A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils
Abstract
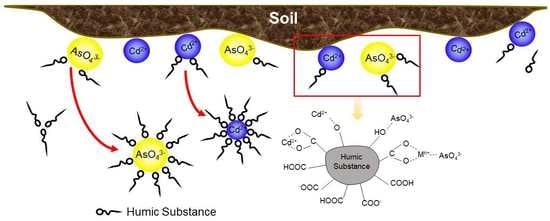
:1. Introduction
2. Materials and Methods
2.1. Soil Samples, Spiking Treatment, and Laboratory Analysis
2.2. Humic Substance and Its Analysis
2.3. Soil Washing Experiment
2.4. Kinetics of Cd and As Removal by Humic Substance
| Pseudo-first-order equation: | (1) | |
| Pseudo-second-order equation: | (2) | |
| Elovich: | (3) | |
| Parabolic diffusion: | (4) | |
| Power function: | (5) |
3. Results and Discussion
3.1. The Basic Properties of Spiked Soils and Humic Substances
3.2. Effect of pH on the Removal of Cd and As
3.3. Effect of Liquid–Solid Ratio on the Removal of Cd and As
3.4. Effect of Humic Substance Concentration on the Removal of Cd and As
3.5. Effect of Humic Substance Washing Cycles on Cd and As Removal
3.6. FTIR Analysis of Humic Substance Before and After Washing Contaminated Soils
3.7. Kinetics of Cd and As Removal by Humic Substance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministry of Environmental Protection. Report on the National Soil Contamination Survey. Available online: http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/W020140417558995804588.pdf (accessed on 13 November 2019).
- Guo, X.F.; Wei, Z.B.; Wu, Q.T.; Li, C.P.; Qian, T.W.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Cardellach, E.; Cañadas, I.; Rodríguez, J. Solar thermal vitrification of mining contaminated soils. Int. J. Miner. Process. 2013, 119, 65–74. [Google Scholar] [CrossRef]
- Sylvain, B.; Mikael, M.H.; Florie, M.; Emmanuel, J.; Marilyne, S.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.D.; Yuan, G.D.; Wei, J.; Bi, D.X.; Ok, Y.S.; Wang, H.L. Humic substances as a washing agent for Cd-contaminated soils. Chemosphere 2017, 181, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Chen, J.J.; Wang, X.W. Removal of arsenic and cadmium with sequential soil washing techniques using Na2EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef]
- Yuan, G.D. Nanomaterials to the rescue. Nano Today 2008, 3, 61. [Google Scholar] [CrossRef]
- Beiyuan, J.Z.; Tsang, D.C.W.; Valix, M.; Baek, K.; Ok, Y.S.; Zhang, W.H.; Bolan, N.S.; Rinklebe, J.; Li, X.D. Combined application of EDDS and EDTA for removal of potentially toxic elements under multiple soil washing schemes. Chemosphere 2018, 205, 178–187. [Google Scholar] [CrossRef]
- Gitipour, S.; Ahmadi, S.; Madadian, E.; Ardestani, M. Soil washing of chromium- and cadmium-contaminated sludge using acids and ethylenediaminetetra acetic acid chelating agent. Environ. Technol. 2016, 37, 145–151. [Google Scholar] [CrossRef]
- Kim, J.O.; Lee, Y.W.; Chung, J. The role of organic acids in the mobilization of heavy metals from soil. KSCE J. Civ. Eng. 2013, 17, 1596–1602. [Google Scholar] [CrossRef]
- Makino, T.; Maejima, Y.; Akahane, I.; Kamiya, T.; Takano, H.; Fujitomi, S.; Ibaraki, T.; Kunhikrishnan, A.; Bolan, N. A practical soil washing method for use in a Cd-contaminated paddy field, with simple on-site wastewater treatment. Geoderma 2016, 270, 3–9. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gusiatin, Z.M.; Klimiuk, E. Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 2012, 86, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Ali, A.; Wang, P.; Li, R.H.; Tian, X.H.; Zhang, Z.Q. Comparison of the feasibility of different washing solutions for combined soil washing and phytoremediation for the detoxification of cadmium (Cd) and zinc (Zn) in contaminated soil. Chemosphere 2019, 230, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, V.; Kastelec, D.; Lestan, D.; Grcman, H. Soil physical characteristics after EDTA washing and amendment with inorganic and organic additives. Environ. Pollut. 2014, 186, 56–62. [Google Scholar] [CrossRef]
- Torres, L.G.; Lopez, R.B.; Beltran, M. Removal of As, Cd, Cu, Ni, Pb, and Zn from a highly contaminated industrial soil using surfactant enhanced soil washing. Phys. Chem. Earth 2012, 30–36, 37–39. [Google Scholar] [CrossRef]
- Ash, C.; Tejnecky, V.; Boruvka, L.; Drabek, O. Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrol. 2016, 187, 18–30. [Google Scholar] [CrossRef]
- Tan, K.H. Humic matter in Soil and the Environment, Principles and Controversies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kulikowska, D.; Gusiatin, Z.M.; Bulkowska, K.; Klik, B. Feasibility of using humic substances from compost to remove heavy metals (Cd, Cu, Ni, Pb, Zn) from contaminated soil aged for different periods of time. J. Hazard. Mater. 2015, 300, 882–891. [Google Scholar] [CrossRef]
- Yuan, G.D.; Theng, B.K.G. Clay-organic interactions in soil environments. In Handbook of Soil Science: Resource Management and Environmental Impacts, 2nd ed.; Huang, P.M., Sumner, M., Li, Y.C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; Volume 2, pp. 2.1–2.20. [Google Scholar]
- Lu, R.K. Analytical Methods for Soil and Agricultural Chemistry; Agricultural Science and Technology Press: Beijing, China, 1999; pp. 477–479. [Google Scholar]
- International Humic Substances Society. Acidic Functional Groups of IHSS Samples. Available online: http://humic-substances.org/acidic-functional-groups-of-ihss-samples/ (accessed on 13 November 2019).
- Scholz, N.; Behnke, T.; Resch, G.U. Determination of the critical micelle concentration of neutral and ionic surfactants with fluorometry, conductometry, and surface tension-a method comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; Gharous, E.M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Mulligan, C.N. Effects of three low-molecular-weight organic acids (LMWOAs) and pH on the mobilization of arsenic and heavy metals (Cu, Pb, and Zn) from mine tailings. Environ. Geochem. Health 2013, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Neupane, G.; Donahoe, R.J. Calcium–phosphate treatment of contaminated soil for arsenic immobilization. Appl. Geochem. 2013, 28, 145–154. [Google Scholar] [CrossRef]
- Mao, X.H.; Jiang, R.; Xiao, W.; Yu, J.G. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Dousova, B.; Buzek, F.; Herzogova, L.; Machovic, V.; Lhotka, M. Effect of organic matter on arsenic(V) and antimony(V) adsorption in soils. Eur. J. Soil Sci. 2015, 66, 74–82. [Google Scholar] [CrossRef]
- Carbonaro, R.F.; Atalay, Y.B.; Di Toro, D.M. Linear free energy relationships for metal-ligand complexation: Bidentate binding to negatively-charged oxygen donor atoms. Geochim. Cosmochim. Acta 2011, 75, 2499–2511. [Google Scholar] [CrossRef] [Green Version]
- Fakour, H.; Lin, T.F. Experimental determination and modeling of arsenic complexation with humic and fulvic acids. J. Hazard. Mater. 2014, 279, 569–578. [Google Scholar] [CrossRef]
- Wang, S.; Song, X.Y.; Wang, N.; Li, C.X.; Wang, W.; Zhang, J.J. Characteristics of soil humic substances as determined by conventional and synchrotron Fourier Transform Infrared Spectroscopy. J. Appl. Spectrosc. 2014, 81, 843–849. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, Y.T.; Xiong, H.B. Removal of heavy metals from polluted soil using the citric acid fermentation broth: A promising washing agent. Environ. Sci. Pollut. Res. 2017, 24, 9506–9514. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Pacheco, C.; Hay, M.B.; Myneni, S.C.B. Structural environments of carboxyl groups in natural organic molecules from terrestrial systems. Part 2: 2D NMR spectroscopy. Geochim. Cosmochim. Acta 2007, 71, 3533–3544. [Google Scholar] [CrossRef]
- Pospíšilová, L.; Komínková, M.; Zítka, O.; Kizek, R.; Barančíková, G.; Litavec, T.; Lošák, T.; Hlušek, J.; Martensson, A.; Liptaj, T. Fate of humic acids isolated from natural humic substances. Acta Agric. Scand. Sect. B 2015, 65, 517–528. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, J.L.; Wang, R.Q.; Li, F.S.; Wang, W.X. Adsorption and desorption of divalent mercury (Hg2+) on humic acids and fulvic acids extracted from typical soils in China. Colloids Surf. A 2009, 335, 194–201. [Google Scholar] [CrossRef]
- Abdulla, H.A.N.; Minor, E.C.; Dias, R.F.; Hatcher, P.G. Changes in the compound classes of dissolved organic matter along an estuarine transect: A study using FTIR and 13C NMR. Geochim. Cosmochim. Acta 2010, 74, 3815–3838. [Google Scholar] [CrossRef]
- Zhou, Y.; Selvam, A.; Wong, J.W.C. Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and arsenate binding to dissolved humic acids: Influence of pH, type of humic acid, and aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Habibul, N.; Liu, X.Y.; Sheng, G.P.; Yu, H.Q. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto dissolved organic matter. Environ. Sci. Technol. 2015, 49, 2052–2058. [Google Scholar] [CrossRef]
- Nakamoto, K. Applications in coordination, organometallic, and bioinorganic chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Li, H.; Wang, J.H.; Zhao, B.Y.; Gao, M.S.; Shi, W.J.; Zhou, H.J.; Xie, Z.L.; Zhou, B.; Lu, C.W.; He, J. The role of major functional groups: Multi-evidence from the binding experiments of heavy metals on natural fulvic acids extracted from lake sediments. Ecotoxicol. Environ. Saf. 2018, 162, 514–520. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Usman, A.R.A.; Ahmad, M.; Kim, K.R.; Vithanage, M.; Ok, Y.S. Role of chelating agents on release kinetics of metals and their uptake by maize from chromated copper arsenate-contaminated soil. Environ. Technol. 2013, 34, 747–755. [Google Scholar] [CrossRef]
- Fonseca, B.; Pazos, M.; Figueiredo, H.; Tavares, T.; Sanromán, M.A. Desorption kinetics of phenanthrene and lead from historically contaminated soil. Chem. Eng. J. 2011, 167, 84–90. [Google Scholar] [CrossRef]
- Tsai, S.C.; Wang, T.H.; Wei, Y.Y.; Yeh, W.C.; Jan, Y.L.; Teng, S.P. Kinetics of Cs adsorption/desorption on granite by a pseudo first order reaction model. J. Radioanal. Nucl. Chem. 2007, 275, 555–562. [Google Scholar] [CrossRef]
- Meng, F.D.; Yuan, G.D.; Larson, S.L.; Ballard, J.H.; White, J.R.; Arslan, Z.; Han, F.X. Kinetics and thermodynamics of uranium (VI) adsorption onto humic acid derived from leonardite. Int. J. Environ. Res. Public Health 2019, 16, 1552. [Google Scholar] [CrossRef] [Green Version]






| Parameters | Unit | Red Soil | Black Soil | Fluvo-Aquic Soil |
|---|---|---|---|---|
| Sand content | % | 31.53 ± 0.09 | 32.79 ± 0.05 | 36.36 ± 0.05 |
| Silt content | % | 55.77 ± 0.15 | 55.41 ± 0.14 | 51.56 ± 0.14 |
| Clay content | % | 12.71 ± 1.04 | 11.80 ± 0.59 | 12.08 ± 2.13 |
| Texture 1 | - | Silty loam | Silty loam | Silty loam |
| CEC | Cmol (+)/kg | 11.01 ± 0.13 | 26.05 ± 0.21 | 18.41 ± 0.17 |
| Soil organic carbon | g/kg | 2.72 ± 0.03 | 16.43 ± 0.14 | 10.52 ± 0.07 |
| pH (H2O) | - | 5.68 | 7.89 | 8.20 |
| Total Cd | mg/kg | 47.50 ± 0.55 | 49.10 ± 0.93 | 40.47 ± 0.93 |
| Total As | mg/kg | 450.86 ± 10.12 | 584.25 ± 8.56 | 566.88 ± 10.47 |
| Parameters | C | H | O | N | Ash Content | pH | CMC 1 | Phenolic-OH | -COOH | Total Ca | Total As | Total Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | % | - | mg C/L | mol/kg | mg/g | mg/kg | ||||||
| Humic substance | 40.90 | 2.82 | 23.71 | 0.52 | 31.78 | 10.72 | 1575.46 | 2.84 | 7.55 | 12.13 | 3.69 | 0.09 |
| Models | Parameters | Metal | Red Soil | Black Soil | Fluvo-Aquic Soil |
|---|---|---|---|---|---|
| Pseudo-first-order equation | q1 | Cd | 41.960 | 29.820 | 25.710 |
| k1 | 5.294 | 0.846 | 0.457 | ||
| R2 | 0.997 | 0.952 | 0.882 | ||
| q1 | As | 203.320 | 258.650 | 303.070 | |
| k1 | 0.461 | 1.342 | 0.217 | ||
| R2 | 0.918 | 0.931 | 0.990 | ||
| Pseudo-second-order equation | q2 | Cd | 41.48 | 25.05 | 19.45 |
| k2 | 0.847 | 0.145 | 0.144 | ||
| R2 | 0.996 | 0.842 | 0.737 | ||
| q2 | As | 222.340 | 276.780 | 321.030 | |
| k2 | 0.003 | 0.008 | 0.001 | ||
| R2 | 0.968 | 0.983 | 0.972 | ||
| Elovich | α | Cd | 5260.98 | 252.05 | 70.39 |
| β | 0.222 | 0.214 | 0.209 | ||
| R2 | 0.804 | 0.913 | 0.950 | ||
| α | As | 424.160 | 7861.220 | 214.630 | |
| β | 0.024 | 0.029 | 0.014 | ||
| R2 | 0.944 | 0.903 | 0.939 | ||
| Parabolic diffusion | a | Cd | 23.870 | 9.620 | 5.500 |
| kp | 5.650 | 5.740 | 5.400 | ||
| R2 | 0.240 | 0.674 | 0.864 | ||
| a | As | 40.530 | 99.760 | 14.210 | |
| kp | 43.490 | 47.820 | 67.370 | ||
| R2 | 0.837 | 0.600 | 0.919 | ||
| Power function | b | Cd | 40.040 | 19.120 | 12.980 |
| kf | 0.025 | 0.179 | 0.256 | ||
| R2 | 0.999 | 0.956 | 0.986 | ||
| b | As | 99.930 | 187.340 | 91.210 | |
| kf | 0.266 | 0.143 | 0.405 | ||
| R2 | 0.956 | 0.971 | 0.939 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L.; Meng, F.; Wang, J. A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils. Int. J. Environ. Res. Public Health 2019, 16, 4999. https://doi.org/10.3390/ijerph16244999
Bi D, Yuan G, Wei J, Xiao L, Feng L, Meng F, Wang J. A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils. International Journal of Environmental Research and Public Health. 2019; 16(24):4999. https://doi.org/10.3390/ijerph16244999
Chicago/Turabian StyleBi, Dongxue, Guodong Yuan, Jing Wei, Liang Xiao, Lirong Feng, Fande Meng, and Jie Wang. 2019. "A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils" International Journal of Environmental Research and Public Health 16, no. 24: 4999. https://doi.org/10.3390/ijerph16244999
APA StyleBi, D., Yuan, G., Wei, J., Xiao, L., Feng, L., Meng, F., & Wang, J. (2019). A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils. International Journal of Environmental Research and Public Health, 16(24), 4999. https://doi.org/10.3390/ijerph16244999