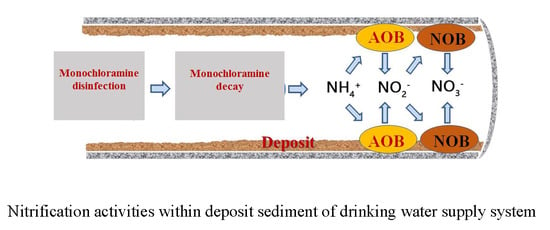
Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microsensor Fabrication
2.3. Microsensor Measurements
2.4. Flux Calculation
2.5. Diffusion Analysis
3. Results and Discussion
3.1. Microsensor Measurements of DO and Nitrogen Species
3.2. Estimation of Production and Consumption Rates
3.3. Diffusion Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azara, A.; Castiglia, A.; Piana, A.; Masia, M.D.; Palmieri, A.; Arru, B.; Maida, G.; Dettori, M. Derogation from drinking water quality standards in Italy according to the European Directive 98/83/EC and the Legislative Decree 31/2001—A look at the recent past. Ann. Ig. 2018, 30, 517–526. [Google Scholar]
- Dettori, M.; Azara, A.; Loria, E.; Piana, A.; Masia, M.D.; Palmieri, A.; Cossu, A.; Castiglia, P. Population distrust of drinking water safety. Community outrage analysis, prediction and management. Int. J. Environ. Res. Public Health 2019, 16, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rak, J.R.; Pietrucha-Urbanik, K. An approach to determine risk indices for drinking water–study investigation. Sustainability 2019, 11, 3189. [Google Scholar] [CrossRef] [Green Version]
- Pietrucha-Urbanik, K.; Żelazko, A. Approaches to assess water distribution failure. Period Polytech-Civ. 2017, 61, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.L.; Li, X.; Yang, Y.L.; Luo, J.H.; Li, G.B.; Wang, C.H. Advances of nitrification studies in drinking water distribution system. China Water Wastew. 2012, 28, 24–28. (In Chinese) [Google Scholar]
- Zhou, L.L. Biofilm and Nitrification Control in Water Supply System. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2010. (In Chinese). [Google Scholar]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 1–24. [Google Scholar] [CrossRef]
- Sun, H.F.; Shi, B.Y.; Wang, D.S. Characteristics of biofilm formed on the wall of drinking water supply piping system and its impact on water quality. China Water Wastew. 2011, 27, 40–45. (In Chinese) [Google Scholar]
- Zhang, X.Y.; Liu, W.J.; Gao, S.H.; Zhang, L. Simulation impact of piping material and residual chlorine on biofilm formation in drinking water distribution system. China Environ. Sci. 2006, 26, 303–306. (In Chinese) [Google Scholar]
- LeChevallier, M.W.; Lowry, C.D.; Lee, R.G. Disinfecting biofilm in a model distribution system. J. Am. Water Work. Ass. 1990, 82, 8799. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Cawthon, C.D.; Lee, R.G. Factors promoting survival of bacteria in chlorinated water supplies. Appl. Environ. Microb. 1988, 54, 649–654. [Google Scholar] [CrossRef] [Green Version]
- AWWAF. Fundamentals and Control of Nitrification in Chloraminated Drinking Water Distribution Systems (AWWA Manual M56); American Water Works Association: Denver, CO, USA, 2006. [Google Scholar]
- Wahman, D.G.; Pressman, J.G. Nitrification in chloraminated drinking water distribution systems: factors affecting occurrence. Compreh. Water Qual. Purif. 2014, 2, 283–294. [Google Scholar]
- Zhang, Y.; Love, N.; Edwards, M. Nitrification in drinking water systems. Crit. Rev. Environ. Sci. Technol. 2009, 39, 153–208. [Google Scholar] [CrossRef]
- Zhou, L.L.; Liu, W.J.; Zhang, Y.J. Comparison of activity between attached bacteria and suspended bacteria in drinking water distribution system. China Water Wastew. 2007, 23, 37–40. (In Chinese) [Google Scholar]
- Chiao, T.H.; Clancy, T.M.; Pinto, A.; Xi, C.; Raskin, L. Differential resistance of drinking water bacterial populations to monochloramine disinfection. Environ. Sci. Technol. 2014, 48, 4038–4047. [Google Scholar] [CrossRef]
- Cochran, W.; McFeters, G.; Stewart, P. Reduced susceptibility of thin Pseudomonas aeruginosa biofilms to hydrogen peroxide and monochloramine. J. Appl. Microbiol. 2000, 88, 22–30. [Google Scholar] [CrossRef] [Green Version]
- van der Wielen, P.W.J.J.; Lut, M.C. Distribution of microbial activity and specific microorganisms across sediment size fractions and pipe wall biofilm in a drinking water distribution system. Water Sci. Technol. Water Sup. 2016, 16, 896–904. [Google Scholar] [CrossRef]
- Reagan, J.M. Microbial Ecology of Nitrification in Chloraminated Drinking Water Distribution Systems. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 2001. [Google Scholar]
- Wang, J.; Yu, J. Nitrification and Its Control Strategies in Water Supply System. Master’s Thesis, Hunan University, Changsha, China, 2009. (In Chinese). [Google Scholar]
- Zhang, Y.G.; Zhou, L.L.; Li, W.Y. Nitrification in chloraminated drinking water distribution system and its control. China Water Wastew. 2008, 24, 6–9. (In Chinese) [Google Scholar]
- Zhang, Y.; Zhou, L.; Zeng, G. Impact of total organic carbon and chlorine to ammonia ratio on nitrification in a bench-scale drinking water distribution system. Front. Environ. Sci. Eng. 2010, 4, 430–437. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, Y.J.; Li, G.B. Effect of pipe material and low level disinfectants on biofilm development in a simulated drinking water distribution system. J. Zhejiang Univ. Sci. A 2009, 10, 725–731. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhou, Y.G.; Ye, H.X. Controlling Effect of Chloramines, and Chlorite on Nitrification in Simulated Drinking Water Distribution System. J. Hunan Univ. 2012, 39, 69–73. (In Chinese) [Google Scholar]
- Lu, J.; Struewing, I.; Yelton, S.; Ashbolt, N. Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. J. Appl. Microbiol. 2015, 119, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.M.; Harrington, G.W.; Baribeau, H. Diversity of nitrifying bacteria in full-scale chloraminated distribution systems. Water Res. 2003, 37, 197–205. [Google Scholar] [CrossRef]
- de Beer, D.; Srinivasan, R.; Stewart, P.S. Direct measurement of chorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 1994, 60, 4339–4344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Pressman, J.G.; Wahman, D.G.; Bishop, P.L. Characterization and application of a chlorine microelectrode for measuring monochloramine within a biofilm. Sens. Actuat. B Chem. 2010, 145, 734–742. [Google Scholar] [CrossRef]
- Lee, W.H.; Wahman, D.G.; Bishop, P.L.; Pressman, J.G. Free chlorine and monochloramine application to nitrifying biofilm: Comparison of biofilm penetration, activity, and viability. Environ. Sci. Technol. 2011, 45, 1412–1419. [Google Scholar] [CrossRef]
- Lee, W.H.; Wahman, D.G.; Pressman, J.G. Monochloramine sensative amperometric microelectrode: Optimization of gold, platimum, and carbon fiber sensing materials for removal of dissolved oxygen interference. Ionics 2015, 21, 2663–2674. [Google Scholar] [CrossRef]
- Pressman, J.G.; Lee, W.H.; Bishop, P.L.; Wahman, D.G. Effect of free ammonia concentration on monochloramine penetration within a nitrifying biofilm and its effect on activity, viability, and recovery. Water Res. 2012, 46, 882–894. [Google Scholar] [CrossRef]
- Lu, R.; Yu, T. Fabrication and evaluation of an oxygen microelectrode applicable to environmental engineering and science. J. Environ. Eng. Sci. 2002, 1, 225–235. [Google Scholar] [CrossRef]
- Liu, H.; Tan, S.; Sheng, Z.; Liu, Y.; Yu, T. Bacterial community structure and activity of sulfate reducing bacteria in a membrane aerated biofilm analyzed by microsensor and molecular techniques. Biotechnol. Bioeng. 2014, 111, 2155–2162. [Google Scholar] [CrossRef]
- Tan, S.Y.; Yu, T.; Shi, H.C. Microsensor determination of multiple microbial processes in an oxygen-based membrane aerated biofilm. Water Sci. Technol. 2014, 69, 909–914. [Google Scholar] [CrossRef]
- Liu, H.; Tan, S.; Yu, T.; Liu, Y. Sulfate reducing bacterial community and in situ activity in mature fine tailings analyzed by real time qPCR and microsensor. J. Environ. Sci. China 2016, 44, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wahman, D.G.; Pressman, J.G. Penetration and activity of monochloramine and free chlorine in sediment from drinking water storage tank. Environ. Sci. Technol. 2019, 53, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Larsen, L.H.; Kjaer, T.; Revsbech, N. Biosensor determination of the microscale distribution of nitrate, nitrate assimilation, nitrification, and denitrification in a diatominhabited freshwater sediment. Appl. Environ. Microb. 1998, 64, 3264–3269. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.L.; Kjar, T.; Revsbech, N.P. Use of NOx- micorsensors to estimate the activity of sediment nitrification and NOx- consumption along an estuarine salinity, nitrate, and light gradient. Aquat. Microb. Ecol. 2001, 26, 181–193. [Google Scholar] [CrossRef]
- Okabe, S.; Ito, T.; Satoh, H. Analyses of spatial distribution of sulphate reducing bacteria and their activity in aerobic wastewater biofilms. Appl. Microbiol. Biot. 1999, 65, 5107–5116. [Google Scholar]
- Kreft, J.U.; Picioreanu, C.; Wimpenny, J.W.; van Loosdrecht, M.C. Individual-based modeling of biofilms. Microbiology 2001, 147, 2897–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, H.; Ding, N. Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System. Int. J. Environ. Res. Public Health 2020, 17, 772. https://doi.org/10.3390/ijerph17030772
Liu X, Liu H, Ding N. Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System. International Journal of Environmental Research and Public Health. 2020; 17(3):772. https://doi.org/10.3390/ijerph17030772
Chicago/Turabian StyleLiu, Xun, Hong Liu, and Ning Ding. 2020. "Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System" International Journal of Environmental Research and Public Health 17, no. 3: 772. https://doi.org/10.3390/ijerph17030772
APA StyleLiu, X., Liu, H., & Ding, N. (2020). Chloramine Disinfection-Induced Nitrification Activities and Their Potential Public Health Risk Indications within Deposits of a Drinking Water Supply System. International Journal of Environmental Research and Public Health, 17(3), 772. https://doi.org/10.3390/ijerph17030772