Effect of Potassium Chlorate on the Treatment of Domestic Sewage by Achieving Shortcut Nitrification in a Constructed Rapid Infiltration System
Abstract
:1. Introduction
2. Materials and Methods
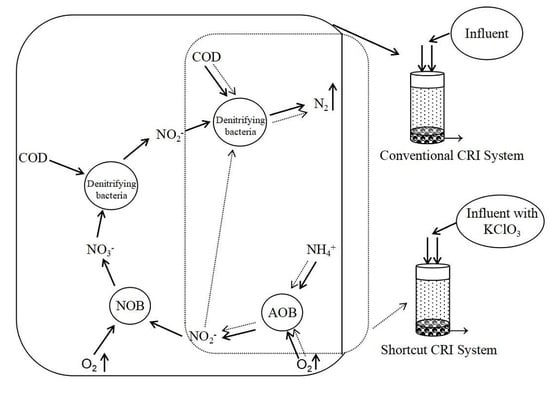
2.1. Experimental Design
2.2. Sewage and Operational Conditions
2.3. Analytical Methods
2.4. Scanning Electron Microscope Detection
3. Results and Discussion
3.1. Effect of Potassium Chlorate on Removal Efficiency of Ammonium Nitrogen
3.2. Effect of Potassium Chlorate on Nitrate Accumulation in a CRI System
3.3. Effect of Potassium Chlorate and pH on Nitrite Accumulation in a CRI System
3.4. Prospects for the Achievement of Shortcut Nitrification–Denitrification in a CRI System
4. Conclusions
- (1)
- The addition of 3 mM KClO3 to influent at a constant pH of 8.4 is not sufficient to inhibit that of NOB so that shortcut nitrification does not take place in the CRI system.
- (2)
- Adjusting the pH of influent to 8.4 alone did not contribute much to establish shortcut nitrification in CRI.
- (3)
- Although, the addition of 5 mM KClO3 in influent could both inhibit the activity of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), the inhibition of NOB was so strong that made the NO2−-N to be the dominant product of total oxidized nitrogen in effluent for a long period, showing that shortcut nitrification could be achieved and maintained successfully in a CRI system.
- (4)
- According to the data of nitrate and nitrite in Figure 6 and Figure 7, the consumption of external carbon source (CH3OH) for subsequent denitrification was calculated and analysed by using Equations (1) and (2), the results showed that the consumption of carbon source (CH3OH) of Test 3 (pH 8.4, 5 mM KClO3) was only 38.73% of the consumption of Test 2 (pH 8.4). Therefore, compared with conventional sewage treatment methods, achievement of the shortcut nitrification–denitrification process in the CRI system will take both the advantages of the CRI system and shortcut nitrification–denitrification process; it will not only have a unique structure and feeding mode to construct aerobic, facultative, and anaerobic environments for microorganism enriching in the filling medium, but also improve the denitrification rate and save the carbon source consumption during the reaction process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, X.J.; Fu, Y.S.; Liu, F.; Xue, D.; Xu, W. Total nitrogen removal efficiency of improved constructed rapid infiltration system. Technol. Water Treat. 2009, 10, 021. [Google Scholar]
- Ronald, W.C.; Sherwood, C.R.; Robert, K.B. Applying treatment wastewater to land. Bio. Cycle 2001, 4, 32–35. [Google Scholar]
- He, J.T.; Zhong, Z.S.; Tang, M.G. New method of solving contradiction of rapid infiltration system land using. Geoscience 2001, 15, 339–345. [Google Scholar]
- Xu, W.L.; Zhang, W.; Jian, Y. Analysis of nitrogen removal performance of constructed rapid infiltration system (CRIS). Appl. Ecol. Environ. Res. 2017, 15, 199–206. [Google Scholar] [CrossRef]
- Xu, W.L.; Yang, Y.N.; Cheng, C. Treat Phoenix River water by constructed rapid Infiltration system. J. Coast. Res. 2015, 73, 386–390. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhang, H.Z.; Zhang, X.; Li, W. Development of total nitrogen removing technology in constructed rapid infiltration systems. Ind. Water Treat. 2013, 33, 1–4. [Google Scholar]
- Ling, Y.; Fan, L.K.; Min, X.; Yue, L.; Sen, W. Environmental economic value calculation and sustainability assessment for constructed rapid infiltration system based on emergy analysis. J. Clean Prod. 2017, 167, 582–588. [Google Scholar]
- Wang, L.; Yu, Z.P.; Zhao, Z.J. The removal mechanism of ammoniac nitrogen in constructed rapid infiltration system. China Environ. Sci. 2006, 26, 500–504. [Google Scholar]
- Zhang, J.B. Study on Constructed Rapid Infiltration for Wastewater Treatment. Ph.D. Thesis, University of Geosciences, Beijing, China, 2002. [Google Scholar]
- Song, Z.X.; Zhang, H.Z.; Wang, Z.L.; Ping, Y.H.; Liu, G.Y.; Zhao, Q. Treating sewage by strengthened constructed rapid infiltration system. Chin. J. Environ. Eng. 2016, 10, 3491–3495. [Google Scholar]
- Xu, G.J.; Xu, X.C.; Yang, F.L.; Liu, S.T. Selective inhibition of nitrite oxidation by chlorate dosing in aerobic granules. J. Hazard. Mater. 2011, 185, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Xia, L.; Ma, T.; Zhang, Y.Q.; Zhou, Y.Y.; He, X.G. Achieving short-cut nitrification and denitrification in modified intermittently aerated constructed wetland. Bioresour. Technol. 2017, 232, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.J.; Xu, X.C.; Yang, F.L.; Liu, S.T.; Gao, Y. Partial nitrification adjusted by hydroxylamine in aerobic granules under high DO and ambient temperature and subsequent Anammox for low C/N wastewater treatment. Chem. Eng. J. 2012, 213, 338–345. [Google Scholar] [CrossRef]
- Sukru, A.; Erdal, S. Influence of salinity on partial nitrification in a submerged biofilter. Bioresour. Technol. 2012, 118, 24–29. [Google Scholar]
- Chen, J.; Zhang, J.Q.; Wen, H.Y.; Zhang, Q.; Yang, X.; Li, J. The effect of hydroxylamine inhibition and pH control on achieving shortcut nitrification in constructed rapid infiltration system. Acta Sci. Circumst. 2016, 36, 3728–3735. [Google Scholar]
- Cui, Y.W.; Peng, Y.Z.; Gan, X.Q.; Ye, L.; Wang, Y.Y. Achieving and maintaining biological nitrogen removal via nitrite under normal conditions. J. Environ. Sci. 2005, 17, 794–798. [Google Scholar]
- Ge, L.P.; Qiu, L.P.; Liu, Y.Z.; Zhang, S.B. Effect of Free Chlorine on Shortcut Nitrification in Biological Aerated Filter. J. Univ. Jinan Sci. Technol. 2011, 25, 336–339. [Google Scholar]
- Lees, H.; Simpson, J.R. The biochemistry of the nitrifying organisms. 5. Nitrite oxidation by Nitrobacter. Biochem. J. 1957, 65, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Banashri, S.A.P.A. Partial nitrification—Operational parameters and microorganisms involved. Rev. Environ. Sci. Biotechnol. 2007, 6, 285–313. [Google Scholar]
- Wei, F.S. The Standard Methods for the Examination of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 211, 254–279. ISBN 9787801634009. [Google Scholar]
- Ni, H.; Xiong, Z.; Zhang, S.; Zeng, S.Q.; Li, L. Effect of porous ceramic on the immobilized microorganisms and scaning electron microscopy. J. Hubei Univ. Nat. Sci. 2011, 33, 182–186. [Google Scholar]
- Glass, C.; Silverstein, J. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- Chen, J.W. Study on Nitrogen Removal in Partial Nitrification Denitrification Biological Filters. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2017. [Google Scholar]
- Yan, N.; Jin, X.B.; Zang, J.Q. A comparison between the processes of denitrification with glucose and methanol as carbon sourse. J. Shanghai Teach. Univ. Nat. Sci. 2002, 31, 41–44. [Google Scholar]
- Zhang, Z.L. Selection of External Carbon Sources for Denitrification. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2009. [Google Scholar]
- Gómez, M.A.; Gonzálezlópez, J.; Hontoria-García, E. Influence of carbon source on nitrate removal of contaminated ground-water in a denitrifying submerged filter. J. Hazard. Mater. 2000, 80, 69–80. [Google Scholar] [CrossRef]








| Water Quality Parameters | Mean Concentration (mg/L) |
|---|---|
| Chemical Oxygen Demand (COD) | 245.22 ± 27.11 |
| NH4+-N | 53.93 ± 3.81 |
| NO3−-N | 1.15 ± 0.67 |
| NO2−-N | 0.14 ± 0.09 |
| Total Nitrogen (TN) | 55.35 ± 6.01 |
| pH | 7.3 ± 0.14 (control), 8.4 (Tests 2–4) |
| Temperature (°C) | 34.2 ± 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Xu, W.; Yan, Z.; Qian, L. Effect of Potassium Chlorate on the Treatment of Domestic Sewage by Achieving Shortcut Nitrification in a Constructed Rapid Infiltration System. Int. J. Environ. Res. Public Health 2018, 15, 670. https://doi.org/10.3390/ijerph15040670
Fang Q, Xu W, Yan Z, Qian L. Effect of Potassium Chlorate on the Treatment of Domestic Sewage by Achieving Shortcut Nitrification in a Constructed Rapid Infiltration System. International Journal of Environmental Research and Public Health. 2018; 15(4):670. https://doi.org/10.3390/ijerph15040670
Chicago/Turabian StyleFang, Qinglin, Wenlai Xu, Zhijiao Yan, and Lei Qian. 2018. "Effect of Potassium Chlorate on the Treatment of Domestic Sewage by Achieving Shortcut Nitrification in a Constructed Rapid Infiltration System" International Journal of Environmental Research and Public Health 15, no. 4: 670. https://doi.org/10.3390/ijerph15040670
APA StyleFang, Q., Xu, W., Yan, Z., & Qian, L. (2018). Effect of Potassium Chlorate on the Treatment of Domestic Sewage by Achieving Shortcut Nitrification in a Constructed Rapid Infiltration System. International Journal of Environmental Research and Public Health, 15(4), 670. https://doi.org/10.3390/ijerph15040670