A Feasibility Study of Ammonia Recovery from Coking Wastewater by Coupled Operation of a Membrane Contactor and Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
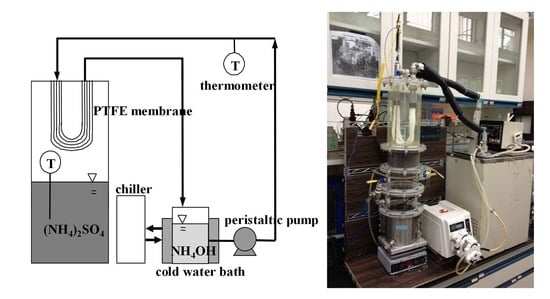
2.1. HFMC Module
2.2. Modified MD Module
2.3. Characteristics of Coking Wastewater
3. Results and Discussion
3.1. Evaluation of HFMC Membrane Materials and Receiving Solutions
3.1.1. PP Hollow Fiber Membranes
3.1.2. PTFE Hollow Fiber Membranes
3.1.3. Evaluation of Distilled Water as the Receiving Solution
3.2. Effects of Feed pH Level for the PTFE HFMC Module
3.3. Ammonia Solution Recovered by the Modified MD Module
3.3.1. Accumulation of Concentrated (NH4)2SO4 Solution
3.3.2. Ammonia Solution Recovery
3.3.3. Economic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Ghose, M.K. Complete physico-chemical treatment for coke plant effluents. Water Res. 2002, 36, 1127–1134. [Google Scholar] [CrossRef]
- Güçlü, D.; Şirin, N.; Şahinkaya, S.; Sevimli, M.F. Advanced treatment of coking wastewater by conventional and modified fenton processes. Environ. Prog. Sustain. Energy 2013, 32, 176–180. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Z.; Song, L. Study on production of an auxiliary agent of coagulation using waste polystyrene foam and its application to remove phenol from coking plant effluent. Environ. Prog. Sustain. Energy 2010, 29, 494–498. [Google Scholar] [CrossRef]
- Chang, I.-S.; Chung, C.-M. Pollution prevention for manufacturing of ammonium chloride—An ecperimental study of wastewater recycling. Desalination 2000, 127, 145–153. [Google Scholar] [CrossRef]
- Hung, C.-M.; Lou, J.-C.; Lin, C.-H. Removal of ammonia solutions used in catalytic wet oxidation processes. Chemosphere 2003, 52, 989–995. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reus, 4th ed.; McGraw-Hill Science: New York, NY, USA, 2014. [Google Scholar]
- Chen, J.; Shi, H.; Lu, J. Electrochemical treatment of ammonia in wastewater by RuO2-IrO2-TiO2/Ti electrodes. J. Appl. Electrochem. 2007, 37, 1137–1144. [Google Scholar] [CrossRef]
- Ratnayaka, D.D.; Brandt, M.J.; Johnson, M. Water Supply, 6th ed.; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Griffin, A.E.; Chamberlin, N.S. Relation of ammonia-nitrogen to break-point chlorination. Am. J. Public Health Nations Health 1941, 31, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sorensen, B.; Jorgensen, S.E. The Removal of Nitrogen Compounds from Wastewater; Elsevier Science: Amsterdam, The Netherlands, 1993; Volume 54. [Google Scholar]
- Koyuncu, I.; Topacik, D.; Turan, M.; Celik, M.S.; Sarikaya, H.Z. Application of the membrane technology to control ammonia in surface water. Water Sci. Technol. 2001, 1, 117–124. [Google Scholar]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: A review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Pressley, T.A.; Bishop, D.F.; Roan, S.G. Ammonia-nitrogen removal by breakpoint chlorination. Environ. Sci. Technol. 1972, 6, 622–628. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Qu, D.; Sun, D.; Wang, H.; Yun, Y. Experimental study of ammonia removal from water by modified direct contact membrane distillation. Desalination 2013, 326, 135–140. [Google Scholar] [CrossRef]
- Xie, Z.; Duong, T.; Hoang, M.; Nguyen, C.; Bolto, B. Ammonia removal by sweep gas membrane distillation. Water Res. 2009, 43, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, L.; Li, Z.; Ma, R.; Yang, Z. Experimental study of ammonia removal from water by membrane distillation (MD): The comparison of three configurations. J. Membr. Sci. 2006, 286, 93–103. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J. Membr. Sci. 2013, 430, 263–303. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.N.; Khorasani, Z. Ammonia removal from aqueous solutions using hollow-fiber membrane contactors. Chem. Eng. J. 2010, 162, 242–249. [Google Scholar] [CrossRef]
- Shirazian, S.; Ashrafizadeh, S.N. Mass transfer simulation of carbon dioxide absorption in a hollow-fiber membrane contactor. Sep. Sci. Technol. 2010, 45, 515–524. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, S.-H.; Yang, M.-C. Mass transfer analysis on air stripping of VOCs from water in microporous hollow fibers. J. Membr. Sci. 2005, 255, 79–87. [Google Scholar] [CrossRef]
- Johnson, R.A. Perstraction with Chemical Reaction. Australian Patent No. WO1994016800A1, 4 August 1994. [Google Scholar]
- El-Bourawi, M.S.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of vacuum membrane distillation for ammonia removal. J. Membr. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Ghaffour, N.; Li, J.D.; Gray, S.; Francis, L.; Maab, H.; Amy, G.L. Modeling of air-gap membrane distillation process: A theoretical and experimental study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Lin, Y.-H.; Chen, J. Enhanced air gap membrane desalination by novel finned tubular membrane modules. J. Membr. Sci. 2011, 378, 398–406. [Google Scholar] [CrossRef]
- Wang, J.; Fan, B.; Luan, Z.; Qu, D.; Peng, X.; Hou, D. Integration of direct contact membrane distillation and recirculating cooling water system for pure water production. J. Clean. Prod. 2008, 16, 1847–1855. [Google Scholar] [CrossRef]
- Chafidz, A.; Kerme, E.D.; Wazeer, I.; Khalid, Y.; Ajbar, A.; Al-Zahrani, S.M. Design and fabrication of a portable and hybrid solar-powered membrane distillation system. J. Clean. Prod. 2016, 133, 631–647. [Google Scholar] [CrossRef]
- Tan, X.; Tan, S.P.; Teo, W.K.; Li, K. Polyvinylidene fluoride (PVDF) hollow fibre membranes for ammonia removal from water. J. Membr. Sci. 2006, 271, 59–68. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]








| Parameter, Unit | PP | PTFE |
|---|---|---|
| Pore diameter (µm) | 0.1–0.3 | 0.2–0.3 |
| Membrane thickness (µm) | 45 | 600 |
| Outer diameter (mm) | 0.4 | 2.2 |
| Surface area (m2) | 0.64 | 0.15 |
| Porosity | Not available | 0.53 |
| COD (mg/L) | pH | NH3-N (mg/L) | Conductivity (μs/cm) | Phenol (mg/L) | CN− (mg/L) | SCN− (mg/L) |
|---|---|---|---|---|---|---|
| 5211 ± 640 | 5.5–8.2 | 648 ± 158 | 6495 ± 895 | 945 ± 168 | 2.5 ± 0.2 | 512 ± 55 |
| Feed Solution | Receiving Solution | |
|---|---|---|
| Component | Coking wastewater | 0.1 M H2SO4 |
| Volume (mL) | 2000 | 500 |
| Initial pHs | 9.7, 10.5, 11.5, 12.5 | <1 |
| Circulation flow rate (mL/min) | 830 | 830 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-H.; Horng, R.-Y.; Hsu, S.-F.; Chen, S.-S.; Ho, C.-H. A Feasibility Study of Ammonia Recovery from Coking Wastewater by Coupled Operation of a Membrane Contactor and Membrane Distillation. Int. J. Environ. Res. Public Health 2018, 15, 441. https://doi.org/10.3390/ijerph15030441
Lin P-H, Horng R-Y, Hsu S-F, Chen S-S, Ho C-H. A Feasibility Study of Ammonia Recovery from Coking Wastewater by Coupled Operation of a Membrane Contactor and Membrane Distillation. International Journal of Environmental Research and Public Health. 2018; 15(3):441. https://doi.org/10.3390/ijerph15030441
Chicago/Turabian StyleLin, Po-Hsun, Ren-Yang Horng, Shu-Fang Hsu, Shiao-Shing Chen, and Chia-Hua Ho. 2018. "A Feasibility Study of Ammonia Recovery from Coking Wastewater by Coupled Operation of a Membrane Contactor and Membrane Distillation" International Journal of Environmental Research and Public Health 15, no. 3: 441. https://doi.org/10.3390/ijerph15030441
APA StyleLin, P.-H., Horng, R.-Y., Hsu, S.-F., Chen, S.-S., & Ho, C.-H. (2018). A Feasibility Study of Ammonia Recovery from Coking Wastewater by Coupled Operation of a Membrane Contactor and Membrane Distillation. International Journal of Environmental Research and Public Health, 15(3), 441. https://doi.org/10.3390/ijerph15030441