Using Microbiological Sampling to Evaluate the Efficacy of Nasofibroscope Disinfection: The Tristel Trio Wipes System in Ear–Nose–Throat (ENT) Endoscopy
Abstract
:1. Introduction
- ✓
- patient and personnel safety
- ✓
- a high level of standardization in reprocessing
- ✓
- documentation of several important endoscope reprocessing parameters (printed automatically)
- ✓
- audible and visual alarms activated when a safety fault is detected
- ✓
- a lower work-load compared to full manual reprocessing.
- ✓
- the specialized AWs require a separate reprocessing room
- ✓
- if AWs are not kept properly, they may themselves become an infection hazard by contamination of endoscopes during reprocessing. Systematic maintenance and validation of is compulsory
- ✓
- potentially high costs
- ✓
- the endoscopy procedure may have to be cancelled if AWs break down
- ✓
- the washing process may decrease the clarity of the optical image within a short period of time.
2. Materials and Methods
2.1. Automated Mechanical Washer (AW)
- acidic pre-disinfectant cleaner, compatible with peracetic acid; then
- five minutes of high-level disinfection with a 5% peracetic acid-based disinfectant with anticorrosive additive; then
- rinsing with filtered water (0.2 µm) to ensures that bacteria free water is supplied to the FFNs.
2.2. Tristel Trio Wipes (TTW)
- pre-cleaning with the Tristel Pre-Clean Wipe,
- high-level disinfection with the sporicidal wipe for a contact time of 30 s,
- neutralization of any chemical residues with the rinse wipe.
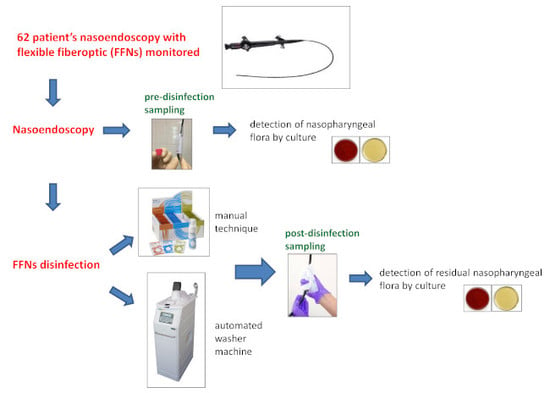
2.3. Sampling Procedure
2.4. Laboratory Analysis
2.4.1. Sample Preparation
- The pre-disinfection samples (tube with 2 mL of Page’s rinsing solution) were vortexed for 30 s.
- The post-disinfection samples were vortexed for 30 sec in order to extract the microbial load from the wipe, then centrifuged (3000× g for 30 min) and finally suspended in 2 mL of Page’s buffer.
2.4.2. Culture and Identification
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008; CDC (Centers for Disease Control and Prevention): Atlanta, GA, USA, 2008; pp. 1–161.
- Spaulding, E. Chemical disinfection and antisepsis in the hospital. J. Hosp. Res. 1957, 9, 5–31. [Google Scholar]
- Muscarella, L.F. Prevention of disease transmission during flexible laryngoscopy. Am. J. Infect. Control. 2007, 35, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Warren, D.; Tomasi, A.M.; Raju, T.N.; Vidyasagar, D. Transmission of neonatal listeriosis in a delivery room. Am. J. Dis. Child. 1985, 139, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Foweraker, J.E. The laryngoscope as a potential source of cross-infection. J. Hosp. Infect. 1995, 29, 315–316. [Google Scholar] [CrossRef]
- Neal, T.J.; Hughes, C.R.; Rothburn, M.M.; Shaw, N.J. The neonatal laryngoscope as a potential source of cross-infection. J. Hosp. Infect. 1995, 30, 315–317. [Google Scholar] [CrossRef]
- Hitchcock, B.; Moynan, S.; Frampton, C.; Reuther, R.; Gilling, P.; Rowe, F. A randomised, single-blind comparison of high-level disinfectants for flexible nasendoscopes. J. Laryngol. Otol. 2016, 130, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration FDA. FDA-Cleared Sterilants and High Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices. 2015. Available online: https://www.fda.gov/medicaldevices/deviceregulationandguidance/reprocessingofreusablemedicaldevices/ucm437347.htm (accessed on 11 November 2019).
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.M.; Hassan, C.; Jung, M.; Kampf, B.; Neumann, C.; et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA)—Update 2018. Endoscopy 2018, 50, 1205–1234. [Google Scholar] [CrossRef] [PubMed]
- Tristel. Tristel User Guide. Available online: http://www.tristel.com/sites/default/files/trl_247-2_trio_user_guide_gb.pdf (accessed on 20 February 2019).
- ENT, UK. Guidance on the Decontamination and Sterilization of Rigid and Flexible Endoscopes; ENT UK: London, UK, 2010. [Google Scholar]
- Ditommaso, S.; Giacomuzzi, M.; Ricciardi, E.; Zotti, C. Experimental Study to Develop a Method for Improving Sample Collection to Monitor Laryngoscopes after Reprocessing. Clin. Endosc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Carbonnelle, E.; Mesquita, C.; Bille, E.; Day, N.; Dauphin, B.; Beretti, J.L.; Ferroni, A.; Gutmann, L.; Nassif, X. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin. Biochem. 2011, 44, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rolain, J.-M. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lowry, P.W.; Jarvis, W.R.; Oberle, A.D.; Bland, L.A.; Silberman, R.; Bocchini, J.A., Jr.; Dean, H.D.; Swenson, J.M.; Wallace, R.J., Jr. Mycobacterium chelonae causing otitis media in an ear-nose-and-throat practice. N. Engl. J. Med. 1988, 319, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Sorin, M.; Segal-Maurer, S.; Mariano, N.; Urban, C.; Combest, A.; Rahal, J.J. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect. Control. Hosp. Epidemiol. 2001, 22, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Shimono, N.; Takuma, T.; Tsuchimochi, N.; Shiose, A.; Murata, M.; Kanamoto, Y.; Uchida, Y.; Morita, S.; Matsumoto, H.; Hayashi, J. An outbreak of Pseudomonas aeruginosa infections following thoracic surgeries occurring via the contamination of bronchoscopes and an automatic endoscope reprocessor. J. Infect. Chemother. 2008, 14, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Gastmeier, P.; Vonberg, R.-P. Klebsiella spp. in endoscopy-associated infections: we may only be seeing the tip of the iceberg. Infection 2014, 42, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.; Block, C.; Schwartz, C.; Gross, I.; Cohen, M.; Fridlender, Z.G.; Moses, A.E.; Berkman, N.; Benenson, S. Cluster of Fusarium solani isolations in a Bronchoscopy Unit. Clin. Microbiol. Infect. 2016, 22, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.; Smith, A.; Anderson, M.; Stewart, J.; Hamilton, K.; McNamee, S.; Curran, E.T. Transmission of Salmonella enteritidis after endoscopic retrograde cholangiopancreatography because of inadequate endoscope decontamination. Am. J. Infect. Control 2017, 45, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, K.; Choudhury, N.; Bhat, S.; Weerasinghe, A.; Marais, J. Evaluation of disinfection of flexible nasendoscopes using Tristel wipes: a prospective single blind study. Ann. R. Coll. Surg. Engl. 2012, 94, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J.M. Efficient methods for large-area surface sampling of sites contaminated with pathogenic microorganisms and other hazardous agents: current state, needs, and perspectives. Appl. Microbiol. Biotechnol. 2009, 84, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Essential Elements of a Reprocessing Program for Flexible Endoscopes—Recommendations of the HICPAC; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017; p. 12.
- National Health Service. Management and Decontamination of Flexible Endoscopes (HTM 01-06). 2016. Available online: https://www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes (accessed on 26 September 2018).
- Beilenhoff, U.; Neumann, C.S.; Rey, J.F.; Biering, H.; Blum, R.; Schmidt, V.; null and the ESGE Guidelines Committee. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy 2007, 39, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Public Health Agency of Canada. Infection Prevention and Control Guideline for Flexible Gastrointestinal Endoscopy and Flexible Bronchoscopy; Public Health Agency of Canada: Ottawa, ON, Canada, 2011. Available online: http://www.deslibris.ca/ID/229834 (accessed on 11 November 2019).


| Bacterium | No. of Samples with the Indicated Isolate (%) |
|---|---|
| Staphylococcus epidermidis | 45 (73) |
| Staphylococcus aureus | 23 (37) |
| Coagulase-negative staphylococci (CoNS) | 8 (13) |
| Streptococcus viridans | 27 (44) |
| Streptococcus pneumoniae | 5 (8) |
| Corynebacterium spp | 19 (31) |
| Corynebacterium striatum | 3 (5) |
| Other gram-positive bacteria | 10 (16) |
| Pseudomonas aeruginosa | 7 (11) |
| Stenotrophomonas maltophilia | 1 (2) |
| Neisserie | 6 (10) |
| Haemophilus influenzae | 5 (8) |
| Haemophilus parainfluenzae | 8 (13) |
| Enterobacteriaceae | 8 (13) |
| Non-fermenting Gram-negative bacilli | 2 (3) |
| Other Gram-negative bacteria | 1 (2) |
| Candida albicans | 2 (3) |
| Type of Samples | TVCs (Geometric Mean CFU ± ds) |
|---|---|
| AW | 2.54 × 102 ± 5.42 × 102 |
| TTW | 2.41 × 102 ± 4.71 × 102 |
| Code/Groups | Bacterium (Colony Count) |
|---|---|
| 24 AW pre-disinfection | Corynebacterium spp., S. epidermidis (≅100) * |
| 24 AW post-disinfection | Kokuria spp. (2) |
| 42 AW pre-disinfection | S. viridans, S. epidermidis (≅200) * |
| 42 AW post-disinfection | Rothia mucillaginosa (4), S. viridans (1) |
| 1 TTW pre-disinfection | S. epidermidis, S. viridans, H. parainflenzae, N. subflava (≅400) * |
| 1 TTW post-disinfection | S. hominis (1) |
| 15 TTW pre-disinfection | S. epidermidis (100) |
| 15 TTW post-disinfection | S. epidermidis (1) |
| 22 TTW pre-disinfection | S. epidermidis (6) |
| 22 TTW post-disinfection | S. epidermidis (1) |
| 23 TTW pre-disinfection | S. aureus, S. epidermidis (≅500) * |
| 23 TTW post-disinfection | S. epidermidis (2) |
| 26 TTW pre-disinfection | S. aureus, C. striatum, P.aeruginosa, S. epidermidis, S. viridans (≅1300) * |
| 26 TTW post-disinfection | Micrococcus luteus, S. viridans (≅600) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditommaso, S.; Giacomuzzi, M.; Cipriani, R.; Zaccaria, T.; Cavallo, R.; Boggio, V.; Albera, R.; Zotti, C.M. Using Microbiological Sampling to Evaluate the Efficacy of Nasofibroscope Disinfection: The Tristel Trio Wipes System in Ear–Nose–Throat (ENT) Endoscopy. Int. J. Environ. Res. Public Health 2019, 16, 4583. https://doi.org/10.3390/ijerph16224583
Ditommaso S, Giacomuzzi M, Cipriani R, Zaccaria T, Cavallo R, Boggio V, Albera R, Zotti CM. Using Microbiological Sampling to Evaluate the Efficacy of Nasofibroscope Disinfection: The Tristel Trio Wipes System in Ear–Nose–Throat (ENT) Endoscopy. International Journal of Environmental Research and Public Health. 2019; 16(22):4583. https://doi.org/10.3390/ijerph16224583
Chicago/Turabian StyleDitommaso, Savina, Monica Giacomuzzi, Raffaella Cipriani, Teresa Zaccaria, Rossana Cavallo, Valeria Boggio, Roberto Albera, and Carla M. Zotti. 2019. "Using Microbiological Sampling to Evaluate the Efficacy of Nasofibroscope Disinfection: The Tristel Trio Wipes System in Ear–Nose–Throat (ENT) Endoscopy" International Journal of Environmental Research and Public Health 16, no. 22: 4583. https://doi.org/10.3390/ijerph16224583
APA StyleDitommaso, S., Giacomuzzi, M., Cipriani, R., Zaccaria, T., Cavallo, R., Boggio, V., Albera, R., & Zotti, C. M. (2019). Using Microbiological Sampling to Evaluate the Efficacy of Nasofibroscope Disinfection: The Tristel Trio Wipes System in Ear–Nose–Throat (ENT) Endoscopy. International Journal of Environmental Research and Public Health, 16(22), 4583. https://doi.org/10.3390/ijerph16224583