Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old
Abstract
:1. Introduction
2. Methodology
2.1. Sampling Frame
2.2. Microactivity Data Collection
2.3. Observer Training
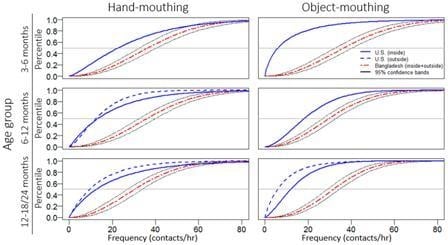
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Inter-Observer Agreement
3.2. Observational Results
4. Discussion
5. Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

| Object Super-Category | Mouthing Frequency (Contacts/h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | ||||||||||||
| 3–6 Months (n = 21) | 6–12 Months (n = 104) | 12–18 Months (n = 23) | ||||||||||
| Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | |
| Hands | 39.5 | 37.3 | 6.5 | 93.6 | 35.8 | 34.4 | 3.7 | 90.0 | 32.3 | 29.7 | 7.9 | 80.7 |
| Objects | 29.6 | 23.1 | 4.7 | 73.6 | 31.6 | 29.6 | 2.0 | 94.6 | 17.0 | 15.2 | 2.7 | 42.4 |
| Cloth | 10.9 | 10.4 | 3.4 | 24.7 | 9.3 | 8.3 | 0.0 | 45.1 | 5.1 | 3.9 | 1.7 | 13.4 |
| Other objects | 18.7 | 9.0 | 0.0 | 52.6 | 22.2 | 20.7 | 1.3 | 79.5 | 12.0 | 8.8 | 0.9 | 38.5 |
| Dietary intake (mouthfuls) | 6.8 | 6.9 | 0.0 | 26.2 | 14.5 | 12.8 | 0.0 | 56.6 | 20.0 | 16.6 | 6.1 | 46.5 |
| Water ingestion (# of times) | 1.6 | 0.7 | 0.0 | 7.4 | 3.2 | 2.4 | 0.0 | 15.1 | 4.6 | 3.8 | 1.1 | 16.3 |
| Soil ingestion | 0.1 | 0.0 | 0.0 | 0.7 | 0.3 | 0.0 | 0.0 | 5.5 | 0.3 | 0.0 | 0.0 | 1.6 |
| Feces ingestion | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| (A) Soil Ingestion | |||||||||||
| Study | Age Group | Mouthers | Children Observed | % | Child-Hours of Observation | Frequency (/h) | |||||
| Mean | Median | ||||||||||
| This study | 3–6 months | 2 | 21 | 9.5 | 77 | 0.1 | 0 | ||||
| Ngure [22] | 3–6 months | 1 | 7 | 14.3 | 42 | 1.7 | - | ||||
| This study | 6–12 months | 29 | 104 | 27.9 | 420 | 0.3 | 0 | ||||
| Ngure [22] | 6–12 months | 0 | 7 | 0 | 42 | NA | NA | ||||
| This study | 12–18 months | 8 | 23 | 34.8 | 87 | 0.3 | 0 | ||||
| Ngure [22] | 12–18 months | 2 | 7 | 28.6 | 42 | 2 | - | ||||
| This study | 3–18 months | 39 | 148 | 26.4 | 590 | 1 | 0.5 | ||||
| Ngure [22] | 3–18 months | 3 | 21 | 14.3 | 126 | 1.9 | - | ||||
| George [17] | 6 to 30 months | 38 | 216 | 17.6 | 1080 | >0.2 times/h | |||||
| (B) Feces Ingestion | |||||||||||
| This study | 3–6 months | 0 | 21 | 0 | 77 | 0 | 0 | ||||
| Ngure [22] | 3–6 months | 0 | 7 | 0 | 42 | NA | NA | ||||
| This study | 6–12 months | 3 | 104 | 2.9 | 420 | 0 | 0 | ||||
| Ngure [22] | 6–12 months | 1 | 7 | 14.3 | 42 | 0.2 | - | ||||
| This study | 12–18 months | 1 | 23 | 4.3 | 90 | 0 | 0 | ||||
| Ngure [22] | 12–18 months | 3 | 7 | 42.9 | 42 | 0.5 | - | ||||
| This study | 3–18 months | 4 | 148 | 2.7 | 590 | 0.6 | 0.6 | ||||
| Ngure [22] | 3–18 months | 4 | 21 | 19 | 126 | 0.3 | - | ||||
| George [17] | 6 to 30 months | 1 | 216 | 0.5 | 1080 | > 0.2 times/h | |||||
| Marquis [18] | 0–60 months | not given | 21 | 120 | 0.3 | - | |||||
| Object Super-Category | Median Frequency (Contacts/h) | ||
|---|---|---|---|
| Study Arm | |||
| Control | Sanitation | Water + Sanitation + Hygiene | |
| (n = 49) | (n = 49) | (n = 50) | |
| Hands | 35.8 | 34.6 | 31.0 |
| Objects | 21.9 | 25.2 | 27.1 |
| Food | 12.3 | 13.8 | 10.3 |
| Water | 2.4 | 2.8 | 2.3 |
| Soil | 0.0 | 0.0 | 0.0 |
| Feces | 0.0 | 0.0 | 0.0 |
| Item Mouthed/Location | Age Group | ||||||
|---|---|---|---|---|---|---|---|
| 3–6 Month | 6–12 Month | 12–18 Month | |||||
| Shape | Scale | Shape | Scale | Shape | Scale | ||
| Hand | Inside | 0.79 | 41.08 | 0.57 | 32.92 | 0.31 | 21.05 |
| Outside | 1.29 | 43.73 | 0.66 | 30.52 | 0.83 | 29.22 | |
| Object | Inside | 0.37 | 24.12 | 0.6 | 27.62 | 0.41 | 14.44 |
| Outside | 1.67 | 26.06 | 0.65 | 26.5 | 0.76 | 16.62 | |
References
- U.S. Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition (EPA/600/R-090/052F); U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Canales, R.A.; Leckie, J.O. Application of a stochastic model to estimate children’s short-term residential exposure to lead. Stoch. Environ. Res. Risk Assess. 2007, 21, 737–745. [Google Scholar] [CrossRef]
- Hemond, H.F.; Solo-Gabriele, H.M. Children’s Exposure to Arsenic from CCA-Treated Wooden Decks and Playground Structures. Risk Anal. 2004, 24, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Zartarian, V.G.; Xue, J.; Özkaynak, H.; Dang, W.; Glen, G.; Smith, L.; Stallings, C. A Probabilistic Arsenic Exposure Assessment for Children Who Contact CCA-Treated Playsets and Decks, Part 1: Model Methodology, Variability Results, Model Evaluation. Risk Anal. 2006, 26, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.G.; Özkaynak, H.; Dang, W.; Glen, G.; Smith, L.; Stallings, C. A probabilistic arsenic exposure assessment for children who contact chromated copper arsenate (CCA)-treated playsets and decks, Part 2: Sensitivity and uncertainty analyses. Risk Anal. 2006, 26, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Beamer, P.I.; Canales, R.A.; Ferguson, A.C.; Leckie, J.O.; Bradman, A. Relative Pesticide and Exposure Route Contribution to Aggregate and Cumulative Dose in Young Farmworker Children. Int. J. Environ. Res. Public Health 2012, 9, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Zartarian, V.; Xue, J.; Glen, G.; Smith, L.; Tulve, N.; Tornero-Velez, R. Quantifying children’s aggregate (dietary and residential) exposure and dose to permethrin: Application and evaluation of EPA’s probabilistic SHEDS-Multimedia model. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Young, B.M.; Tulve, N.S.; Egeghy, P.P.; Driver, J.H.; Zartarian, V.G.; Johnston, J.E.; Delmaar, C.J.E.; Evans, J.J.; Smith, L.A.; Glen, G.; et al. Comparison of four probabilistic models (CARES, Calendex, ConsExpo, and SHEDS) to estimate aggregate residential exposures to pesticides. J. Expo. Anal. Environ. Epidemiol. 2012, 22, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.C. M.; Davis, J.; Boehm, A.B. Hand-to-Mouth Contacts Result in Greater Ingestion of Feces than Dietary Water Consumption in Tanzania: A Quantitative Fecal Exposure Assessment Model. Environ. Sci. Technol. 2015, 49, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Özkaynak, H.; Xue, J.; Zartarian, V.G.; Glen, G.; Smith, L. Modeled Estimates of Soil and Dust Ingestion Rates for Children. Risk Anal. 2011, 31, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.; Moya, J.; Freeman, N.; Beamer, P.; Black, K.; Tulve, N.; Shalat, S. A Meta-Analysis of Children’s Hand-to-Mouth Frequency Data for Estimating Nondietary Ingestion Exposure. Risk Anal. 2007, 27, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zartarian, V.; Tulve, N.; Moya, J.; Freeman, N.; Auyeung, W.; Beamer, P. A meta-analysis of children’s object-to-mouth frequency data for estimating non-dietary ingestion exposure. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Black, K.; Shalat, S.L.; Freeman, N.C.; Jimenez, M.; Donnelly, K.C.; Calvin, J.A. Children’s mouthing and food-handling behavior in an agricultural community on the US/Mexico border. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Freeman, N.C.; Jimenez, M.; Reed, K.; Gurunathan, S.; Edwards, R.; Roy, A.; Adgate, J.L.; Pellizzari, E.D.; Quackenboss, J.; Sexton, K.; et al. Quantitative analysis of children’s microactivity patterns: The Minnesota Children’s Pesticide Exposure Study. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Freeman, N.C.; Hore, P.; Black, K.; Jimenez, M.; Sheldon, L.; Tulve, N.; Lioy, P.J. Contributions of children’s activities to pesticide hand loadings following residential pesticide application. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.M.; Kwong, L.H.; Rahman, M.; Ercumen, A.; Pickering, A.; Luby, S.; Ghosh, P.K.; Unicomb, L. E. coli contamination of complementary foods and associations with domestic hygiene in rural Bangladesh. Unpublished work. 2015. [Google Scholar]
- George, C.; Oldja, L.; Biswas, S.; Perin, J.; Lee, G.; Kosek, M.; Sack, S.; Ahmed, S.; Haque, R.; Parvin, T.; et al. Geophagy is Associated with Environmental Enteropathy and Stunting in Children in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2015, 92, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Marquis, G.S.; Ventura, G.; Gilman, R.H.; Porras, E.; Miranda, E.; Carbajal, L.; Pentafiel, M. Fecal contamination of shanty town toddlers in households with non-corralled poultry, Lima, Peru. Am. J. Public Health 1990, 80, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Juberg, D.R.; Alfano, K.; Coughlin, R.J.; Thompson, K.M. An observational study of object mouthing behavior by young children. Pediatrics 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.-C.; Özkaynak, H.; Beamer, P.; Dang, W.; Hsi, H.-C.; Jiang, C.-B.; Chien, L.-C. Mouthing activity data for children aged 7 to 35 months in Taiwan. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, S.; Gialamas, A.; Jones, L.; Edwards, P.; Maynard, E. Child Activity Patterns for Environmental Exposure Assessment in the Home; National Environmental Health Forum: Adelaide, Australia, 1999.
- Ngure, F.M.; Humphrey, J.H.; Mbuya, M.N.N.; Majo, F.; Mutasa, K.; Govha, M.; Mazarura, E.; Chasekwa, B.; Prendergast, A.J.; Curtis, V.; et al. Formative Research on Hygiene Behaviors and Geophagy among Infants and Young Children and Implications of Exposure to Fecal Bacteria. Am. J. Trop. Med. Hyg. 2013, 89, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Cohen Hubal, E.A.; Sheldon, L.S.; Burke, J.M.; McCurdy, T.R.; Berry, M.R.; Rigas, M.L.; Zartarian, V.G.; Freeman, N.C. Children’s Exposure Assessment: A review of factors influencing children’s exposure, and the data available to characterize and assess that exposure. Environ. Health Perspect. 2000, 108, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Cohen Hubal, E.A.; Suggs, J.C.; Nishioka, M.G.; Ivancic, W.A. Characterizing residue transfer efficiencies using a fluorescent imaging technique. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.F.; Null, C.; Luby, S.P.; Unicomb, L.; Stewart, C.P.; Dewey, K.G.; Ahmed, T.; Ashraf, S.; Christensen, G.; Clasen, T.; et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: The WASH Benefits study design and rationale. BMJ Open 2013, 3, e003476. [Google Scholar] [CrossRef] [PubMed]
- AuYeung, W.; Canales, R.A.; Beamer, P.; Ferguson, A.C.; Leckie, J.O. Young Children’s Mouthing Behavior: An Observational Study via Videotaping in a Primarily Outdoor Residential Setting. J. Child. Health 2004, 2, 271–295. [Google Scholar] [CrossRef]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2004. [Google Scholar]
- Smith, S.A.; Norris, B. Reducing the risk of choking hazards: Mouthing behavior of children aged 1 month to 5 years. Inj. Control Saf. Promot. 2003, 10, 145–154. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Guidance on Selecting Age Groups for Monitoring and Assessing Childhood Exposures to Environmental Contaminants; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- Greene, M.A. Mouthing Times amoung Young Children fron Otservational Data; U.S. Product Safety Commision: Bethesda, MD, USA, 2002.
- Tulve, N.S.; Suggs, J.C.; McCurdy, T.; Cohen Hubal, E.A.; Moya, J. Frequency of mouthing behavior in young children. J. Exp. Anal. Envrion. Epidemiol. 2002, 12, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Beamer, P.; Key, M.E.; Ferguson, A.C.; Canales, R.A.; Auyeung, W.; Leckie, J.O. Quantified activity pattern data from 6 to 27-month-old farmworker children for use in exposure assessment. Environ. Res. 2008, 108, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Beamer, P.I.; Luik, C.E.; Canales, R.A.; Leckie, J.O. Quantified outdoor micro-activity data for children aged 7–12-years old. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.J.; Jimenez, M.; Freeman, N.C.; Lioy, P.J. Quantification of children’s hand and mouthing activities through a videotaping methodology. J. Expo. Anal. Environ. Epidemiol. 1999, 9, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Akland, G.G.; Pellizzari, E.D.; Hu, Y.; Roberds, M.; Rohrer, C.A.; Leckie, J.O.; Berry, M.R. Factors influencing total dietary exposures of young children. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M.D.; Galiani, S.; Gertler, P.J.; Martinez, S.; Titiunik, R. Housing, Health, and Happiness. Am. Econ. Assoc. 2009, 1, 75–105. [Google Scholar] [CrossRef]
- Julian, T.R.; Islam, M.A.; Pickering, A.J.; Roy, S.; Fuhrmeister, E.R.; Ercumen, A.; Harris, A.; Bishai, J.; Schwab, K.J. Genotypic and Phenotypic Characterization of Escherichia coli Isolates from Feces, Hands, and Soils in Rural Bangladesh via the Colilert Quanti-Tray System (IDEXX). Appl. Environ. Microbiol. 2015, 81, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Zartarian, V.G.; Ferguson, A.C.; Leckie, J.O. Quantified dermal activity data from a four-child field study. J. Expo. Anal. Environ. Epidemiol. 1997, 7, 543–553. [Google Scholar] [PubMed]
- Ruel, M.T.; Arimond, M. Spot-check Observational Method for Assessing Hygiene Practices: Review of Experience and Implications for Programmes. J. Health Popul. Nutr. 2002, 20, 65–76. [Google Scholar] [PubMed]
- Greene, M.A. Mouthing Times and DINP Risk for Children over Three Years of Age; U.S. Consumer Product Safety Commission: Bethesda, MD, USA, 2002.
- U.S. Consumer Product Safety Commission. Chronic Hazard Advisory Panel on Diisononyl Phthalate (DINP): Report to the U.S. Consumer Product Safety Commission; U.S. Consumer Product Safety Commission: Bethesda, MD, USA, 2001.



| Characteristic | Mean (SD) or Frequency (%) | |||
|---|---|---|---|---|
| Total (n = 148) | 3–6 Months (n = 21) | 6–12 Months (n = 104) | 12–24 Months (n = 23) | |
| Child characteristics | ||||
| Male | 77 (51.7) | 13 (61.9) | 53 (51.0) | 11 (47.8) |
| Age in months | 8.3 (3.1) | 4.5 (0.7) | 7.81 (1.5) | 14.2 (1.4) |
| Mobility | ||||
| Sit only | 82 (55.7) | 21 (100.0) | 60 (57.7) | 1 (4.3) |
| Crawl | 50 (33.6) | 0 (0) | 43 (41.3) | 7 (30.4) |
| Walk | 16 (10.7) | 0 (0) | 1 (1.0) | 15 (65.2) |
| Hand dominance | ||||
| No dominant hand | 80 (54.4) | 9 (42.9) | 55 (52.9) | 16 (69.6) |
| Right hand dominant | 61 (40.9) | 12 (57.1) | 44 (42.3) | 5 (21.7) |
| Left hand dominant | 7 (4.7) | 0 (0) | 5 (4.8) | 2 (8.7) |
| Household characteristics | ||||
| Study arm (Number of households) | ||||
| Control | 49 (32.9) | 7 (33.3) | 36 (34.6) | 6 (26.1) |
| Sanitation | 49 (33.6) | 7 (33.3) | 31 (29.8) | 11 (47.8) |
| Water + sanitation + hygiene | 50 (33.6) | 7 (33.3) | 37 (35.6) | 6 (26.1) |
| Number of people in compound | 12.2 (6.9) | 13.4 (7.0) | 12.8 (7.2) | 8.8 (3.3) |
| Number of children <5 in compound | 2.0 (1.1) | 1.8 (1.2) | 2.0 (1.2) | 1.7 (0.8) |
| Number of children <5 in household | 1.4 (0.6) | 1.3 (0.7) | 1.4 (0.6) | 1.3 (0.6) |
| Mother’s years of formal education | 5.8 (3.5) | 5.0 (2.6) | 5.6 (3.6) | 7.7 (2.4) |
| Has electricity | 84 (56.4) | 13 (61.9) | 57 (54.8) | 14 (60.9) |
| Roof of tin | 91 (61.7) | 18 (85.7) | 67 (64.4) | 6 (26.1) |
| Owns a mobile phone | 121 (81.9) | 18 (85.7) | 81 (77.9) | 22 (95.7) |
| Household has dirt floor | 126 (85.2) | 20 (95.2) | 91 (87.5) | 15 (65.2) |
| Courtyard has dirt floor | 146 (98.7) | 21 (100.0) | 103 (99.0) | 22 (95.7) |
| Child feces observed in courtyard | 7 (4.7) | 0 (0) | 5 (4.8) | 2 (8.7) |
| Animal feces observed in courtyard | 147 (99.3) | 21 (100.0) | 103 (99.0) | 23 (100.0) |
| (A) Hand-Mouthing Frequency (Contacts/h) | |||||||||||
| Demographics | Location | ||||||||||
| Study | Country | Age Group | Inside | Outside | Inside + Outside | ||||||
| n | Mean | Median | n | Mean | Median | n | Mean | Median | |||
| This Study | Bangladesh | 3–6 months | 21 | 43.6 | 39.0 | 21 | 40.0 | 30.0 | 21 | 39.5 | 37.3 |
| Xue [11] | USA | 3–6 months | 23 | 28.0 | 23.0 | - | - | - | - | - | - |
| Greene * [30] | USA | 3–6 months | 23 | 28.0 | 23.0 | - | - | - | - | - | - |
| This Study | Bangladesh | 6–12 months | 103 | 40.5 | 31.6 | 103 | 34.0 | 29.4 | 104 | 35.8 | 34.4 |
| Xue [11] | USA | 6–12 months | 119 | 18.9 | 14.0 | 10 | 14.5 | 11.6 | |||
| Greene * [30] | USA | 6–12 months | 88 | 19.8 | 14.5 | - | - | - | - | - | - |
| Tulve,* [31] | USA | 6–12 months | 9 | 14.4 | 5.0 | - | - | - | - | - | - |
| Black * [13] | USA | 6–12 months | 11 | 19.1 | 13.7 | - | - | - | - | - | - |
| Beamer * [32] | USA | 6–12 months | 11 | 14.6 | 17.0 | - | - | - | - | - | - |
| Tsou [20] | Taiwan | 7–12 months | 8 | 16.7 | 16.3 | - | - | - | - | - | - |
| Brinkman [21] | Australia | 5–12.5 months | - | - | - | - | - | - | 22 | 13.1 | 9.9 |
| This Study | Bangladesh | 12–18 months | 23 | 38.7 | 28.2 | 23 | 30.9 | 23.1 | 23 | 32.3 | 29.7 |
| Xue [11] | USA | 12–24 months | 245 | 19.6 | 14.0 | 32 | 13.9 | 8.0 | - | - | - |
| Greene * [30] | USA | 12–24 months | 123 | 20.1 | 14.0 | - | - | - | - | - | - |
| Tulve,* [31] | USA | 12–24 months | 84 | 18.5 | 14.0 | 10 | 13.3 | 11.0 | - | - | - |
| Black * [13] | USA | 12–24 months | 20 | 17.1 | 14.6 | 12 | 12.6 | 7.0 | - | - | - |
| Tsou [20] | Taiwan | 12–24 months | 30 | 12.2 | 8.9 | - | - | - | - | - | - |
| Brinkman [21] | Australia | 12.5–20.5 months | - | - | - | - | - | - | 20 | 23.3 | 8.2 |
| (B) Object-Mouthing Frequency (Contacts/h) | |||||||||||
| This Study | Bangladesh | 3–6 months | 21 | 38.9 | 24.1 | 21 | 23.1 | 21.6 | 21 | 29.6 | 23.1 |
| Xue [12] | USA | 3–6 months | 19 | 11.2 | 9.3 | - | - | - | - | - | - |
| Greene ^ [30] | USA | 3–6 months | 19 | 11.2 | 9.3 | - | - | - | - | - | - |
| This Study | Bangladesh | 6–12 months | 103 | 33.7 | 26.6 | 103 | 30.0 | 25.6 | 104 | 31.6 | 29.6 |
| Xue [12] | USA | 6–12 months | 82 | 20.3 | 19.0 | - | - | - | - | - | - |
| Greene ^ [30] | USA | 6–12 months | 82 | 20.3 | 19.0 | - | - | - | - | - | - |
| Tulve ^ [31] | USA | 6–12 months | 9 | 72.3 | 84.0 | - | - | - | - | - | - |
| Beamer ^ [32] | USA | 6–12 months | 11 | 43.9 | 38.6 | - | - | - | - | - | - |
| Tsou [20] | Taiwan | 7–12 months | 8 | 65.7 | 60.7 | - | - | - | - | - | - |
| Brinkman [21] | Australia | 5 to 12.5 months | - | - | - | - | - | - | 22 | 50.4 | 47.0 |
| This Study | Bangladesh | 12–18 months | 23 | 21.2 | 17.3 | 23 | 18.4 | 13.2 | 23 | 17.0 | 15.2 |
| Xue [12] | USA | 12–24 months | 137 | 14.2 | 12.3 | 21 | 8.8 | 6.0 | - | - | - |
| Greene ^ [30] | USA | 12–24 months | 134 | 14.5 | 13.3 | - | - | - | - | - | - |
| Tulve ^ [31] | USA | 12–24 months | 84 | 46.7 | 40.5 | 10 | 9.2 | 5.5 | - | - | - |
| Beamer ^ [32] | USA | 12–24 months | 7 | 26.8 | 29.0 | - | - | - | |||
| AuYeung ^ [26] | USA | 12–24 months | - | - | - | 7 | 7.5 | 7.9 | - | - | - |
| Tsou [20] | Taiwan | 12–24 months | 30 | 18.5 | 11.8 | - | - | - | - | - | - |
| Brinkman [21] | Australia | 12.5–20.5 months | - | - | - | - | - | - | 20 | 29.7 | 12.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwong, L.H.; Ercumen, A.; Pickering, A.J.; Unicomb, L.; Davis, J.; Luby, S.P. Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old. Int. J. Environ. Res. Public Health 2016, 13, 563. https://doi.org/10.3390/ijerph13060563
Kwong LH, Ercumen A, Pickering AJ, Unicomb L, Davis J, Luby SP. Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old. International Journal of Environmental Research and Public Health. 2016; 13(6):563. https://doi.org/10.3390/ijerph13060563
Chicago/Turabian StyleKwong, Laura H., Ayse Ercumen, Amy J. Pickering, Leanne Unicomb, Jennifer Davis, and Stephen P. Luby. 2016. "Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old" International Journal of Environmental Research and Public Health 13, no. 6: 563. https://doi.org/10.3390/ijerph13060563
APA StyleKwong, L. H., Ercumen, A., Pickering, A. J., Unicomb, L., Davis, J., & Luby, S. P. (2016). Hand- and Object-Mouthing of Rural Bangladeshi Children 3–18 Months Old. International Journal of Environmental Research and Public Health, 13(6), 563. https://doi.org/10.3390/ijerph13060563