Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses
Abstract
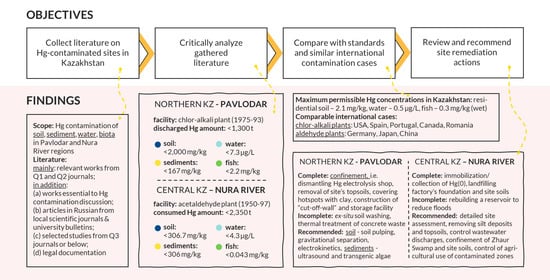
:1. Introduction
2. Human Exposure to Hg, Regulations on Hg, and Hg Mobility
2.1. Human Exposure to Mercury, Regulations
2.2. Effect of Site-Specific Conditions on Hg Mobility
3. Site #1: Lake Balkyldak and Pavlodar Region
3.1. Soils
3.2. Sediments
3.3. Water
3.4. Biota
3.5. Air and Snow
3.6. Hg in Population and Food
3.7. Human Health Risk Assessment by Hg Exposure
4. Site #2: Nura River and Temirtau Region
4.1. Soils
4.2. Sediments
4.3. Water
4.4. Biota
4.5. Hg in Population
4.6. Comparison Between Cases of Pavlodar and Nura
5. Comparison with Cases from Literature
6. Remediation Responses
6.1. Demercuration of Lake Balkyldak
6.2. Demercuration of Nura River
7. Conclusions and Recommendations
- Sediments from Lake Balkyldak are severely contaminated in contrast to the nearby Irtysh River, which was less affected by Hg pollution.
- Several hotspots with high Hg levels, mainly located on the chemical plant’s territory, might still be significant sources of pollution, and as a result, Hg from soil can affect the atmosphere, groundwater, and other media.
- Human health risks are mainly related to fish consumption from the lake and homegrown vegetables with the possibility of soil ingestion.
- The first 10–20 km of the riverbed from the wastewater discharge point was confirmed to be the most contaminated part of the river in terms of Hg in soil, sediments, and technogenic silts.
- Special attention should be paid to the river sediments that may contaminate water during annual floods.
- The local population might be exposed to Hg due to the consumption of locally caught fish from the river and reservoirs; hence, it is necessary to prohibit fishing and consumption in the region.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EEA (European Environment Agency). Hazardous Substances in Europe’s Fresh and Marine Waters—An Overview; EEA Technical Report No 8/2011; EEA: Copenhagen, Denmark, 2011. [Google Scholar]
- UNEP (United Nations Environmental Protection) and WHO (World Health Organization). Guidance for Identifying Populations at Risk from Mercury Exposure; UNEP (United Nations Environmental Protection) and WHO (World Health Organization): Geneva, Switzerland, 2008. [Google Scholar]
- Hsiao, H.-W.; Ullrich, S.M.; Tanton, T.W. Burdens of mercury in residents of Temirtau, Kazakhstan. Sci. Total Environ. 2011, 409, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Swedish Work Environment Authority. Occupational Exposure Limit Values, AFS 2011:18. The Swedish Work Environment Authority’s Provisions and General Recommendations on Occupational Exposure Limit Values. 2011. Available online: https://old.liu.se/medfak/bkv/arbetsmiljo/laboratoriesakerhetshandboken/kemikaliehantering/forbud-och-tillstand/dokument-om-om-forbud-gransvarden-mm/1.692615/OccupationalExposureLimitValues_AFS2011_18.Eng.pdf (accessed on 30 November 2020).
- Xu, J.; Bravo, A.G.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Bueno, P.C.; Bellido, E.; Rubí, J.A.M.; Ballesta, R.J. Concentration and spatial variability of mercury and other heavy metals in surface soil samples of periurban waste mine tailing along a transect in the Almadén mining district (Spain). Environ. Earth Sci. 2008, 56, 815–824. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.; Li, P.; Yu, B.; Lin, C.-J.; Sommar, J.; Feng, X. Re-emission of legacy mercury from soil adjacent to closed point sources of Hg emission. Environ. Pollut. 2018, 242, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.L.; Puccini, M. Leather tanning: Life cycle assessment of retanning, fatliquoring and dyeing. J. Clean. Prod. 2019, 226, 720–729. [Google Scholar] [CrossRef]
- AMAP (Arctic Monitoring and Assessment Programme)/UNEP (United Nations Environment Programme). Technical Background Report for the Global Mercury Assessment 2013; UNEP: Geneva, Switzerland, 2013. [Google Scholar]
- Qureshi, A.; MacLeod, M.; Scheringer, M.; Hungerbühler, K. Mercury cycling and species mass balances in four North American lakes. Environ. Pollut. 2009, 157, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Acquavita, A.; Biasiol, S.; Lizzi, D.; Mattassi, G.; Pasquon, M.; Skert, N.; Marchiol, L. Gaseous Elemental Mercury Level and Distribution in a Heavily Contaminated Site: The Ex-chlor Alkali Plant in Torviscosa (Northern Italy). Water Air Soil Pollut. 2017, 228, 62–75. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2004; Volume 1, Available online: http://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf (accessed on 30 November 2020).
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Porewater Distribution and Benthic Flux Measurements of Mercury and Methylmercury in the Gulf of Trieste (Northern Adriatic Sea). Estuar. Coast. Shelf Sci. 1999, 48, 415–428. [Google Scholar] [CrossRef]
- Guney, M.; Welfringer, B.; De Repentigny, C.; Zagury, G.J. Children’s Exposure to Mercury-Contaminated Soils: Exposure Assessment and Risk Characterization. Arch. Environ. Contam. Toxicol. 2013, 65, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.R.; Shannon, M.W. The Committee on Environmental Health Technical report: Mercury in the environment: Implications for pediatricians. Pediatrics 2001, 108, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozheyeva, G. The Pavlodar chemical weapons plant in Kazakhstan: History and legacy. Nonproliferation Rev. 2000, 7, 136–145. [Google Scholar] [CrossRef]
- Woodruff, S.; Dack, S. Analysis of risk from mercury contamination at the Khimprom Plant in Kazakhstan. Land Contam. Reclam. 2004, 12, 213–218. [Google Scholar] [CrossRef]
- Ilyushchenko, M.A.; Yakovlyeva, L.V.; Heaven, S.; Lapshin, E.V. Mercury pollution in Nura River. Promyshlennost Kazakhstana 2001, 6, 56–59. (In Russian) [Google Scholar]
- Hsiao, H.-W.; Ullrich, S.M.; Tanton, T.W. Burdens of mercury in residents of Temirtau, KazakhstanII: Verification of methodologies for estimating human exposure to high levels of Hg pollution in the environment. Sci. Total Environ. 2010, 408, 4033–4044. [Google Scholar] [CrossRef]
- Tanton, T.W.; Ilyushchenko, M.A.; Heaven, S. Some water resources issues of Central Kazakhstan. Proc. Inst. Civ. Eng.—Water Marit. Eng. 2001, 148, 227–233. [Google Scholar] [CrossRef]
- EC (European Council). Directive 2000/60/EC of the European Parliament and the Council. L327; EC: Brussels, Belgium, 2000. [Google Scholar]
- EC (European Council). Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy; EC: Brussels, Belgium, 2008. [Google Scholar]
- Chan, H.M. Mercury in fish: Human health risks. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, NY, USA, 2011; pp. 697–704. [Google Scholar]
- Shao, D.; Liang, P.; Kang, Y.; Wang, H.-S.; Cheng, Z.; Wu, S.; Shi, J.; Lo, S.C.L.; Wang, W.; Wong, M.H. Mercury species of sediment and fish in freshwater fish ponds around the Pearl River Delta, PR China: Human health risk assessment. Chemosphere 2011, 83, 443–448. [Google Scholar] [CrossRef]
- Calvo, A.C.; Cutanda, F.; Esteban, M.; Pärt, P.; Navarro, C.; Gómez, S.; Rosado, M.; Lopez, A.M.R.; López, E.; Exley, K.; et al. Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environ. Res. 2015, 141, 58–68. [Google Scholar]
- Morel, F.M.M.; Kraepiel, A.M.L.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef] [Green Version]
- OSHA (Occupational Safety and Health Administration). 2020. Available online: https://www.osha.gov/SLTC/mercury/index.html (accessed on 14 April 2020).
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Mercury; ATSDR: Atlanta, GA, USA, 1999. [Google Scholar]
- Leal, L.T.C.; Guney, M.; Zagury, G.J. In vitro dermal bioaccessibility of selected metals in contaminated soil and mine tailings and human health risk characterization. Chemosphere 2018, 197, 42–49. [Google Scholar] [CrossRef]
- U.S. EPA (United States Environmental Protection Agency). Integrated Risk Information System (IRIS) Mercury, Elemental Quickview. 1995. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?&substance_nmbr=370 (accessed on 17 April 2020).
- U.S. EPA (United States Environmental Protection Agency). Integrated Risk Information System (IRIS) Mercuric Chloride (HgCl2) Quickview. 1995. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=692 (accessed on 17 April 2020).
- U.S. EPA (United States Environmental Protection Agency). Integrated Risk Information System (IRIS) Methylmercury (MeHg). 2001. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?&substance_nmbr=73 (accessed on 17 April 2020).
- Ministry of National Economy. Order of the Minister of National Economy of the Republic of Kazakhstan Dated February 28 2015. No. 168 “On Approval of Hygienic Standards for Atmospheric Air in Urban and Rural Settlements”; Ministry of National Economy of the Republic of Kazakhstan: Nur-Sultan, Kazakhstan, 2015. (In Russian) [Google Scholar]
- WHO (World Health Organization). Air Quality Guidelines for Europe; World Health Organization Regional Office for Europe: Denmark, Copenhagen, 2000. [Google Scholar]
- U.S. EPA (United States Environmental Protection Agency). 2018 Edition of the Drinking Water Standards and Health Advisories; U.S. EPA: Washington, DC, USA, 2018.
- FPT CDW (Federal-Provincial-Territorial Committee on Drinking Water). Guidelines for Canadian Drinking Water Quality—Summary Table; FPT CDW: Ottawa, ON, Canada, 2010. [Google Scholar]
- EC (European Council). Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption; EC: Brussels, Belgium, 1998. [Google Scholar]
- Ministry of National Economy. Order of the Minister of National Economy of the Republic of Kazakhstan dated March 16, 2015 No. 209 “On Approval of the Sanitary Rules ‘Sanitary and Epidemiological Requirements for Water Sources, Places for Water Intake for Household and Drinking Purposes, Domestic and Drinking Water Supply and Places for Cultural and Domestic Water Use and the Safety of Water Bodies’”; Ministry of National Economy of the Republic of Kazakhstan: Nur-Sultan, Kazakhstan, 2015. (In Russian)
- DTSC (California Department of Toxic Substances Control) and HERO (Human and Ecological Risk Office). HERO Human Health Risk Assessment (HHRA) Note 3, DTSC-Modified Screening Levels (DTSC-SLs). 2019. Available online: https://dtsc.ca.gov/wp-content/uploads/sites/31/2019/04/HHRA-Note-3-2019-04.pdf (accessed on 30 November 2020).
- CCME (Canadian Council of Ministers of the Environment). Canadian soil quality guidelines for the protection of environmental and human health: Mercury (inorganic) (1999). In Canadian Environmental Quality Guidelines, 1999; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Lame, F.; Maring, L. Into Dutch Soils; Ministry of Infrastructure and the Environment of The Netherlands: Brussels, Belgium, 2014.
- Ministry of National Economy. Order of the Minister of National Economy of the Republic of Kazakhstan dated June 25, 2015 No. 452 “On Approval of Hygienic Standards for Environmental Safety (Soil)”; Ministry of National Economy of the Republic of Kazakhstan: Nur-Sultan, Kazakhstan, 2015. (In Russian)
- Zillioux, E.J. Mercury in Fish: History, Sources, Pathways, Effects, and Indicator Usage. Environ. Indic. 2015, 743–766. [Google Scholar]
- Ministry of Health. Order of the Acting Minister of Health of the Republic of Kazakhstan dated August 6, 2010 No. 611 “On Approval of Sanitary Rules Sanitary and Epidemiological Requirements for Food Products”; Ministry of Health of the Republic of Kazakhstan: Nur-Sultan, Kazakhstan, 2010. (In Russian)
- Neculita, C.M.; Zagury, G.J.; Deschênes, L. Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J. Environ. Qual. 2005, 34, 255–262. [Google Scholar] [PubMed]
- Eckley, C.S.; Gilmour, C.C.; Janssen, S.; Luxton, T.P.; Randall, P.M.; Whalin, L.; Austin, C. The assessment and remediation of mercury contaminated sites: A review of current approaches. Sci. Total Environ. 2020, 707, 136031. [Google Scholar] [CrossRef]
- Guney, M.; Karatas, T.; Ozkul, C.; Akyol, N.H.; Acar, R.U. Contamination by As, Hg, and Sb in a region with geogenic As anomaly and subsequent human health risk characterization. Environ. Monit. Assess. 2019, 192, 50. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Ilyushchenko, M.A.; Kamberov, I.M.; Tanton, T.W. Mercury contamination in the vicinity of a derelict chlor-alkali plant. Part I: Sediment and water contamination of Lake Balkyldak and the River Irtysh. Sci. Total Environ. 2007, 381, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agriculture Organization of the United Nations). FAO/UNESCO Soil Map of the World: Central Asia. 1992. Available online: http://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/faounesco-soil-map-of-the-world/en/ (accessed on 20 April 2020).
- Sposito, G. Soil, Encyclopedia Britannica. 2020. Available online: https://www.britannica.com/science/soil/ (accessed on 5 May 2020).
- Heaven, S. Mercury in the River Nura and its floodplain, Central Kazakhstan: I. River sediments and water. Sci. Total Environ. 2000, 260, 35–44. [Google Scholar] [CrossRef]
- Heaven, S.; A Ilyushchenko, M.; Kamberov, I.M.; I Politikov, M.; Tanton, T.W.; Ullrich, S.M.; Yanin, E.P. Mercury in the River Nura and its floodplain, Central Kazakhstan: II. Floodplain soils and riverbank silt deposits. Sci. Total Environ. 2000, 260, 45–55. [Google Scholar] [CrossRef]
- Yanin, E.P. Mercury in the Epiphyte-Retained Suspension of the Nura River (Kazakhstan) as an Indicator of Technogenic Pollution. Geol. Geophys. 2000, 41, 1074–1077. (In Russian) [Google Scholar]
- Ullrich, S.M.; Ilyushchenko, M.A.; Uskov, G.A.; Tanton, T.W. Mercury distribution and transport in a contaminated river system in Kazakhstan and associated impacts on aquatic biota. Appl. Geochem. 2007, 22, 2706–2734. [Google Scholar] [CrossRef]
- Ilyushchenko, M.A.; Uskov, G.A.; Zyryanova, N.A. Mercury (Hg) contamination of fish fauna of Bylkyldak technical pond. KazNU Sci. J. Environ. Ser. 2002, 11, 102–105. (In Russian) [Google Scholar]
- Ilyushchenko, M.A.; Lapshin, E.V.; Delibare, A.; Tanton, T.W. Influence of Fly Ash of KarGRES-1 on decrease of risk posed by mercury pollution of the Nura River. Promyshlennost Kazakhstana 2005, 30, 60–63. [Google Scholar]
- Panin, M.S.; Geldymamedova, E.A. Ecological and geochemical characteristics of the soils of Pavlodar of the Republic of Kazakhstan. Vestn. TSU 2006, 292, 171–177. (In Russian) [Google Scholar]
- Ullrich, S.M.; Ilyushchenko, M.A.; Tanton, T.W.; Uskov, G.A. Mercury contamination in the vicinity of a derelict chlor-alkali plant. Part II: Contamination of the aquatic and terrestrial food chain and potential risks to the local population. Sci. Total Environ. 2007, 381, 290–306. [Google Scholar] [CrossRef]
- Shaimardanova, B.H.; Korogod, N.P.; Bigaliyev, A.B.; Assylbekova, G.E. Heavy Metals Accumulation in Children Hair. Novosibirsk State University Bulletin. Ser. Biol. Clin. Med. 2009, 8, 107–111. (In Russian) [Google Scholar]
- Ilyushchenko, M.A.; Daukeyev, G.B.; Tanton, T.W. Post-Containment Management And monitoring of Mercury Pollution in site of Former PO ‘Khimprom’ and Assessment of Environmental Risk Posed by Contamination of Groundwater and Adjacent Water Bodies of the Northern Industrial Area of Pavlodar; JSC Almaty University of Power Engineering and Telecommunications: Almaty, Kazakhstan, 2011; Available online: http://hg-pavlodar.narod.ru/ (accessed on 30 November 2020).
- Shakhova, T.S.; Talovskaya, A.V.; Yazikov, E.G.; Filimonenko, E.A.; Lyapina, E.E. Evaluation of mercury contamination in the vicinity of enterprises of the petrochemical complex in the winter period (based on the example of Pavlodar, Republic of Kazakhstan). Bulletin of the Tomsk Polytechnic University. Geo Assets Eng. 2016, 327, 16–25. (In Russian) [Google Scholar]
- Randall, P.; Ilyushchenko, M.A.; Lapshin, E.; Kuzmenko, L. Case study: Mercury pollution near a chemical plant in Northern Kazakhstan. Air Waste Manag. Assoc. 2005, 2, 19–24. [Google Scholar]
- Ilyushchenko, M.A.; Abdrashitova, S.A.; Tanton, T.W.; Heaven, S.; Yanin, E.P. Results of research into mercury pollution of the river Nura in Central Kazakhstan and proposals for demercurisation. In Materials of the Second Congress in memory of B.A.Beremzhanov in Chemistry and Chemical Technologies; Chemistry Series; Vestnik KazGU: Almaty, Kazakhstan, 1999; pp. 18–21. [Google Scholar]
- Yanin, E.P. Mercury in industrial river sludges: Features of redistribution, location, geochemical mobility. In Proceedings of the Second International Symposium “Mercury in the Biosphere: Ecological and Geochemical Aspects”, Novosibirsk, Russia, 21–25 September 2015; pp. 401–406. (In Russian). [Google Scholar]
- Hintelmann, H.; Hempel, M.; Wilken, R.D. Observation of Unusual Organic Mercury Species in Soils and Sediments of Industrially Contaminated Sites. Environ. Sci. Technol. 1995, 29, 1845–1850. [Google Scholar] [CrossRef]
- Kruger, O.; Ebinghaus, R.; Kock, H.H.; Richter-Politz, I.; Geilhufe, C. Estimation of gaseous mercury emissions in Germany: Inverse modelling of source strengths at the contaminated industrial site BSL Werk Schkopau. In Mercury Contaminated Sites. Environmental Science; Ebinghaus, R., Turner, R.R., de Lacerda, L.D., Vasiliev, O., Salomons, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Hasche, R.L.; Boundy, R.H. Report on Manufacture of Acetaldehyde at I.G. Farbenindustrie; U.S. Technical Intelligence Committee: Schkopau, Germany, 1945. [Google Scholar]
- Tomiyasu, T.; Matsuyama, A.; Eguchi, T.; Marumoto, K.; Ōki, K.; Akagi, H. Speciation of mercury in water at the bottom of Minamata Bay, Japan. Mar. Chem. 2008, 112, 102–106. [Google Scholar] [CrossRef]
- Nakata, H.; Shimada, H.; Yoshimoto, M.; Narumi, R.; Akimoto, K.; Yamashita, T.; Matsunaga, T.; Nishimura, K.; Tanaka, M.; Hiraki, K.; et al. Concentrations and Distribution of Mercury and Other Heavy Metals in Surface Sediments of the Yatsushiro Sea including Minamata Bay, Japan. Bull. Environ. Contam. Toxicol. 2007, 80, 78–84. [Google Scholar] [CrossRef]
- Matsuyama, A.; Eguchi, T.; Sonoda, I.; Tada, A.; Yano, S.; Tai, A.; Marumoto, K.; Tomiyasu, T.; Akagi, H. Mercury Speciation in the Water of Minamata Bay, Japan. Water, Air Soil Pollut. 2010, 218, 399–412. [Google Scholar] [CrossRef]
- Yasuda, Y.; Matsuyama, A.; Yasutake, A.; Yamaguchi, M.; Aramaki, R.; Xiaojie, L.; Pin, J.; Yumin, A.; Li, L.; Mei, L.; et al. Mercury distribution in farmlands downstream from an acetaldehyde producing chemical company in Qingzhen City, Guizhou, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2004, 72, 445–451. [Google Scholar] [CrossRef]
- Horvat, M.; Nolde, N.; Fajon, V.; Jereb, V.; Logar, M.; Lojen, S.; Jaćimović, R.; Falnoga, I.; Liya, Q.; Faganeli, J.; et al. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci. Total Environ. 2003, 304, 231–256. [Google Scholar] [CrossRef]
- Matsuyama, A.; Yasuda, Y.; Yasutake, A.; Xiaojie, L.; Pin, J.; Li, L.; Mei, L.; Yumin, A.; Liya, Q. Detailed Pollution Map of an Area Highly Contaminated by Mercury Containing Wastewater from an Organic Chemical Factory in People’s Republic of China. Bull. Environ. Contam. Toxicol. 2006, 77, 82–87. [Google Scholar] [CrossRef]
- Rudd, J.W.; Bodaly, R.; Fisher, N.S.; Kelly, C.A.; Kopec, A.D.; Whipple, C. Fifty years after its discharge, methylation of legacy mercury trapped in the Penobscot Estuary sustains high mercury in biota. Sci. Total Environ. 2018, 642, 1340–1352. [Google Scholar] [CrossRef]
- Turner, R.; Kopec, A.; Charette, M.; Henderson, P. Current and historical rates of input of mercury to the Penobscot River, Maine, from a chlor-alkali plant. Sci. Total Environ. 2018, 637-638, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kopec, A.D.; Kidd, K.A.; Fisher, N.S.; Bowen, M.; Francis, C.; Payne, K.; Bodaly, R. Spatial and temporal trends of mercury in the aquatic food web of the lower Penobscot River, Maine, USA, affected by a chlor-alkali plant. Sci. Total Environ. 2019, 649, 770–791. [Google Scholar] [CrossRef]
- Esbrí, J.M.; López-Berdonces, M.A.; Fernández-Calderón, S.; Higueras, P.; Díez, S. Atmospheric mercury pollution around a chlor-alkali plant in Flix (NE Spain): An integrated analysis. Environ. Sci. Pollut. Res. 2015, 22, 4842–4850. [Google Scholar] [CrossRef]
- Fernández-Martínez, R.; Esbrí, J.M.; Higueras, P.; Rucandio, M.I. Comparison of mercury distribution and mobility in soils affected by anthropogenic pollution around chloralkali plants and ancient mining sites. Sci. Total Environ. 2019, 671, 1066–1076. [Google Scholar] [CrossRef]
- Palanques, A.; Grimalt, J.O.; Belzunces, M.; Estrada, F.; Puig, P.; Guillén, J. Massive accumulation of highly polluted sedimentary deposits by river damming. Sci. Total Environ. 2014, 497–498, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.X.; Roig, R.; Gacia, E.; Catalan, J. Differential accumulation of mercury and other trace metals in the food web components of a reservoir impacted by a chlor-alkali plant (Flix, Ebro River, Spain): Implications for biomonitoring. Environ. Pollut. 2011, 159, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Inácio, M.; Pereira, V.; Pinto, M. Mercury contamination in sandy soils surrounding an industrial emission source (Estarreja, Portugal). Geoderma 1998, 85, 325–339. [Google Scholar] [CrossRef]
- Reis, A.T.; Rodrigues, S.M.; Araújo, C.; Coelho, J.P.; Pereira, E.; Duarte, A.C. Mercury contamination in the vicinity of a chlor-alkali plant and potential risks to local population. Sci. Total Environ. 2009, 407, 2689–2700. [Google Scholar] [CrossRef]
- Ramalhosa, E.; Pato, P.; Monterroso, P.; Pereira, E.; Vale, C.; Duarte, A.C. Accumulation versus remobilization of mercury in sediments of a contaminated lagoon. Mar. Pollut. Bull. 2006, 52, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Chmura, G.L. An inventory of historical mercury emissions in Maritime Canada: Implications for present and future contamination. Sci. Total Environ. 2000, 256, 39–57. [Google Scholar] [CrossRef]
- Garron, C.; Gagné, F.; Ernst, W.; Julien, G.; Bernier, M.; Caldwell, C. Mercury Contamination of Marine Sediments and Blue Mussels (Mytilus edulis) in the Vicinity of a Mercury Cell Chlor-Alkali Plant in Dalhousie, New Brunswick, Canada. Water Qual. Res. J. 2005, 40, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.R. Mercury concentrations in marine sediments near a former mercury cell chlor-alkali plant in eastern Canada. Mar. Pollut. Bull. 2016, 107, 398–401. [Google Scholar] [CrossRef]
- Sensen, M.; Richardson, D.H.S. Mercury levels in lichens from different host trees around a chlor-alkali plant in New Brunswick, Canada. Sci. Total Environ. 2002, 293, 31–45. [Google Scholar] [CrossRef]
- Bravo, A.G.; Loizeau, J.-L.; Ancey, L.; Ungureanu, V.G.; Dominik, J. Historical record of mercury contamination in sediments from the Babeni Reservoir in the Olt River, Romania. Environ. Sci. Pollut. Res. 2008, 16, S66–S75. [Google Scholar] [CrossRef]
- Bravo, A.G.; Cosio, C.; Amouroux, D.; Zopfi, J.; Chevalley, P.-A.; Spangenberg, J.E.; Ungureanu, V.-G.; Dominik, J. Extremely elevated methyl mercury levels in water, sediment and organisms in a Romanian reservoir affected by release of mercury from a chlor-alkali plant. Water Res. 2014, 49, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.B. Disposition of toxic metals in the agricultural food chain. 2. Steady-state bovine tissue biotransfer factors. Environ. Sci. Technol. 1992, 26, 1915–1921. [Google Scholar] [CrossRef]
- Song, Z.; Li, P.; Ding, L.; Li, Z.; Zhu, W.; He, T.; Feng, X. Environmental mercury pollution by an abandoned chlor-alkali plant in Southwest China. J. Geochem. Explor. 2018, 194, 81–87. [Google Scholar] [CrossRef]
- Bolaños-Alvarez, Y.; Alonso-Hernandez, C.M.; Morabito, R.; Díaz-Asencio, M.; Pinto, V.; Gómez-Batista, M. Mercury contamination of riverine sediments in the vicinity of a mercury cell chlor-alkali plant in Sagua River, Cuba. Chemosphere 2016, 152, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Pedrero, Z.; Lima, L.; Olivares, S.; De La Rosa, D.; Berail, S.; Tessier, E.; Pannier, F.; Amouroux, D. Assessment of Hg contamination by a Chlor-Alkali Plant in riverine and coastal sites combining Hg speciation and isotopic signature (Sagua la Grande River, Cuba). J. Hazard. Mater. 2019, 371, 558–565. [Google Scholar] [CrossRef]
- Perrot, V.; Epov, V.N.; Pastukhov, M.V.; Grebenshchikova, V.I.; Zouiten, C.; Sonke, J.E.; Husted, S.; Donard, O.F.X.; Amouroux, D. Tracing Sources and Bioaccumulation of Mercury in Fish of Lake Baikal—Angara River Using Hg Isotopic Composition. Environ. Sci. Technol. 2010, 44, 8030–8037. [Google Scholar] [CrossRef]
- Navrátil, T.; Šimeček, M.; Shanley, J.B.; Rohovec, J.; Hojdová, M.; Houška, J. The history of mercury pollution near the Spolana chlor-alkali plant (Neratovice, Czech Republic) as recorded by Scots pine tree rings and other bioindicators. Sci. Total Environ. 2017, 586, 1182–1192. [Google Scholar] [CrossRef]
- Covelli, S.; Emili, A.; Acquavita, A.; Koron, N.; Faganeli, J. Benthic biogeochemical cycling of mercury in two contaminated northern Adriatic coastal lagoons. Cont. Shelf Res. 2011, 31, 1777–1789. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediation of mercury: An essence review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Duan, P.; Khan, S.; Ali, N.; Shereen, M.A.; Siddique, R.; Ali, B.; Iqbal, H.M.; Nabi, G.; Sajjad, W.; Bilal, M. Biotransformation fate and sustainable mitigation of a potentially toxic element of mercury from environmental matrices. Arab. J. Chem. 2020, 13, 6949–6965. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Kumar, A.; Maiti, S.K. Mercury remediation potential of Brassica juncea (L.) Czern. for clean-up of flyash contaminated sites. Chemosphere 2020, 248, 125857. [Google Scholar] [CrossRef] [PubMed]
- Bestetti, G.; Gandolfi, I.; Franzetti, A. Microbiological Mercury Removal from Contaminated Materials. WO2009125341A2, 15 October 2009. [Google Scholar]
- Lestan, D.; Finzgar, N.; Gerl, M.; Gluhar, S.; Lakovic, G.; Hamiti, B. Soil and Sedement Remediation. CA2942367A1, 19 September 2016. [Google Scholar]
- Alden, D.F.; Birk, G.M.; Bang, S.; Harwell, J.H.; Shiau, B.J. Method and a Chemical Composition for Accelerated In-Situ Biochemical Remediation. U.S. Patent Application 20200261954A1, 20 August 2020. [Google Scholar]
- Nick, L.; Liangdong, W.; Li, L. Stabilisation Curing Agent and Administering Method that Heavy-Metal Contaminated Soil or Solid Waste are Administered. CN104312591B, 13 October 2014. [Google Scholar]
- Xia, M.; Chen, Z.; Li, Y.; Li, C.; Ahmad, N.M.; Cheema, W.A.; Zhu, S. Removal of Hg(ii) in aqueous solutions through physical and chemical adsorption principles. RSC Adv. 2019, 9, 20941–20953. [Google Scholar] [CrossRef] [Green Version]
- Mahbub, K.R.; Bahar, M.; Labbate, M.; Krishnan, K.; Andrews, S.; Naidu, R.; Megharaj, M.; Bahar, M.; Krishnan, K. Bioremediation of mercury: Not properly exploited in contaminated soils! Appl. Microbiol. Biotechnol. 2017, 101, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grujić, S.; Vasić, S.; Radojević, I.; Čomić, L.; Ostojić, A. Comparison of the Rhodotorula mucilaginosa Biofilm and Planktonic Culture on Heavy Metal Susceptibility and Removal Potential. Water Air Soil Pollut. 2017, 228, 73. [Google Scholar] [CrossRef]
- Looney, B.B.; Denham, M.E.; Vangelas, K.M.; Bloom, N.S. Removal of Mercury from Low-Concentration Aqueous Streams Using Chemical Reduction and Air Stripping. J. Environ. Eng. 2003, 129, 819–825. [Google Scholar] [CrossRef]
- He, F.; Gao, J.; Pierce, E.; Strong, P.J.; Wang, H.; Liang, L. In situ remediation technologies for mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 8124–8147. [Google Scholar] [CrossRef]
- Richard, J.-H.; Biester, H. Mercury removal from contaminated groundwater: Performance and limitations of amalgamation through brass shavings. Water Res. 2016, 99, 272–280. [Google Scholar] [CrossRef]
- Huttenloch, P.; Roehl, K.E.; Czurda, K. Use of Copper Shavings to Remove Mercury from Contaminated Groundwater or Wastewater by Amalgamation. Environ. Sci. Technol. 2003, 37, 4269–4273. [Google Scholar] [CrossRef]
- Subirés-Muñoz, J.; García-Rubio, A.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Garcia-Herruzo, F.; Paz-García, J.M. Feasibility study of the use of different extractant agents in the remediation of a mercury contaminated soil from Almaden. Sep. Purif. Technol. 2011, 79, 151–156. [Google Scholar] [CrossRef]
- Randall, P.M.; Chattopadhyay, S. Mercury contaminated sediment sites—An evaluation of remedial options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef] [Green Version]
- Park, C.M.; Katz, L.E.; Liljestrand, H.M. Mercury speciation during in situ thermal desorption in soil. J. Hazard. Mater. 2015, 300, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2017, 15, 1123–1138. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Brassica juncea (L.) Czern. (Indian mustard): A putative plant species to facilitate the phytoremediation of mercury contaminated soils. Int. J. Phytoremed. 2020, 22, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Kraus, T.E.; Fleck, J.A.; Krabbenhoft, D.P.; Horwath, W.R.; Bachand, S.M.; Herzog, M.P.; Hartman, C.A.; Bachand, P.A.M. Experimental Dosing of Wetlands with Coagulants Removes Mercury from Surface Water and Decreases Mercury Bioaccumulation in Fish. Environ. Sci. Technol. 2015, 49, 6304–6311. [Google Scholar] [CrossRef]
- Rumayor, M.; Lopez-Anton, M.A.; Somoano, M.D.; Maroto-Valer, M.; Richard, J.-H.; Biester, H.; Martinez-Tarazona, M.; Maroto-Valer, M.M. A comparison of devices using thermal desorption for mercury speciation in solids. Talanta 2016, 150, 272–277. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Siripornadulsil, S.; Sayre, R.T.; Traina, S.J.; Weavers, L.K. Removal of mercury from sediment by ultrasound combined with biomass (transgenic Chlamydomonas reinhardtii). Chemosphere 2011, 83, 1249–1254. [Google Scholar] [CrossRef]
- Akimat of Pavlodar City. Report on the Implementation of the Development Program of the Pavlodar City Territory for 2016–2020. 2018. Available online: https://www.akimat-pvl.gov.kz/post.php?id=2427 (accessed on 11 May 2020).
- World Bank. Nura River Clean-Up Project. The World Bank Group. 2010. Available online: https://projects.vsemirnyjbank.org/ru/projects-operations/project-detail/P059803?lang=ru (accessed on 12 May 2020).
- Dushkina, Y.N.; Urazgalieva, A.A.; Mustafina, V.V. Mercury contamination in the Republic of Kazakhstan: Current situation and measures for its minimization. Chem. Saf. 2018, 2, 237–243. (In Russian) [Google Scholar] [CrossRef]



| Medium | World Health Organization | USA | Canada | E.U. | Kazakhstan |
|---|---|---|---|---|---|
| In air within working zones (μg/m3) | not available (n.a.) | 100 (8 h and ceiling) [29] | (n.a.) | elemental and inorganic, Sweden: 30 (8 h) organic: 10 (8 h) [5] | 5 [35] |
| In ambient air of populated areas (μg/m3) | 1 (annual) [36] | (n.a.) | (n.a.) | (n.a.) | 0.3 [35] |
| In water for sanitary and domestic use (μg/L) | 1 [14] | 2 (maximum contaminant level) [37] | 1 [38] | drinking water: 1 (parametric value) [39] surface waters: 0.07 (maximum contaminant level) [39] | 0.5 [40] |
| In soil for agricultural use and in residential places (mg/kg of soil) | (n.a.) | California: 1 [41] | 6.6 [42] | Netherlands: 0.83 [43] | 2.1 [44] |
| In soil of other areas (mg/kg of soil) | (n.a.) | industrial, California: 4.4 [41] | industrial: 50 [42] | industrial, Netherlands: 4.8 [43] | 10 [44] |
| In biota (mg/kg wet weight) | fish: 0.5 (MeHg) predatory fish: 1 (MeHg) [45] | 1 (MeHg) [45] | all fish except shark, swordfish and tuna: 0.5 [45] | fish: 0.5 predatory fish: 1 [45] | fish: 0.3 predatory fish: 0.6 [46] |
| Study | Title | Objective | Sampling | Analysis | Main Findings | Conclusions | Recommendations |
|---|---|---|---|---|---|---|---|
| 2000a Heaven et al. [54] | Mercury in the River Nura and its floodplain, Central Kazakhstan: I. River sediments and water | Establish the location, extent, and nature of the contaminated sediments and evaluate the potential for sediment transport in the Nura river | River sediments (n = 156); water (n = n/a); Surveying of the riverbed and backwaters with sediments thickness | Sediments: acid digestion + CV-AAS; Water (on site): Au pre-concentration and SnCl2 reduction + portable AAS | Sediments: very high concentrations in first 15 km downstream, average 150–240 mg/kg; Estimated total silts between Temirtau and Intumak Reservoir (75 km) 463,500 m3 or 9.4 t Hg; A major part of polluted sediment not transported far downstream (except for floods) Hg in backwaters reflect changes of Hg in riverbed silts, in first 5–15 km average 86 mg/kg, falling to 2.6 mg/kg near Intumak Water: mean <1 μg/L (EU limit for inland surface waters & WHO (1984) in drinking water); but > 0.3 μg/L local limit for fish-inhabited waters; No general relationship between amount of suspended particulate matter (SPM) and total, dissolved or suspended Hg | Results were lower than expected; most of the contaminated silts do not appear to be readily transported for long distances downstream (also confirmed by hydraulic modeling work (not presented)); Hg in the water leaving the reservoir would suggest that more than 100–200 kg Hg/year could be moving downstream (mainly dissolved form) | The desired option for reclamation is to dredge silts from the outfall canal and remove highly polluted sediments from its banks; dredge technogenic silts from the first 25–30 km of riverbed below Temirtau and limit further distribution; remove silts with >10 mg/kg Hg deposited on banks Management of discharge: to reduce flood size and prevent disturbance of sediment |
| 2000b Heaven et al. [55] | Mercury in the River Nura and its floodplain, Central Kazakhstan: II. Floodplain soils and riverbank silt deposits | A detailed survey of the floodplain to investigate the extent of pollution and to assess the need for remediation | A survey covering 160 km2 of the floodplain of River Nura (72 lakes in total); Topsoil samples 0–15 cm (n = 1100); Silts at highly-contaminated Zhaur Swamp (n = 157 from 28 boreholes); Additional soil samples from irrigated areas in 1998 (n = 10) | Acid digestion + AAS Silts—preliminary sequential extraction tests: 0.1 M HCl | Topsoils (53 t Hg): from 0.01 to >100 mg/kg, Hg > 21 mg/kg (tenfold local limit) mainly in first 25 km of river, > 10 mg/kg (Dutch intervention value) up to 60 km downstream, River bank deposits/silts (65 t Hg): mean 73.3 mg/kg in most contaminated section; mean 13.4 mg/kg 70 km downstream Zhaur swamp formerly used as waste disposal area (62 t Hg): up to 1974 mg/kg at the surface; fall rapidly with increasing depth, mean total Hg 306.7 mg/kg in upper 20 cm In older silts w/total Hg 10.2–10.7 mg/kg: highly mobile 71.6–87.9%, rel. mobile (oxides) 2.1–3.5%, insoluble 7.2–24.6%; In sediment from riverbed below Intumak with total 0.017 mg/kg: highly mobile 15.5%, relatively mobile 10%, insoluble 74.5% | The contamination is severe but relatively localized, with >70% of mercury in topsoils and >90% of mercury in riverbank deposits located within 25 km from the source. | Removal of the silt deposits from banks in the first 30 km below outfall would remove >90% of Hg; isolate Hg by stockpiling silts under a meter of inert cover material in a location safe from groundwater intrusion and flooding; cease cultivation of Zhaur Swamp; remove and isolate upper 40 cm of soil; soils with > 10 mg/kg should be taken out of agricultural production; minimize flooding of contaminated areas of the Nura valley by regulating the discharge from Samarkand Reservoir |
| 2000 Yanin [56] | Mercury in the epiphyte retained of the Nura River (Kazakhstan) as an indicator of technogenic pollution. | To evaluate the effectiveness of epiphytic suspension in assessing the level and scale of waterbodies pollution by mercury | Epiphytic suspension from Myrio phyllum specatum L. (dried and separated) Technogenic silts (dried and sieved) | AAS (IMGRE-900 mercury analyzer) | Maximum total Hg concentrations in technogenic silts near wastewater discharge (about 6–10 km); 0.05 km from outfall mean total Hg in silts 33.54 mg/kg, 10 km away mean Hg is maximum 47.62 mg/kg; Hg in technogenic silts > Hg in epipihtytes; epyphytes can be used as an indicator | Epiphyte suspension (which intensively concentrates Hg) reflects the influence of various Hg sources to watercourses and shows the extent of pollution. Hg in technogenic silts is mostly deposited in the vicinity of the wastewater discharge | It is proposed to use epiphytic suspension, i.e., suspension precipitated on macrophytes, to estimate the level and scale of the technogenic pollution of Hg’s rivers. |
| 2007b Ullrich et al. [57] | Mercury distribution and transport in a contaminated river system in Kazakhstan and associated impacts on aquatic biota | To investigate the transport, fate, and bioavailability of Hg in the Nura river system by analyzing sediments, water, plants, and fish sampled from the river system | Sediments from different years, locations, and depths along the river; water, unfiltered and filtered (0.45 μm); plants (cattail and reed); fish from the river, lakes, local market (n = 130, 20, 6) | Sediments: acid digestion + CV-AAS (Perkin-Elmer AAnalyst 100), acid digestion + CV-AFS, MeHg—modified Westöö procedure, GC-ECD; water: total Hg and suspended solids—BrCl, SnCl2 reduction + CV-AFS (Millennium Merlin); plants: acid digestion + CV-AAS (), CV-AFS; fish: acid digestion + CV-AFS | Sediments within 20 km downstream of effluent—highly polluted, a strong source of water contamination; THg in most contaminated section = 9.95 to 306 mg/kg; Highest MeHg in surface sediments (4.9–39 ug/kg) <0.1% THg; the significant inverse relationship between THg and MeHg% formed in sediments Unfiltered surface water during flood peak THg = 1600–4300 ng/L Background concentrations of Hg in surface water are not reached for 200 km downstream, even in wetlands during flood In aquatic plants Hg in most contaminated section = 15–20x background; fish impacted for >125 km downstream from the source—significant transport of dissolved MeHg to downstream areas, in situ MeHg production | Elevated Hg concentrations in water, fish, and aquatic plants near impoundments appear to indicate that Hg’s availability for methylation may be increased in these areas. The high immobilization of mercury by industrial sludge, the basis of which was the ash of a thermal power station, makes debatable the rationale for cleaning up the mercury-containing bottom sediments of the Nura River under the project of the International Bank for Reconstruction and Development. | Studies on terminal wetlands of the Nura, methylation capacity at Intumak and Samarkand barrage Prevent further transport of Hg to downstream reaches |
| 2010a Hsiao et al. [3] | Burdens of mercury in residents of Temirtau, Kazakhstan I: Hair mercury concentrations and factors of elevated hair mercury levels | To evaluate Hg exposure levels through concentrations in hair of the local population; to describe the relationship between Hg concentrations in hair and dietary intake and other factors; to identify group at high risk of Hg exposure | Hair from Temirtau and Almaty (n = 289 and 13), fish purchased or caught locally (n = 111), food (veg, milk, beef) (n = 24) | Hair: Rigaku Mercury Analyzer SP-3 or MA-2; fish, food: acid digestion + CV-AFS (Millennium Merlin) | Hg in hair = 0.009−5.184 μg/g, mean 0.577 μg/g; in ~17% of population >1 μg/g A positive correlation between Hg in hair and frequencies of river fish consumption Subgroups of males, people >45 y.o and fishermen or anglers—elevated levels | The mean concentration of Hg in the river fish being 0.43 μg/g and an average bodyweight of 67 kg of the local people; Hg in hair at a moderate level, exposure levels not very severe | Raise awareness of the dangers of consuming fish caught in River Nura and its oxbow lakes below Temirtau, or at least decrease consumption rate to no more than once a week, especially for pregnant women |
| 2010b Hsiao et al. [21] | Burdens of mercury in residents of Temirtau, Kazakhstan. II: Verification of methodologies for estimating human exposure to high levels of Hg pollution in the environment | To evaluate the exposure risk posed by Hg waste from a disused acetaldehyde plant at Temirtau, to identify the adaptability of these approaches, and discuss the uncertainty and variability generating in the methodologies of exposure assessments | Fish (n = 21), food (vegetables, milk, beef) (n = 24), soils (n = 27), loose dust (n = 38), hair (n = 289); questionnaire (n = 232) | Fish, food: acid digestion + CV-AFS (Millennium Merlin), soil and dust: acid digestion + CV-AAS (Perkin-Elmer AAnalyst 100), hair: Rigaku Mercury Analyzer SP-3 or MA-2; HQ = Average daily intake/RfD | Probabilistic (Monte-Carlo): ADD of MeHg mean 0.08 (0.003–12.233) μg/kg body weight/day—75% of MeHg intake via fish; 19% population exceeded 0.1 ug/kg BW/day Non-carcinogenic risk due to MeHg contamination: HI = 0.36 at 50 percentile, but HI = 2.53 at 95 percentile Deterministic: HI = sum of HQ = 7.92, where HQ = 7.62 from fish consumption | The probabilistic approach (MC simulation) is slightly overestimated, but the stable and reliable prediction for the high-end exposed population, while the deterministic approach overestimated ADD 1.5 times than values derived from hair. Fish and shellfish consumption—major route of MeHg exposure | Probabilistic approach robust, useful, and reliable in assessing accurate levels of exposure to Hg |
| 2002 Ilyushchenko et al. [58] | Mercury (Hg) contamination of fish fauna of Balkyldak technical pond | To investigate the extent of mercury contamination in the fish fauna of the Balkyldak lake | Fish from Balkyldak (n = 55): tench, common perch, silver crucian carp, Siberian dace | Acid, bromide-bromate digestion + CV-AFS (PSA 10.025 Millennium-Merlin) | In 50 out of 55 Hg in muscle tissue > 0.3 mg/kg (max allowable concentration in dace/crucian carp/tench) Average total Hg = 4.36/3.18/1.98 | Limited sample size does not allow to draw exact conclusions | Further research of Hg accumulation in other aquatic organisms and Hg migration along the food chains of the ecosystem of Balkyldak (hydrobiological and trophological research methods) |
| 2004 Woodruff and Dack [19] | Analysis of risk from mercury contamination at the Khimprom Plant in Kazakhstan | To examine mercury contamination at the chlor-alkali plant at Pavlodar and to establish whether risks to human health exist from this contamination via vegetable consumption and soil ingestion | Surface soils from site and Pavlodarskoye village, groundwater (n = unknown) | Risk assessment (ingestion of food and soil): (1) UK Contaminated Land Exposure Assessment (CLEA) model; (2) The Netherlands, Van Hall Institute Risc-Human Model Version 3.0 | Hg in soil from plant far and close to contaminated zones (1997-8 and 2001-2) = 0.0067 and 835.9 mg/kg respectively; Hg in soil from Pavlodarskoye village (2001-2) = 1.5 mg/kg; Hg in groundwater from plant (1997-8 and 2001-2) = 0.00022 and 18 mg/L, respectively; Hg in groundwater from Pavlodarskoye village (2001-2) = 0.005 mg/L; CLEA model—vegetable uptake and ingestion of soil on vegetables; risk not related to increased Hg concentrations in the latter years Risc-Human model—ingestion of soil by children, ingestion of meat and vegetables children and adults | Possible health risks to the local population at Pavlodarskoye from the consumption of homegrown vegetable uptake and with ingestion of soil attached to homegrown vegetables | To obtain a more representative value of calculated risk, factoring in these differences recommended that vegetable uptake be studied further |
| 2005 Ilyushchenko et al. [59] | Activities for prevention of the threat of river Irtysh mercury pollution in Pavlodar, Kazakhstan | Report on the results and strategies for preventing pollution | - | Four large hotspots with Hg in soil = >500 × 2.1 mg/kg 2931 kg of Hg for the industrial site No.1, 16,022 kg of Hg for the area between the industrial site of former PO “Khimprom” and lake Balkyldak; In the tissue of fish from the lake 0.18-2.2 mg/kg The plume of Hg-contaminated groundwater: up to 150 ug/L, decreasing with distance from hotspots In surface water: (i) atmospheric precipitations in lagoons ≤50 mg/L; (ii) surface water to the south from lagoons 3–30 ug/L; (iii) surface water in a ditch along lake 2–18 ug/L; (iv) surface water of lake 3.4 ug/L (near lagoons) to 0.1–0.3 ug/L (along the rest of the shore) In unfinished emergency canal from the lake to west ≤0.01 ug/L; in Irtysh river <0.002 μg/L; in oxbow lakes near village <0.009 ug/L; in predatory fish from river 0.075–0.16 mg/kg | Four scenarios of Hg transport with groundwater until 2030: (1) direction of plume does not change—no severe threat to village and river; a limited amount of Hg might enter the emergency canal; (2) cut-off wall around building 31; (3) containment of both sources of pollution—eliminate groundwater contamination; (4) changes of hydrogeological conditions in the northern industrial area of Pavlodar depending on industrial development or degradation | Instead of expensive and ineffective recovery of Hg from highly contaminated wastes, the containment strategy was proposed assuming isolation of major hotspots from the atmosphere, surface run-off, and groundwater. A cut-off wall built-in 2003–2005 around four major hotspots; topsoil to 0.5 m excavated and removed to isolated sites; building 31 demolished; monolith storage | |
| 2006 Panin and Geldymamedova [60] | Ecological and geochemical characteristics of soils in Pavlodar, Republic of Kazakhstan | To identify the presence of heavy metals and other chemical elements in soils of Pavlodar city | Soil from Pavlodar and surrounding areas (n = 609) | Acid digestion + AAS (Perkin Elmer 403 + HGA-74) | Hg in the city was in the range of 0.08–18.96 mg/kg Mean total Hg in northern industrial zone 3.51 mg/kg, in the north part of the city 0.21 mg/kg Mean concentration of elements 1.6–22.5 times higher than background (especially Hg, Cd, Co, Mo) Northern industrial zone: max Hg, V, Sr, Ni compared to other areas Zc > 128 (ecological disaster area) on the territory of chemical plant | Maps of the distribution of chemical elements in the soils are compiled. The highest concentrations of chemical elements in the northern industrial zone | |
| 2007a Ullrich et al. [50] | Mercury contamination in the vicinity of a derelict chlor-alkali plant. Part I: Sediment and water contamination of Lake Balkyldak and the River Irtysh | To investigate the impact of Hg emissions from the chlor-alkali plant on the surrounding environment and, in particular, the lake (sediments, water, and biota) | Sediments (n = 55) and water from Balkyldak (n = 38); sediments and water from Irtysh (n = 32), water from oxbow lakes (n = 18); soil from 6 locations around lake | Acid digestion + CV-AAS, CV-AFS | Hg in sediments in the lake, near wastewater outfall pipe up to 1500 mg/kg; Hg in lake water in the range of 0.11–1.39 ug/L (mainly in the southern part); on windy days, concentration up to 7.3 ug/L; Hg in river sediments up to 0.046 mg/kg in the old river channel & up to 0.36 mg/kg in floodplain oxbow lakes; Hg in river water—not detected, in oxbow lakes—trace (3-9 ng/L) Hg in soil around lake—2.65 mg kg−1 (0.22–5.72 mg kg−1) at 0–10 cm depth, 1.81 mg kg−1 at 10–20 cm, and 1.14 mg kg−1 at 20–50 cm | Balkyldak sediments are heavily contaminated. Thus, the lake poses a threat and needs remediation; Hg does not significantly impact the Irtysh river A cut-off wall around lagoons and clay cover eliminated a major source of Hg to the lake. However, the lake still receives Hg via an old outfall pipe | Recommendations: ex-situ dredging or disposal; thermal desorption; capping and dredging Preliminary tests carried out on a sediment sample taken from the south of Lake Balkyldak indicated that most Hg was present as elemental Hg, and >95% of the Hg could be removed by ultrasound (unpublished data). |
| 2007c Ullrich et al. [61] | Mercury contamination in the vicinity of a derelict chlor-alkali plant Part II: Contamination of the aquatic and terrestrial food chain and potential risks to the local population | To gain a preliminary insight into the potential for contamination of the terrestrial food chain and the associatedlevel of risk. | Water from lake Balkyldak (n = 55), from Irtysh and oxbow lakes (n = 30), water from wells (n = 30); Cow milk from village (n = 15), liver and kidney from 1 cow; soil (n = 24) | Water: acid digestion + CV-AFS | Fish from Balkyldak seriously contaminated by Hg (dace>carp>tench) Mean (range) Hg in perch from Balkyldak 0.89 (0.16–2.20 mg/kg) >>> in perch from Irtysh 0.112 (0.075–0.125 mg/kg) (limited sample size) Hg in 91% of fish exceed the permissible level Mean (range) Hg in soil = 1.04 (0.10 and 3.30) mg/kg Hg in groundwater < LoD (5 ng/L) Hg in bovine milk samples < 2 ug/kg, tissue = 10.96 ug/kg | Hg in fish from lake Balkyldak exceed current human health limits; so, consumption of contaminated fish appears to be the main route of exposure for humans | Eliminate the current fish population by using rotenone (fish poison) Environmental and human health impacts associated with cattle grazing on contaminated land around the plant and drinking contaminated surface waters To investigate Hg uptake from vegetables grown in contaminated soil |
| 2009 Shaimardanova et al. [62] | Heavy Metals Accumulation in Children Hair | To justify the accumulation rate of chemicals (Hg, Zn, Se, Rb) in children’s hair living in Pavlodar as a method of an environmental assessment of the quality of urban ecosystem under conditions of long technogenic impact | Children hair 12–14 y.o. (n = 100) | Instrumental neutron activation analysis (INAA) | Highest Hg accumulation in W, SW, NW districts = 0.5–0.7 mg/kg due to proximity to the industrial zone Uneven distribution of toxic elements in human biosubstrates | High Hg and Zn content due to high mobility in “soil–snow– plants–biosubstrates (hair)” system Two groups of main exposure sources: coal energy and metal industry (Hg, Zn); chemical (Hg, Se, Rb) and petrochemicals (Zn, Se); Most contaminated districts: NW, W, SW | |
| 2011 Ilyuchshenko et al. [63] | Final technical report | (1) Risk assessment on the flow direction of groundwater polluted with oil products and Hg, including its passage through sampling wells in Pavlodarskoye village, joining River Irtysh and/or resurfacing at pastures. In case of high risk, building strategy to control and minimize it; (2) Building risk management strategy for the environment from Hg pollution of Lake Balkyldak, including pollutants bioaccumulation in the food chain. | Surface and ground water (n = 800), bottom sediment (n = 334), soil (n = 610), grass (4 g), biota from Balkyldak (n = 132); water for MeHg (n = 3), Hydrogeologic modeling for risk assessment and management of groundwater pollution via ModFlow GMS 5.0. Sampling plan of top 3 soil layers (0-10, 10-20, 20-50 cm) in a regular grid with a varying sampling step | AAS (Lumex RA 915+); AFS (PS Analytical Millennium Merlin System) | Computer model of Hg contamination of groundwater verified by Hg analysis -> predicted Hg transport within 30 y Hg plume in groundwater continues to spread in N-NW direction of the plant -> high risk of pollution of topsoil layers Hg levels in GW in the eastern direction are falling as predicted by computer modeling Hg levels on plant’s territory are unpredictable, but in topsoils of most parts are very high despite demercuration efforts Waste storage facilities of PCP show good isolation results The estimated amount of Hg discarded in Lake Balkyldak by the plant—135,336 kg Hg levels of surface waters of Balkyldak decreased after the burial of Hg waste Hg levels of biota fell as well, but some fish specimen had high Hg levels in 2007 (1–1.5 mg/kg) | Topsoils and vegetation: >2.1 mg/kg (MPC) in selected sites with Hg-bearing groundwater; in soils on PCP site extremely high—up to x1000 MPC Groundwater: extremely irregular decrease; high risk of formation of new hotspots of soil contamination on PCP territory due to the transport of soluble Hg to aeration zone; no risk of the Irtysh and water-supply wells of Pavlodarskoye village contamination if hydro-geological conditions remain same; | (1) Create a monitoring laboratory for PCP to complete implementation of post-demercuration monitoring programs (2) Treatment for soils: pulpation + gravitational separation (3) Bioremediation of groundwater to immobilize Hg (4) Sediments: pump using a dredger and move to an isolated pond with subsequent evaporation and burial (5) Ban the consumption of fish from Balkyldak (6) 2nd phase of demercuration plan to address: (i) treatment and remediation of soils on plant’s territory (ii) Hg immobilization in groundwater (iii) treatment of Balkyldak’s sediments |
| 2016 Shakhova et al. [64] | Evaluation of mercury contamination in the vicinity of enterprises of the petrochemical complex in the winter period (based on the example of Pavlodar, Republic of Kazakhstan) | To evaluate mercury pollution in the vicinity of petrochemical complex enterprises during the winter period (on the example of Pavlodar) according to the study of/investigating/analyzing the snow cover as storage of solid particles. | Snow from 11 locations (1 sample of 10-12 L), number of samples at the closest residential area = 5 | AAS (Lumex RA 915+ and PYRO 915) | (1) Hg concentration in solid fraction of snow exceeds max allowable concentration by 1.5–7 times. In NE zone: 0.31–1.04 mg/kg, SW: 0.22 mg/kg, NW: 0.03–0.26 mg/kg, background 0.15 mg/kg; in Pavlodarskoe village close to background Daily mean Hg deposited on snow cover 4.9–221 mg/(km2 × day); max—1.5 km from the plant, NE zone | High Hg concentrations in the NE zone might be related to technogenic Hg contamination and wind directions; Hg depositions and concentrations in snow covers are high (0.03–1.04 mg/kg) in the vicinity (0.5–2.5 km) from PCP | The data obtained can be used for planning of environmental activities, such as air monitoring in the northern industrial zone of Pavlodar, as well as for further monitoring of health risks of the Pavlodar region population |
| Case | Pavlodar (Includes Balkyldak Lake and Irtysh River) | Nura River |
|---|---|---|
| Source of contamination | Chlorine and caustic soda production at the JSC “Pavlodar Chemical Plant,” Hg used in electrolysis | Acetaldehyde production at the Temirtau chemical plant by direct C2H2 hydration in the presence of HgSO4 |
| Years of operation | 1975–1993 | 1950–1997 |
| Contaminated zones | The territory of the plant, lake Balkyldak for wastewater discharge, Shoptykol | Nura river (mainly close to the discharge point), Intumak and Samarkand reservoirs, Swamp Zhaur, old ash lagoon of KarGRES-1 and wastewater treatment facilities, terminal wetlands of Korgalzhyn National Park |
| Hg quantities discharged | 1300 t [65]; 1000 t [50]; 19 t on the territory of the site [59]; discarded into lake 135 t [63]; | 2351.6 tons of Hg consumed [19] |
| Hg concentrations in soil and groundwater (mg/kg for soils, mg/L for water) | Soils:
| Soil: |
| Hg concentrations in water (mean) |
| |
| Hg concentrations in sediments (mean) | ||
| Hg in air, biota, hair, blood (mean) |
|
|
| Facility | Years of Operation | Production Capacity | Estimated Discharge | Hg in Soils (mg/kg) | Hg in Sediments (mg/kg) | Hg in Water (ng/L) | Main Remarks | Reviewed References | |
|---|---|---|---|---|---|---|---|---|---|
| Temirtau, Kazakhstan | Acetaldehyde | 1950–1997 | Not reported in reviewed references | 1200 t [67] | 0.01 to over 100 [55] up to 1974 [66] | 150–240 [54] 9.95–306 [50] | 500–1250 [54] 1600–4300 [50] | Reviewed in detail in the present study (Refer to Table 3). | [50,66,67] |
| Schkopau, Germany | Acetaldehyde | Before 1942–1989 | 142,800–300,000 t/y | Not reported in reviewed references | 14 to over 1000 [68] | Not reported in reviewed references | Not reported in reviewed references | An industrial complex with three chlor-alkali plants and one acetaldehyde plant [68]; A strong source of atmospheric Hg emissions (0.2–1.7 kg/day) [69]; High levels of organic Hg compounds in the plant’s soils, possibly due to reaction of Hg0 with carbide (CaC2) dust covering the site’s soils, reaction of Hg with soil’s humus, intermediate reactions in acetaldehyde production [68]. | [68,69,70] |
| Minamata, Japan | Acetaldehyde | 1932–1968 | Not reported in reviewed references | 250 t [71] | Not reported in reviewed references | Before dredging: up to 2000 [71] After dredging: 0.49–3.4 [72] 0.61–6.73 [71] | 1.3–4.3 (total [71]) 0.10–0.95 (dissolved [73]) | >2200 registered cases of Minamata disease in the area by 2003 [72]; Dredging in 1974–1990 to decrease sediment Hg below 25 mg/kg [72]; Hg in sediments still 70 times higher than background levels [71]; 254% of total Hg in the bottom water layer is MeHg -> MeHg is the main form of Hg from sediment to water [71]. | [71,72,73] |
| Qingzhen, Guizhou, China | Acetic acid and acetaldehyde | 1971–2000 | Not reported in reviewed references | 134.6 t [74] | 14.3+−0.1 to 354+−15 [75] 0.06–321.38 [74] 0.14–259.56 [76] | Not reported in reviewed references | 450–1830 (total) 12.7–16.1 (dissolved [75] | Site conditions similar to Nura River; Total daily intake in μg/kg of 60 kg body weight for rice (inorganic Hg—0.001–0.003, MeHg—0.005–0.019) irrigated by polluted river water and fish (inorganic Hg—0.000–0.003, MeHg—0.01–0.13) were estimated and exceeded values recommended by U.S. EPA [75]. | [74,75,76] |
| Pavlodar, Kazakhstan | Hg-cell chlor-alkali plant | 1975–1993 | 100,000 t of Cl2/y | 1000–1300 t [50,65] | 0.0067–835.9 [19] 0.22–5.72 (around the lake, excluding the plant’s site, [50]) up to 2000 [63] | 0.11–617 [50] | 110–7300 [50] | Reviewed in detail in the present study (Refer to Table 3). | [19,50,63,65] |
| Penobscot River, Maine, US | Hg-cell chlor-alkali plant | 1967–2012 | 65,700 t of Cl2/y | 9 t | Not reported in reviewed references | 0.35–1.10 [77] | 2 (dissolved [77]) | Slow drainage of Hg from the site into the river (5.4 g/day) with increased loading during major storm events [78]; site groundwater input of Hg amounted to 17 g/day in the 1990s and decreased to 0.11 g/day in 2009 due to capture and treatment of site groundwater [78]; slow recovery of river’s aquatic food web, need for longer monitoring [79]. | [77,78,79] |
| Flix, Spain | Hg-cell chlor-alkali plant | 1949–2017 | 115,200 t of Cl2/y | Not reported in reviewed references | 0.044–12.9 [80] 1.7–61.6 [81] | 0.098–495 [80] up to 640 [82] | Not reported in reviewed references | The current pollution source is a sludge deposit formed at the riverbank close to the dam and containing approximately 10-18 Mg of Hg [83]; Several dams placed upstream and downstream of the plant resulted in accumulation of waste containing Hg at the base of the downstream dam in the absence of natural dilution and burial with river sediment material [82]; removal of the contaminated deposit has started [81,82]. Elevated atmospheric Hg levels in the region (229 ng/m3 in the vicinity of the plant) exceeding guideline thresholds for a residential area in Flix town [80]. | [80,81,82,83] |
| Estarreja, Portugal | Hg-cell chlor-alkali plant | 1950–2002 | Not reported in reviewed references | over 50 t | 0.18–49.23 [84] 0.01–90.8 [85] | Up to 180 [84] 0–48 [86] | 7-84 (dissolved [86]) 12–847 (total [85]) | About 8 km2 area around the plant has been identified as a heavily contaminated zone [84]; Hg tightly binds to topsoils with a size fraction <0.063 mm, possibility of groundwater contamination [84]; Hg concentrations in surface sediments were lower than those in deeper parts, indicating higher historical Hg releases with pieces of evidence of remobilization of Hg in water in reducing environments [86]. Agricultural soils located close to the plant’s past effluent discharge spot contain high Hg levels and recommended to be restricted from use and remediated [85]. | [84,85,86] |
| Dalhousie, New Brunswick, Canada | Hg-cell chlor-alkali plant | 1963–2008 | 34,300 t of Cl2/y | 141–163 t (2 chlor-alkali plants of Canada [87]) | Not reported in reviewed references | <0.1–8.1 [88] 0.02–1.96 [88] 0.04–0.28 [89] | 840–4320 (total, in effluents [88]) | Atmospheric Hg emissions from the facility in 1988–1996 were in the range 31–70 kg/y, with 2.5 times higher Hg quantities discharged in the facility’s landfilled sludge [90]; The sludge pile close to the plant contains approximately 2.5 μg of Hg/g in the form of Hg sulfides [90]; Hg content in sediment samples close to the plant exceed Canadian guideline values (0.13 mg/kg) [88]; Recent observations indicate natural recovery due to sediment deposition [89]. | [87,88,89,90] |
| Rm Valcea, Romania | Hg-cell chlor-alkali plant | 1968-present [91] | 210,000 t of Cl2/y | 36–53 t (estimated based on data from [91]) | Not reported in reviewed references | 0.5–45 [91] | 9–88 (dissolved [92]) | Fluctuations in Hg concentration in sediment cores -> flooding cause the transport of contaminated soil from the site to the river sediments [91]; Bravo et al. [91] strongly discouraged any treatment actions involving rework or dredging of the sediments due to the risk of resuspension of buried highly contaminated particles. | [91,92] |
| Remediation/Treatment Technology | Media | Description | Removal Values | References |
|---|---|---|---|---|
| Adsorption by activated carbon | Water | A universal adsorbent material to reduce flux to the environment | 60–95% | [6,100,108] |
| Biochar | Sediment, Water | Sorption of the contaminant by biochar—charcoal produced from plant matter | up to 95% in pore water | [48,102] |
| Bioremediation: bio-treatment, biofunctionalized zeolite, genetically engineered bacteria | Water | A process that generally utilizes microorganisms, plants, or their enzymes to decrease the toxicity of the contaminant | 91–95% | [6,109,110] |
| Chemical reduction and stripping | Water | Injecting chemically reductive additives into contaminated media/chemical reductant in the contaminant plume; physical separation from the aquatic stream by vapor | >94% | [100,102,111] |
| Containment in-place | Soil, Sediment, Water | Covering contaminated media with clean soil and/or other low permeability material | not applicable | [6,112] |
| Copper or brass shavings | Water | Removal of Hg2+ from water by the amalgamation | 96–98% | [48,113,114] |
| Ex situ soil washing | Soil | Washing the excavated soils with a special solution, scrubbing, and separating clean soil | up to 99% | [6,100,115] |
| Excavation or dredging with removal | Soil, Sediment | Removal and off-site storage of the contaminated material | not applicable | [48,116] |
| Immobilized algae | Water | Accumulation of the contaminant from aquatic media in certain species of algae | up to 90% | [100,102,117] |
| In situ thermal desorption | Soil | On-site heating soil to very high temperatures to release contaminant in gaseous/vapor phase | 99% | [6,112,118] |
| In situ flushing/washing | Soil | Flooding a zone with a flushing solution to mobilize contaminant | 35–90% | [50,112] |
| In situ electrochemical/electrokinetic recovery | Soil | Applying low-intensity direct current across electrodes to drive ions migration to the opposite sign electrode using a mobilizing solution | 30–92% | [102,112] |
| Monitored natural attenuation | Soil, Sediment | Natural physio-chemical/biological processes reduce concentration/toxicity/mobility | not applicable | [6,116] |
| Nanotechnology | Soil, Sediment, Water | Injected FeS nanoparticles to contaminated soil immobilize Hg via ion exchange/adsorption | ~92% Ag-Zn > 99% | [102,109,112] |
| Permeable reactive barrier and/or funnel/gate system | Water | A subsurface construction used to channel the contaminated plume into a gate with reactive material to adsorb/decompose/transform the contaminant | variable (material- and site-specific) | [119] |
| Phytoremediation (phytostabilization, phytoextraction, phytovolatilization) | Soil | A process that uses plants to remove, stabilize, or destroy the contaminant | >99%, 2.62 mg/kg max removal efficiency | [100,103,116,120] |
| Precipitation, co-precipitation, chelating agents | Water | A chelating reagent is added to the contaminated water in soluble form, and the contaminant is removed after its flocculation and/or precipitation | variable (reagent-specific) | [111,121] |
| Pump and treat | Water | Pumping contaminated groundwater to treatment system, discharging back to environment | variable (technology specific) | [112] |
| Solidification/Stabilization | Soil, Waste | Physically encapsulating or chemically stabilizing the contaminant in the soil | 90–98% | [6,109,112] |
| Thermal treatment: (1) batch retorting, (2) ex situ thermal desorption, (3) vitrification | Soil, Waste | (1) Heating contaminated material under vacuum to volatilize Hg volatilization, (2) heating excavated soil for volatilization, (3) melting and cooling soils to immobilize contaminant | up to 99% | [6,109,122] |
| Ultrasound remediation | Soil, Sediment, Water | High ultrasonic sound (150–2000 kHz) leading to desorption produced by local turbulence and/or to degradation due to free radical oxidation reactions | ~5%, usually used with bioremediation | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guney, M.; Akimzhanova, Z.; Kumisbek, A.; Beisova, K.; Kismelyeva, S.; Satayeva, A.; Inglezakis, V.; Karaca, F. Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses. Int. J. Environ. Res. Public Health 2020, 17, 8936. https://doi.org/10.3390/ijerph17238936
Guney M, Akimzhanova Z, Kumisbek A, Beisova K, Kismelyeva S, Satayeva A, Inglezakis V, Karaca F. Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses. International Journal of Environmental Research and Public Health. 2020; 17(23):8936. https://doi.org/10.3390/ijerph17238936
Chicago/Turabian StyleGuney, Mert, Zhanel Akimzhanova, Aiganym Kumisbek, Kamila Beisova, Symbat Kismelyeva, Aliya Satayeva, Vassilis Inglezakis, and Ferhat Karaca. 2020. "Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses" International Journal of Environmental Research and Public Health 17, no. 23: 8936. https://doi.org/10.3390/ijerph17238936
APA StyleGuney, M., Akimzhanova, Z., Kumisbek, A., Beisova, K., Kismelyeva, S., Satayeva, A., Inglezakis, V., & Karaca, F. (2020). Mercury (Hg) Contaminated Sites in Kazakhstan: Review of Current Cases and Site Remediation Responses. International Journal of Environmental Research and Public Health, 17(23), 8936. https://doi.org/10.3390/ijerph17238936