Impacts of Artificial Underground Reservoir on Groundwater Environment in the Reservoir and Downstream Area
Abstract
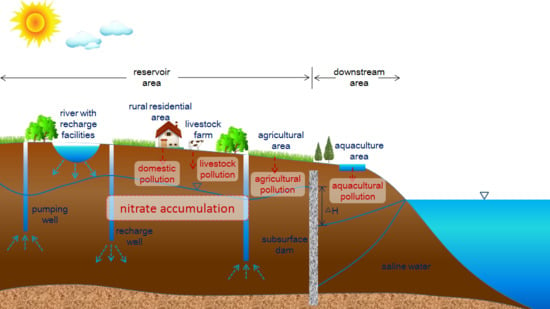
:1. Introduction
2. Materials and Methods
2.1. Geology and Hydrogeology
2.2. Land Use and Artificial Underground Reservoir Project
2.3. Groundwater Sampling and Analysis
2.4. Sources of Nitrogen Pollution in Stored Water
2.4.1. Agricultural Pollution
2.4.2. Domestic Pollution
2.4.3. Livestock and Poultry Farming Pollution
3. Results and Discussion
3.1. Groundwater Flow Field Change and Water Level Fluctuation
3.2. Groundwater Tritium Level and Renewal Rate
3.3. Groundwater Nitrogen in the Reservoir Area
3.4. Groundwater Nitrogen in Downstream Area
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Reservoir Name | Reservoir Area (km2) | Total Storage Capacity (103m3) | Active Storage (103m3) | Reservoir Structure | Construction Method of Cut-Off Wall | Vertical Configuration of Cut-Off Wall | Wall Length (m) | Seepage Control Area of Cut-Off Wall (103 m2) | Construction Period | Location |
|---|---|---|---|---|---|---|---|---|---|---|
| Nangong | 206 | 460,000 | 84,000 | plain palaeochannel | NAp | NAp | NAp | NAp | 1977–1982 | Hebei |
| Wanghe | 68.49 | 56,930 | 32,730 | coastal alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 13,593 | 27.2 | 1999–2006 | Shandong |
| Jiahe | 63.26 | 205,200 | 65,000 | intermontane valley alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 2511 | 4.5 | 2000–2001 | Shandong |
| Daguhe | 421.69 | 384,130 | 237,800 | intermontane valley alluvial plain | high pressure jet grouting, geomembrane wall, permeation grouting | keyed-into low permeability layer | 4350 | 3.5 | 1997–1998 | Shandong |
| Hutuohe | 436.5 | 100.4 | NAp | alluvial fan, cone of depression | NAp | NAp | NAp | NAp | 2012–2014 | Hebei |
| Longhe * | 8.5 | 865 | NA | intermontane valley alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 544.6 | 0.5 | 1998–2000 | Liaoning |
| Jianbaohe | 26.09 | 6904.1 | 6525.2 | intermontane valley alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 930 | 1.5 | 2001–2003 | Liaoning |
| Huangshuihe | 53 | 53,590 | 39,290 | coastal alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 5996 | 16 | 1993–1995 | Shandong |
| Balishahe | 0.7 | 398 | 355 | coastal alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 756 | 5500 | 1988–1989 | Shandong |
| Maguan | NAp | 1325.42 | 1325.42 | karst subterranean river (surface–subsurface reservoir) | damming underground river | NAp | NAp | NAp | 1990–1990 | Guizhou |
| Pengbao | 75 | 84,380 | 7280 | cone of depression | plastic concrete slurry wall, permeation grouting grouting | keyed-into low permeability layer | 4050 | 16.2 | 2009–2011 | Ningxia |
| Liangchenghe | 12.3 | 23,050 | 10,600 | coastal alluvial plain | deep soil mixing | keyed-into low permeability layer | 3990 | 4.8 | 2016–2017 | Shandong |
| Rushanhe | 19.6 | 21,970 | 16,860 | coastal alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 7600 | 11.4 | 2017–2018 | Shandong |
| Shilin | NAp | 6725.5 | 5508.7 | karst subterranean river (surface-subsurface reservoir) | permeation grouting | keyed-into low permeability layer | 4840 | 10 | 2012–2013 | Yunnan |
| Shangba | NAp | 2800 | 2680 | karst subterranean river | damming underground river | keyed-into low permeability layer | NAp | NAp | 2006–2007 | Guizhou |
| Wulichong | NAp | 79,490 | 79,490 | karst subterranean river (surface-subsurface reservoir) | high pressure jet grouting, damming underground river, reinforced concrete slurry wall, damming at outfall of underground river | hanging wall | 1383 | 26.2 | 1991–1996 | Yunnan |
| Baixi | NA | NA | NA | coastal alluvial plain | high pressure jet grouting | keyed-into low permeability layer | 514 | 8153 | 2009–2009 | Zhejiang |
| Sanguanmiao | 1 | 638.1 | 1472.46 | intermontane valley alluvial plain | high pressure jet grouting | keyed-into low permeability layer | NA | NA | 2001–2002 | Liaoning |
| Laolongwan | NA | NA | 360 | intermontane valley alluvial plain | geomembrane | keyed-into low permeability layer | 391.4 | NA | 1995–1998 | Liaoning |
References
- Gleick, P.H. Chapter 5: China and Water. In The World’s Water 2008–2009: The Biennial Report on Fresh Water Resource, 1st ed.; Island Press: Washington, DC, USA, 2008; pp. 79–100. ISBN 9781597265058. [Google Scholar]
- Yong, J. China’s Water Security: Current Status, Emerging Challenges and Future Prospects. Environ. Sci. Policy 2015, 54, 106–125. [Google Scholar]
- American Society of Civil Engineering (ASCE). Standard Guidelines for Artificial Recharge of Groundwater (EWRI/ASCE 34-01); American Society of Civil Engineering: Reston, VA, USA, 2001; ISBN 0-7844-0548-2. [Google Scholar]
- Nishigaki, M.; Kankam-Yeboah, K.; Komatsu, M. Underground Dam Technology in Some Parts of the World. J. Groundw. Hydrol. 2004, 46, 113–130. [Google Scholar] [CrossRef]
- Hanson, G.; Nilsson, A. Ground-Water Dams for Rural-Water Supplies in Developing Countries. Ground Water 2010, 24, 497–506. [Google Scholar] [CrossRef]
- Li, Y.G. Groundwater Reservoir Construction Research; China Environmental Science Press: Beijing, China, 2007; ISBN 978-7-80209-490-1. (In Chinese) [Google Scholar]
- Du, S.H.; Su, X.S.; Zhang, W.J. Effective Storage Rates Analysis of Groundwater reservoir with Surplus Local and Transferred Water Used in Shijiazhuang City, China. Water Environ. J. 2007, 27, 157–169. [Google Scholar] [CrossRef]
- Goldsmith, E.; Hildyard, N. The Social and Environmental Effects of Large Dams; Sierra Club Books: San Francisco, CA, USA, 1984; ISBN 0-87156-848-9. [Google Scholar]
- Liu, C.; Yu, J.; Eloise, K. Groundwater Exploitation and Its Impact on the Environment in the North China Plain. Water Int. 2001, 26, 265–272. [Google Scholar]
- Apaydın, A. Malibogazi Groundwater Dam: An Alternative Model for Semi-Arid Regions of Turkey to Store and Save Groundwater. Environ. Earth. Sci. 2009, 59, 339–345. [Google Scholar] [CrossRef]
- Netherlands Water Partnership (NWP). Smart Water Harvesting Solutions: Examples of Innovative Low-Cost Technologies for Rain, Fog, Runoff Water and Groundwater; Kit: Amsterdam, Netherlands, 2007; ISBN 978-90-78986-01-0. [Google Scholar]
- Li, W.L.; Liu, C.Y.; Tang, H.Y. Groundwater Reservoir Design Theory and Engineering Practices; Yellow River Water Conservancy Press: Zhengzhou, China, 2012; ISBN 978-7-5509-0283-1. (In Chinese) [Google Scholar]
- Sun, X.M. Sustainable Utilization Study of Groundwater Resources in Circum-Bohai-Sea Region. Thesis for Doctoral Degree, China University of Geosciences, Beijing, China, April 2004. (In Chinese with English Abstract). [Google Scholar]
- Xu, J.G.; Wei, Z.R.; Zhang, T.; Zhu, H.H. Construction Condition Analysis for Groundwater reservoir in the Shandong Sector of the Circum-Bohai-Sea Region. Geol. Surv. Res. 2004, 27, 197–202, (In Chinese with English Abstract). [Google Scholar]
- Du, H.X.; Chang, G.C.; Zhang, Q.S.; Zhuang, Y.G. Preliminary Study on Utilizing Groundwater Storage. Adv. Water Sci. 2002, 13, 618–622, (In Chinese with English Abstract). [Google Scholar]
- Deng, M.J. Kariz Wells in Arid Land and Mountain-front Depressed Ground Reservoir. Adv. Water Sci. 2010, 21, 748–756, (In Chinese with English Abstract). [Google Scholar]
- Li, S.J.; Li, Y.; Zhou, X. Using Underground Reservoir Storage and Storage Function to Build Emergency Standby Groundwater Source. Groundwater 2006, 28, 41–43, (In Chinese with English Abstract). [Google Scholar]
- Abarca, E.; Vázquez-Suñé, E.; Ramírez, J.C.; Capino, B.; Gámez, D.; Batlle, F. Optimal Design of Measures to Correct Seawater Intrusion. Water Resour. Res. 2006, 42, W09415. [Google Scholar] [CrossRef]
- Sugio, S.; Nakada, K.; Urish, D.W.; ASCE. Subsurface Seawater Intrusion Barrier Analysis. J. Hydraul. Eng. 1987, 6, 77–779. [Google Scholar] [CrossRef]
- Kaleris, V.; Ziogas, A.I. The Effect of Cutoff Walls on Saltwater Intrusion and Groundwater Extraction in Coastal Aquifers. J. Hydrol. 2013, 476, 370–383. [Google Scholar] [CrossRef]
- Onder, H.; Yilmaz, M. Underground Dams—A Tool of Sustainable Development and Management of Groundwater Resources. Eur. Water 2005, 11, 35–45. [Google Scholar]
- Bonacci, O. Hazards Caused by Natural and Anthropogenic Changes of Catchment Area in Karst. Nat. Hazard Earth Sys. 2004, 4, 655–661. [Google Scholar] [CrossRef]
- Ishida, S.; Tsuchihara, T.; Yoshimoto, S.; Imaizumi, M. Sustainable Use of Groundwater with Underground Dams. Jpn. Agric. Res. Quarterl. 2011, 45, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Ramsesha, C.S.; Nandakumaran, P.; Suresh, S. Efficacy of Sub Surface Dykes as Groundwater Conservation Structures in Hardrock Terrain of Tamil Nadu, India. Int. Semin. Manag. Artif. Recharge 2002. [Google Scholar] [CrossRef]
- Prinz, D.; Singh, A. Technological Potential for Improvements of Water Harvesting; The World Commission on Dams: Cape Town, South Africa, 2000. [Google Scholar]
- Zarkesh, M.M.K.; Ata, D.; Jamshidi, A. Performance of Underground Dams as a Solution for Sustainable Management of Drought. JBU 2012, 1, 35–45. [Google Scholar]
- Nandakumaran, P.; Suresh, S.; Chakrapaani, R. Ground Water Conservation through Sub-surface Dykes—A Case Study from Villupuram District. In Proceedings of the National Conference on Land Care Movement for Food, Water and Livelihood Security, Chennai, India, 16–17 April 2013; Available online: https://doi.org/10.13140/RG.2.1.2203.6644 (accessed on 29 May 2019).
- Foster, S.; Tuinhof, A. Subsurface Dams to Augment Groundwater Storage in Basement Terrain for Human Subsistence in Brazil and Kenya; World Bank: Washington, DC, USA, 2004. [Google Scholar]
- Telmer, K.; Best, M. Underground Dams: A Practical Solution for the Water Needs of Small Communities in Semi-arid Regions. TERRA 2004, 1, 63–65. [Google Scholar]
- National Research Council. Ground Water Recharge Using Waters of Impaired Quality; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Yeh, G.T. Computational Subsurface Hydrology; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Ginige, M.P.; Kaksonen, A.H.; Morris, C.; Shackelton, M.; Patterson, B.M. Bacterial Community and Groundwater Quality Changes in an Anaerobic Aquifer during Groundwater Recharge with Aerobic Recycled Water. Fems. Microbiol. Ecol. 2013, 85, 553–567. [Google Scholar] [CrossRef]
- Grantham, G.; Lucas, J.L. Monitoring of the Unsaturated Zone as an Aid in Aquifer Protection; International Association of Hydrogeologists: Cambridge, UK, 1985; pp. 70–83. [Google Scholar]
- Abdoulhalik, A.; Ahmed, A.; Hamill, G.A. A new physical barrier system for seawater intrusion control. J. Hydrol. 2017, 549, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Lasage, R.; Aerts, J.C.; Verburg, P.H.; Sileshi, A.S. The Role of Small Scale Sand Dams in Securing Water Supply under Climate Change in Ethiopia. Mitig. Adapt. Strateg. Glob. Change 2013, 20, 317–339. [Google Scholar] [CrossRef]
- Ishida, S.; Tsuchihara, T.; Imaizumi, M. Fluctuation of NO-3-N in Groundwater of the Reservoir of the Sunagawa Subsurface Dam, Miyako Island, Japan. Paddy Water Environ. 2006, 4, 101–110. [Google Scholar] [CrossRef]
- Ishida, S.; Kotoku, M.; Abe, E.; Fazal, M.A.; Tsuchihara, T.; Imaizumik, M. Construction of Subsurface Dams and Their Impact on the Environment. Rmz. Mater. Geoenvironment 2003, 50, 149–152. [Google Scholar]
- Yoshimoto, S.; Tsuchihara, T.; Ishida, S.; Imaizumi, M. Development of a Numerical Model for Nitrates in Groundwater in the Reservoir Area of the Komesu Subsurface Dam, Okinawa, Japan. Environ. Earth Sci. 2011, 70, 2061–2077. [Google Scholar] [CrossRef]
- Lalehzari, R.; Tabatabaei, S.H. Simulating the Impact of Subsurface Dam Construction on the Change of Nitrate Distribution. Environ. Earth Sci. 2015, 74, 3241–3249. [Google Scholar] [CrossRef]
- Fakharinia, M.; Lalehzari, R.; Yaghoobzadeh, M. The Use of Subsurface Barriers in the Sustainable Management of Groundwater Resources. World Appl. Sci. J. 2012, 19, 1585–1590. [Google Scholar]
- Zhao, R.F.; Chen, X.P.; Zhang, F.S. Nitrogen Cycling and Balance in Winter-Wheat-Summer-Maize Rotation System in Northern China Plain. Acta Pedologica Sinica 2009, 46, 684–697, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.H. Geochemistry Evolution and Water Cycle Patterns of Groundwater in Golmud River Basin. Master’s Thesis, Chang’an University, Chang’an, China, 2014. (In Chinese with English Abstract). [Google Scholar]
- Long, W.H.; Chen, H.H.; Duan, Q.M.; Li, Z.; Pan, H.J.; Liu, R.Y. Application of Artificial Neural Network in the Restoration of Tritium Concentration in Precipitation. Geol. Resour. 2008, 17, 208–212, (In Chinese with English Abstract). [Google Scholar]
- IAEA/WMO. Global Network of Isotopes in Precipitation. The GN Database, 2018. Available online: https://nucleus.iaea.org/wiser (accessed on 29 May 2019).
- Leduc, C.; Taupin, J.D.; Le Gal La Salle, C. Estimation de la recharge de la nappe phréatique du Continental Terminal (Niamey, Niger) à partir des teneurs en tritium. CR Acad. Sci. Paris 1996, 323, 599–605, (In French with English Abstract). [Google Scholar]
- Le Gal La Salle, C.; Marlin, C.; Leduc, C.; Taupin, J.D.; Massault, M.; Favreau, G. Renewal Rate Estimation of Groundwater Based on Radioactive Tracers (3H, 14C) in an Unconfined Aquifer in a Semi-arid Area, Iullemeden Basin, Niger. J. hydro. 2001, 254, 145–156. [Google Scholar] [CrossRef]
- Dong, W.H.; Lin, X.Y. Analysis on the Influence Factors of the Nitrogen Pollution in Shallow Groundwater—A Case in the North High Plain of Songhua River in Songnen Basin. J. Jilin Univ. Earth Sci. Ed. 2004, 34, 231–235, (In Chinese with English Abstract). [Google Scholar]
- Kang, P.P.; Xu, S.G. The Impact of Mariculture on Nutrient Dynamics and Identification of the Nitrate Sources in Coastal Waters. Environ. Sci. Pollut. R. 2016, 23, 1300–1311. [Google Scholar] [CrossRef] [PubMed]













| Calendar | Growth Period | Agricultural Practices | N-Fertilizer Application (kgN/ha) | |
|---|---|---|---|---|
| June 10th | summer maize | sowing period | summer maize sowing | / |
| July 10th | jointing period | top application | 80 (chemical fertilizer N) | |
| August 1st | booting period | top application | 120 (chemical fertilizer N) | |
| August 20th | filling period | top application | 40 (chemical fertilizer N) | |
| October 1st | harvest period | summer maize harvest straw mulching and nitrogen fertilizer application | 60 (chemical fertilizer N) | |
| October 5th | winter wheat | sowing period | winter wheat sowing base fertilizer application | 250 (organic fertilizer N) 120 (chemical fertilizer N) |
| April 20th | jointing period | top application | 120 (chemical fertilizer N) | |
| June 5th | harvest period | winter wheat harvest straw mulching and N-fertilizer application | 60 (chemical fertilizer N) | |
| Livestock Species | Amount of Wastewater (L/d/pd) | N Concentration (mg/L) | |
|---|---|---|---|
| TN | -N | ||
| swine | 10 | 400 | 250 |
| beef cattle | 30 | 80 | 50 |
| chicken | 0.25 | 500 | 200 |
| Sampling Site | In/Downstream the Reservoir | Ground Elevation (m) | Groundwater Level (m) | Well Depth (m) | Aquifer Characteristics | Surrounding Environment | EC at 25 ℃ (mS/cm) | 3H Level (TU) | Groundwater Renewal Rate (%/a) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | in | 3.4 | 3.1 | 8 | unconfined | wasteland | 1.82 | 17.3 | 14 |
| T2 | in | 4.2 | 3.6 | 3 | unconfined | cultivated land, near the river | 4.91 | 17.0 | 14 |
| T3 | in | 4.6 | 4.0 | 22 | unconfined | cultivated land | 4.16 | 18.7 | 16 |
| T4 | in | 5.0 | –4.9 | about 25 | unconfined | residential land | 1.79 | 17.9 | 15 |
| T5 | in | 6.5 | 4.6 | 31 | unconfined | vegetable field | 1.78 | 18.7 | 16 |
| T6 | in | 9.0 | 6.1 | 35 | unconfined | residential land, near the river | 1.04 | 17.3 | 14 |
| T7 | in | 5.5 | 0.2 | about 35 | unconfined | cultivated land | 3.84 | 18.4 | 15 |
| T8 | in | 10 | 5.8 | 38 | unconfined | cultivated land | 4.80 | 18.0 | 15 |
| T9 | in | 6.0 | –4.0 | about 40 | unconfined | residential land | 2.52 | 16.9 | 14 |
| T10 | in | 5.2 | –5.4 | 25 | unconfined | cultivated land | 1.50 | 18.7 | 16 |
| T11 | in | 10.0 | –0.8 | 20 | unconfined | cultivated land, near the river | 1.66 | 16.8 | 14 |
| T12 | in | 13.1 | –6.1 | 35 | unconfined | cultivated land | 3.55 | 17.2 | 14 |
| T13 | in | 16.0 | 7.7 | 18 | unconfined | orchard | 1.73 | 17.5 | 15 |
| T14 | in | 4.0 | –6.0 | about 20 | unconfined | cultivated land | 4.61 | 17.9 | 15 |
| T15 | in | 13 | 3.6 | about 35 | unconfined | cultivated land | 2.28 | 17.0 | 14 |
| T16 | in | 9.0 | –6.1 | 35 | unconfined | orchard | 1.88 | 18.8 | 16 |
| T17 | in | 7.5 | –9.1 | 14 | unconfined | cultivated land | 1.77 | 16.8 | 14 |
| T18 | downstream | 0.7 | 0.5 | 26 | unconfined | aquaculture land, near the river | 41.9 | 4 | 2 |
| T19 | downstream | 4.4 | 1.6 | 11 | unconfined | cultivated land | 27.4 | 12.6 | 6 |
| T20 | downstream | 3.2 | 2.6 | 10 | unconfined | waste land | 26.8 | 15.5 | 9 |
| T21 | downstream | 4.2 | 4.0 | about 20 | unconfined | residential land | 31.5 | 16.2 | 11 |
| T22 | downstream | 4.5 | 3.2 | 20 | unconfined | cultivated land | 18.0 | 19.2 | 16 |
| T23 | downstream | 0.2 | 0.0 | 12 | unconfined | aquaculture land | 47.1 | 4 | 2 |
| T24 | downstream | 0.2 | –0.1 | 30 | unconfined | aquaculture land | 46.1 | 3.1 | 2 |
| T25 | downstream | 5 | –4.0 | 23 | unconfined | livestock farm | 19.6 | 16.3 | 12 |
| T26 | downstream | 3.2 | –0.9 | 22 | unconfined | pine forest | 32.2 | 6.4 | 3 |
| T27 | downstream | 5 | –3.8 | 18 | unconfined | wasteland | 26.4 | 13.1 | 6 |
| T28 | downstream | 3.7 | –0.4 | 27 | unconfined | aquaculture land | 39.7 | 5.7 | 3 |
| T29 | downstream | 4.0 | –4.0 | 18 | unconfined | residential land | 16.6 | 14.4 | 8 |
| T30 | downstream | 7.4 | –4.0 | about 40 | unconfined | aquaculture land | 41.5 | 6.2 | 3 |
| T31 | downstream | 6 | –3.6 | 33 | unconfined | aquaculture land | 42.3 | 4.9 | 2 |
| Form of Inorganic-N | 2014.07 | 2015.04 | 2015.08 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inside | Downstream | Inside | Downstream | Inside | Downstream | |||||||
| Range | Average | Range | Average | Range | Average | Range | Average | Range | Average | Range | Average | |
| -N | 97.92–99.97 | 99.57 | 3.91–99.73 | 76.32 | 86.83–99.65 | 98.18 | 0.33–99.20 | 64.52 | 99.17–99.91 | 99.75 | 16.42–99.24 | 79.46 |
| -N | 0.00–0.54 | 0.12 | 0.04–15.56 | 4.69 | 0.04–1.52 | 0.23 | 0.26–36.93 | 6.89 | 0.00–0.22 | 0.04 | 0.03–2.83 | 1.33 |
| -N | 0.02–1.79 | 0.31 | 0.00–80.54 | 18.99 | 0.29–11.65 | 1.59 | 0.27–99.41 | 28.59 | 0.07–0.62 | 0.21 | 0.42–79.45 | 19.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xu, S.G.; Kang, P.P.; Fu, Y.Z.; Wang, T.X. Impacts of Artificial Underground Reservoir on Groundwater Environment in the Reservoir and Downstream Area. Int. J. Environ. Res. Public Health 2019, 16, 1921. https://doi.org/10.3390/ijerph16111921
Sun Y, Xu SG, Kang PP, Fu YZ, Wang TX. Impacts of Artificial Underground Reservoir on Groundwater Environment in the Reservoir and Downstream Area. International Journal of Environmental Research and Public Health. 2019; 16(11):1921. https://doi.org/10.3390/ijerph16111921
Chicago/Turabian StyleSun, Ya, Shi Guo Xu, Ping Ping Kang, Yan Zhao Fu, and Tian Xiang Wang. 2019. "Impacts of Artificial Underground Reservoir on Groundwater Environment in the Reservoir and Downstream Area" International Journal of Environmental Research and Public Health 16, no. 11: 1921. https://doi.org/10.3390/ijerph16111921
APA StyleSun, Y., Xu, S. G., Kang, P. P., Fu, Y. Z., & Wang, T. X. (2019). Impacts of Artificial Underground Reservoir on Groundwater Environment in the Reservoir and Downstream Area. International Journal of Environmental Research and Public Health, 16(11), 1921. https://doi.org/10.3390/ijerph16111921