|---|---|---|
| A: Tiber River, downstream of the sewage treatment plant | Summer 2010 | 21 | 7.7 | 34.7 |
| Winter 2010/2011 | 10 | 7.8 | 84.8 | |
| Spring 2011 | 18 | 7.8 | 52.5 | |
| B: Tiber River, estuarine area | Summer 2010 | 22.5 | 7.7 | 41.1 |
| Winter 2010/2011 | 13 | 7.7 | 81.3 | |
| Spring 2011 | 17 | 7.6 | 70.6 | |
| C: LEEA pond, uncontaminated reference site | Summer 2010 | 24 | 7.9 | 84.9 |
| Winter 2010/2011 | 15 | 8.1 | 97.8 | |
| Spring 2011 | 20 | 7.8 | 82.4 |
3.2. Fish Analysis
| Sampling Site | N. of Fish Examined | Sex of Fish Examined | Fish Size (M-F-GA) | |||
|---|---|---|---|---|---|---|
| M | F | GA | TL (cm) | Weight (g) | ||
| A | 101 | 58 | 36 | 7 | 39.4 ± 11.4 | 702 ± 280 |
| B | 58 | 29 | 26 | 3 | 37.7 ± 13.4 | 671 ± 311 |
| C | 47 | 19 | 28 | 0 | 31 ± 10.5 * | 321 ± 124 * |
| Total | 206 | 106 | 90 | 10 | ||



| Sampling Site | Sampling Season | Number (%) of Fish with: | ||
|---|---|---|---|---|
| Examined | Irregularly Shaped Gonads | Intersex Gonads | ||
| A | Summer 2010 | 35 | 2 (5.7) | 7 (20.0) |
| Winter 2010/2011 | 45 | 0 | 11 (24.4) | |
| Spring 2011 | 21 | 1 (4.8) | 0 | |
| Total | 101 | 3 (2.9) | 18 (17.8) | |
| B | Summer 2010 | 16 | 0 | 2 (12.5) |
| Winter 2010/2011 | 20 | 0 | 4 (20.0) | |
| Spring 2011 | 22 | 0 | 0 | |
| Total | 58 | 0 | 6 (10.3) | |
| TOTAL | 159 | 3 (1.9) | 24 (15.1) | |
3.3. Gross Indices
| Sampling Season | Gross Indices in Fish Collected at: | |||||||
|---|---|---|---|---|---|---|---|---|
| Site A | n | Site B | n | Site C | n | |||
| Summer 2010 | HIS (%) | M | 1.8 ± 0.4 | 14 | 2.9 ± 1.6 * | 8 | 0.8 ± 0.2 | 7 |
| F | 1.7 ± 0.3 | 13 | 1.6 ± 0.6 | 6 | 0.8 ± 0.2 * | 13 | ||
| GA | 2.0 ± 0.3 | 7 | 2.2 ± 0.9 | 2 | - | - | ||
| GSI (%) | M | 0.1 ± 0.1 | 14 | 0.4 ± 0.5 | 10 | 0.4 ± 0.7 | 7 | |
| F | 0.2 ± 0.2 | 13 | 0.1 ± 0.05 | 6 | 0.5 ± 0.3 * | 13 | ||
| GA | 0.1 ± 0.1 | 7 | 0.1 ± 0.1 | 2 | - | - | ||
| Winter 2010/2011 | HIS (%) | M | 1.7 ± 0.4 | 17 | 1.6 ± 0.3 | 9 | 0.7 ± 0.2 * | 9 |
| F | 1.7 ± 0.3 | 17 | 1.6 ± 0.2 | 7 | 1 ± 0.4 * | 6 | ||
| GA | 1.9 ± 0.3 | 11 | 1.7 ± 0.3 | 4 | - | - | ||
| GSI (%) | M | 0.2 ± 0.1 | 17 | 0.1 ± 0.1 | 9 | 1.1 ± 0.5 * | 8 | |
| F | 0.3 ± 0.3 | 17 | 0.1 ± 0.1 | 7 | 2.8 ± 4.6 * | 3 | ||
| GA | 0.2 ± 0.1 | 11 | 0.1 ± 0.04 | 3 | - | - | ||
| Spring 2011 | HIS (%) | M | 1.8 ± 0.3 | 14 | 1.7 ± 0.5 | 9 | 1.4 ± 0.8 | 3 |
| F | 1.9 ± 0.3 | 6 | 1.4 ± 0.2 | 13 | 1.5 ± 0.8 | 9 | ||
| GA | 2.37 | 1 | - | - | - | - | ||
| GSI (%) | M | 0.1 ± 0.05 | 14 | 0.1 ± 0.1 | 9 | 0. 1 ± 0.02 | 3 | |
| F | 0.2 ± 0.1 * | 6 | 0.4 ± 0.2 | 13 | 0. 4 ± 0.05 | 9 | ||
| GA | 0.3 | 1 | - | - | - | - | ||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adams, S.M.; Greeley, M.S., Jr.; Ryon, M.G. Evaluating effects of contaminants on fish health at multiple levels of biological organization. Extrapolating from lower to higher levels. Hum. Ecol. Risk Assess. 2000, 6, 15–27. [Google Scholar]
- Scardi, M.; Tancioni, L.; Cataudella, S. Monitoring methods based on fish. In Biological Monitoring of Rivers: Applications and Perspectives; Ziglio, G., Siligardi, M., Flaim, G., Eds.; Wiley: London, UK, 2006; pp. 134–153. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environment risk assessment: A review. Environ. Toxicol. Pharmacol. 2002, 13, 57–149. [Google Scholar] [CrossRef]
- Mayer, F.L.; Versteeg, D.J.; McKee, M.J.; Folmar, L.C.; Graney, R.L.; McCume, D.C.; Rattner, B.A. Physiological and nonspecific biomarkers. In Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress; Huggett, R.J., Kimerle, R.A., Mehrle, P.M., Bergman, H.L., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 5–86. [Google Scholar]
- Schmitt, C.J.; Dethloff, G.M. Biomonitoring of Environmental Status and Trends (BEST) Program: Selected Methods for Monitoring Chemical Contaminants and Their Effects in Aquatic Ecosystems. U.S. Geological Survey; Information and Technology Report USGS/BRD-2000–0005; Biological Resources Division: Columbia, MO, USA, 2000; p. 81. [Google Scholar]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total. Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Murphy, M.B.; Coady, K.K.; Villeneuve, D.L.; Jones, P.D.; Carr, J.A.; Solomon, K.R.; Smith, E.E.; van der Kraak, G.; Gross, T.; et al. Terminology of gonadal anomalies in fish and amphibians resulting from chemical exposures. Rev. Environ. Contam. Toxicol. 2006, 187, 103–131. [Google Scholar] [PubMed]
- Metcalfe, C.D.; Kidd, K.A.; Sumpter, J.P. Chemically induced alterations to gonadal differentiation in fish. In Fish Diseases and Disorders. Non-Infectious Disorders, 2nd ed.; Leatherland, J.F., Woo, P.T.K., Eds.; CABI Publishing: Preston, UK, 2010; Volume 2, pp. 144–165. [Google Scholar]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.D.; Foster, S.W.; Reeser, S.J. Intersex (testicular oocytes) in smallmouth bass, Micropterus dolomieu, from the Potomac River and selected nearby drainages. J. Aquat. Anim. Health 2007, 19, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Klüver, N. Effects of endocrine disrupters on sexual, gonadal development in fish. Sex. Dev. 2009, 3, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Puy-Azurmendi, E.; Ortiz-Zarragoitia, M.; Villagrasa, M.; Kuster, M.; Aragón, P.; Atienza, J.; Puchades, R.; Maquieira, A.; Domínguez, C.; López de Alda, M.; et al. Endocrine disruption in thicklip grey mullet (Chelon labrosus) from the Urdaibai Biosphere Reserve (Bay of Biscay, Southwestern Europe). Sci. Total. Environ. 2013, 443, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Bizarro, C.; Ros, O.; Vallejo, A.; Prieto, A.; Etxebarria, N.; Cajaraville, M.P.; Ortiz-Zarragoitia, M. Intersex condition and molecular markers of endocrine disruption in relation with burdens of emerging pollutants in thicklip grey mullets (Chelon labrosus) from Basque estuaries (South-East Bay of Biscay). Mar. Environ. Res. 2014, 96, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Global assessment of the state-of-the-science of endocrine disruptors. In International Programme on Chemical Safety; WHO/PCS/EDC/02.2; Damstra, T.; Barlow, S.; Bergman, A.; Kavlock, R.; van der Kraak, G. (Eds.) World Health Organization: Geneva, Switzerland, 2002; pp. 1–133.
- Bahamonde, P.A.; Munkittrick, K.R.; Martyniuk, C.J. Intersex in teleost fish: Are we distinguishing endocrine disruption from natural phenomena? Gen. Comp. Endocr. 2013, 192, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bateman, K.S.; Stentiford, G.D.; Feist, S.W. A ranking system for the evaluation of intersex condition in European flounder (Platichthys flesus). Environ. Toxicol. Chem. 2004, 23, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Van Aerle, R.; Nolan, M.; Jobling, S.; Christiansen, L.B.; Sumpter, J.P.; Tyler, C.R. Sexual disruption in a second species of wild cyprinid fish (the gudgeon, Gobio gobio) in United Kingdom freshwaters. Environ. Toxicol. Chem. 2001, 20, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Harries, J.E.; Janbakshs, A.; Jobling, S.; Matthiessen, P.; Sumpter, J.P.; Tyler, C.R. Estrogenic potency of effluent from two sewage treatment works in the United Kingdom. Environ. Toxicol. Chem. 1999, 18, 932–937. [Google Scholar] [CrossRef]
- Puzzi, C.; Bottero, S.; Cevasco, A.; Massari, A.; Monteverde, M.; Pedemonte, F.; Bertolotti, R.; Viganò, L.; Mandich, A. Fish community characterization in two stretches upstream and downstream of the Lambro River confluence with the Po River. Ann. N.Y. Acad. Sci. 2005, 1040, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; Peres, I.; Pihan, J.C. Comparative study of the estrogenic responses of mirror carp (Cyprinus carpio) exposed to treated municipal sewage effluent (Lisbon) during two periods in different seasons. Sci. Total. Environ. 2005, 349, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Snyder, S.A.; Kelly, K.J.; Gross, T.S.; Villeneuve, D.L.; Fitzgerald, S.D.; Villalobos, S.A.; Geisy, J.P. Reproductive responses of common carp (Cyprinus carpio) exposed in cages to influent of the Las Vegas wash in Lake Mead, Nevada from late winter to early spring. Environ. Sci. Technol. 2004, 38, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Zarragoitia, M.; Bizarro, C.; Rojo-Bartolomé, I.; Diaz de Cerio, O.; Cajaraville, M.P.; Cancio, I. Mugilid fish are sentinels of exposure to endocrine disrupting compounds in coastal and estuarine environments. Mar. Drugs 2014, 12, 4756–4782. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.J.; Roumillat, W.A.; Wenner, C.A. Sexual differentiation and gonad development in striped mullet (Mugil cephalus L.) from South Carolina estuaries. Fish Bull. 2005, 103, 601–619. [Google Scholar]
- Aoki, J.Y.; Nagae, M.; Takao, Y.; Hara, A.; Lee, Y.D.; Yeo, I.K.; Lim, B.S.; Park, C.B.; Soyano, K. Survey of contamination of estrogenic chemicals in Japanese and Korean coastal waters using the wild grey mullet (Mugil cephalus). Sci. Total. Environ. 2010, 408, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Antunes, P.; Gil, O.; Vale, C.; Reis-Henriques, M.A. Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from Douro estuary, and their use as bioindicator species for environmental monitoring. Aquat. Toxicol. 2004, 69, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Tancioni, L.; Caprioli, R.; Dawood Al-Khafaji, A.H.; Mancini, L.; Boglione, C.; Ciccotti, E.; Cataudella, S. Anthropogenic threats to fish of interest in aquaculture: Gonad intersex in a wild population of thinlip grey mullet Liza ramada (Risso, 1827) from a polluted estuary in central Italy. Aquac. Res. 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—The Remane diagram with a suggested revised model for estuaries: A review. Estuar. Coast Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Waltham, N.J.; Teasdale, P.R.; Connolly, R.M. Use of flathead mullet (Mugil cephalus) in coastal biomonitor studies: Review and recommendations for future studies. Mar. Pollut. Bull. 2013, 69, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Cardona, L. Habitat selection by grey mullets (Osteichthyes: Mugilidae) in Mediterranean estuaries: The role of salinity. Sci. Mar. 2006, 70, 443–455. [Google Scholar] [CrossRef]
- Crosetti, D.; Cataudella, S. Grey mullet culture. In World Animal Science 34B: Production of Aquatic Animals; Nash, C.E., Ed.; Elsevier B.V.: Burlington, MA, USA, 1995; pp. 271–288. [Google Scholar]
- Maitland, P.S.; Campbell, R.N. Freshwater Fishes of the British Isles; HarperCollins Publishers: London, UK, 1992; p. 368. [Google Scholar]
- Pombo, L.; Elliot, M.; Rebelo, J.E. Environmental influences on fish assemblage distribution of an estuarine costal lagoon, Ria De Aveiro (Portugal). Sci. Mar. 2005, 69, 143–159. [Google Scholar] [CrossRef]
- ARPA Lazio, Sezione Provinciale di Roma, Servizio Risorse idriche e naturali. Quarto Rapporto Sulla Qualità Delle Acque Superficiali e Sotterranee Della Provincia di Roma. 2007. Available online: http//www.arpalazio.it/dati2007/IV_rapportoAcqua.pdf (accessed on 22 October 2014).
- Patrolecco, L.; Capri, S.; de Angelis, S.; Pagnotta, R.; Polesello, S.; Valsecchi, S. Partition of nonylphenol and related compounds among different aquatic compartments in Tevere River (central Italy). Water Air Soil Poll. 2006, 172, 151–166. [Google Scholar] [CrossRef]
- Mattei, D.; Cataudella, S.; Mancini, L.; Tancioni, L.; Migliore, L. Tiber River quality in the stretch of a sewage treatment plant: Toxicity of river water or disinfectants to Daphnia and alteration of benthic macroinvertebrates community. Water Air Soil Poll. 2006, 177, 441–455. [Google Scholar] [CrossRef]
- Bettinetti, R.; Galassi, S.; Quadroni, S.; Volta, P.; Capoccioni, F.; Ciccotti, E.; de Leo, G.A. Use of Anguilla anguilla for biomonitoring persistent organic pollutants (POPs) in Brackish and Riverine Waters in Central and Southern Italy. Water Air Soil Poll. 2011, 217, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Mancini, L.; Caimi, S.; Ciardullo, S.; Zeiner, M.; Bottoni, P.; Tancioni, L.; Cataudella, S.; Caroli, S. A pilot study on the contents of selected pollutants in fish from the Tiber River (Rome). Microch. J. 2005, 79, 171–175. [Google Scholar] [CrossRef]
- Jafri, S.I.H. Gametogenesis in roach, Rutilus rutilus (L.) (Cyprinidae: Teleostei). Pak. J. Zool. 1990, 22, 361–377. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeont. Electr. 2001, 4, 1–9. [Google Scholar]
- Tetreault, G.R.; Bennett, C.J.; Shires, K.; Knight, B.; Servos, M.; McMaster, M.E. Intersex and reproductive impairment of two species of wild fish exposed to multiple municipal wastewater discharges. Aquat. Toxicol. 2011, 104, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, B.; Acarli, D. Hermaphrodite thin-lipped mullet Liza ramada (Risso, 1810) (Teleostei: Mugilidae) from Homa Lagoon (Izmir Bay-Aegean Sea). Aquac. Res. 2006, 37, 1050–1052. [Google Scholar] [CrossRef]
- Diniz, M.S.; Peres, I.; Magalhaes-Antoine, I.; Falla, J.; Pihan, J.C. Estrogenic effects in crucian carp (Carassius carassius) exposed to treated sewage effluent. Ecotox. Environ. Saf. 2005, 62, 427–435. [Google Scholar] [CrossRef]
- Thomas, P.; Rahman, M.S.; Khan, I.A.; Kummer, J.A. Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proc. R. Soc. B Biol. Sci. 2007, 274, 2693–2702. [Google Scholar] [CrossRef]
- Thomas, P.; Rahman, M.S. Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proc. R. Soc. B 2011, 5. [Google Scholar] [CrossRef]
- Koger, C.S.; Teh, S.J.; Hinton, D.E. Determining the sensitive stages of intersex induction in medaka (Oryzias latipes) exposed to 17 b-estradiol or testosterone. Mar. Environ. Res. 2000, 50, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish? An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Coady, K.K.; Murphy, M.B.; Villeneuve, D.L.; Hecker, M.; Carr, J.A.; Solomon, K.R.; Smith, E.E.; van der Kraak, G.; Kendall, R.J.; Giesy, J.P. Effects of atrazine on metamorphosis, growth, and gonadal and laryngeal development in Xenopus laevis. Ecotox. Environ. Saf. 2005, 62, 160–173. [Google Scholar] [CrossRef]
- Barrett, T.J.; Munkittrick, K.R. Seasonal reproductive patterns and recommended sampling times for sentinel fish species used in environmental effects monitoring programs in Canada. Environ. Rev. 2010, 18, 115–135. [Google Scholar] [CrossRef]
- Harries, J.E.; Runnalls, T.; Hill, E.; Harris, C.A.; Maddix, S.; Sumpter, J.P.; Tyler, C.R. Development of a reproductive performance test for endocrine disrupting chemicals using pair-breeding fathead minnows (Pimephales promelas). Environ. Sci. Technol. 2000, 34, 3003–3011. [Google Scholar] [CrossRef]
- Prado, P.S.; Souza, C.C.; Bazzoli, N.; Rizzo, E. Reproductive disruption in lambari Astyanax fasciatus from a Southeastern Brazilian reservoir. Ecotoxicol. Environ. Saf. 2011, 74, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.E.; Munkittrick, K.R.; Luxon, P.L.; van der Kraak, G.J. Impact of low-level sampling stress on interpretation of physiological responses of white sucker exposed to effluent from a bleached kraft pulp mill. Ecotox. Environ. Saf. 1994, 27, 251–264. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tancioni, L.; Caprioli, R.; Al-Khafaji, A.H.D.; Mancini, L.; Boglione, C.; Ciccotti, E.; Cataudella, S. Gonadal Disorder in the Thinlip Grey Mullet (Liza ramada, Risso 1827) as a Biomarker of Environmental Stress in Surface Waters. Int. J. Environ. Res. Public Health 2015, 12, 1817-1833. https://doi.org/10.3390/ijerph120201817
Tancioni L, Caprioli R, Al-Khafaji AHD, Mancini L, Boglione C, Ciccotti E, Cataudella S. Gonadal Disorder in the Thinlip Grey Mullet (Liza ramada, Risso 1827) as a Biomarker of Environmental Stress in Surface Waters. International Journal of Environmental Research and Public Health. 2015; 12(2):1817-1833. https://doi.org/10.3390/ijerph120201817
Chicago/Turabian StyleTancioni, Lorenzo, Riccardo Caprioli, Ayad Hantoosh Dawood Al-Khafaji, Laura Mancini, Clara Boglione, Eleonora Ciccotti, and Stefano Cataudella. 2015. "Gonadal Disorder in the Thinlip Grey Mullet (Liza ramada, Risso 1827) as a Biomarker of Environmental Stress in Surface Waters" International Journal of Environmental Research and Public Health 12, no. 2: 1817-1833. https://doi.org/10.3390/ijerph120201817
APA StyleTancioni, L., Caprioli, R., Al-Khafaji, A. H. D., Mancini, L., Boglione, C., Ciccotti, E., & Cataudella, S. (2015). Gonadal Disorder in the Thinlip Grey Mullet (Liza ramada, Risso 1827) as a Biomarker of Environmental Stress in Surface Waters. International Journal of Environmental Research and Public Health, 12(2), 1817-1833. https://doi.org/10.3390/ijerph120201817