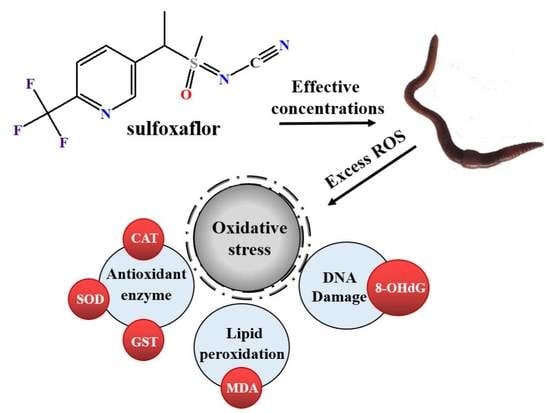
The Toxic Effects of Sulfoxaflor Induced in Earthworms (Eisenia fetida) under Effective Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acute Toxicity Test
2.3. Subchronic Toxicity
2.4. Determination of Effective Concentrations
2.5. Measurement of the ·OH− Content
2.6. Measurement of Enzyme Activity
2.7. Measurement of TBARS Content
2.8. Measurement of 8-OHdG Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Acute Toxicity of Sulfoxaflor on Earthworms
3.2. Effective Concentrations of Sulfoxaflor
3.3. Two-Way ANOVA Results
3.4. Effects of Sulfoxaflor on the ·OH− Content
3.5. Effects of Sulfoxaflor on Antioxidant Enzymes Activities
3.6. Oxidative Damage Effects in Earthworms Induced by Sulfoxaflor
4. Conclusions
- Sulfoxaflor is a supertoxic pollutant to earthworms with the LC50 values of 0.291 µg/cm2 and 6.142 mg/kg, respectively.
- Sulfoxaflor was degraded quickly in the artificial soil with the degradation rate of 0.002–0.017 mg/(kg·d) and a half-life of 12.0–15.4 d at different concentrations.
- The changes in the activity or content of each biomarker in 0.5 mg/kg and 1.0 mg/kg treatment demonstrated that sulfoxaflor caused oxidative damage at the early stage of exposure. At the end of exposure, the toxic effects were reduced due to the degradation of sulfoxaflor.
- 8-OHdG is a sensitive indicator to monitor the toxicity of sulfoxaflor in earthworms.
- The risk of sulfoxaflor to earthworms was reduced because of it was easily degraded in soil. However, high concentrations of sulfoxaflor should not be released into the soil environment as it is a super toxic pollutant to earthworms.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B. Biological characterization of sulfoxaflor, a novel insecticide. Pest. Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Rasoulpour, R.J.; Terry, C.; LeBaron, M.J.; Stebbins, K.; Ellis-Hutchings, R.G.; Billington, R. Mode-of-action and human relevance framework analysis for rat Leydig cell tumors associated with sulfoxaflor. Crit. Rev. Toxicol. 2014, 44, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Phys. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, G.B.; Olson, M.B.; Beavers, K.W.; Loso, M.R.; Sparks, T.C. Characterization of a nicotinic acetylcholine receptor binding site for sulfoxaflor, a new sulfoximine insecticide for the control of sap-feeding insect pests. Pestic. Biochem. Physiol. 2017, 143, 90–94. [Google Scholar] [CrossRef]
- Cutler, P.; Slater, R.; Edmunds, A.J.F.; Maienfisch, P.; Hall, R.G.; Earley, F.G.P.; Pitterna, T.; Pal, S.; Paul, V.L.; Goodchild, J.; et al. Investigating the mode of action of sulfoxaflor: A fourth-generation neonicotinoid. Pest. Manag. Sci. 2013, 69, 607–619. [Google Scholar] [CrossRef]
- Pan, F.X.; Lu, Y.; Wang, L. Toxicity and sublethal effects of sulfoxaflor on the red imported fire ant, Solenopsis invicta. Ecotoxicol. Environ. Saf. 2017, 139, 377–383. [Google Scholar] [CrossRef]
- Siviter, H.; Brown, M.J.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- Garzón, A.; Medina, P.; Amor, F.; Viñuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar]
- Chen, Z.L.; Dong, F.S.; Xu, J.; Liu, X.G.; Cheng, Y.P.; Liu, N.; Tao, Y.; Pan, X.L.; Zheng, Y.Q. Stereoselective separation and pharmacokinetic dissipation of the chiral neonicotinoid sulfoxaflor in soil by ultraperformance convergence chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 6677–6690. [Google Scholar] [CrossRef]
- Wang, Y.; Cang, T.; Zhao, X.P.; Yu, R.X.; Chen, L.P.; Wu, C.X.; Wang, Q. Comparative acute toxicity of twenty-four insecticides to earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2012, 79, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Xiong, K.; Liu, J. Comparative toxicity and bioaccumulation of fenvalerate and esfenvalerate to earthworm Eisenia fetida. J. Hazard. Mater. 2016, 310, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhang, Y.; Wu, D.; McLaughlin, N.; Zhang, S.; Chen, X.; Jia, S.; Liang, A. Temporal Variation of Earthworm Impacts on Soil Organic Carbon under Different Tillage Systems. Int. J. Environ. Res. Public Health 2019, 16, 1908. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar] [CrossRef]
- Roubalová, R.; Dvořák, J.; Procházková, P.; Elhottová, D.; Rossmann, P.; Škanta, F.; Bilej, M. The effect of dibenzo-p-dioxin- and dibenzofuran-contaminated soil on the earthworm Eisenia andrei. Environ. Pollut. 2014, 193, 22–28. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Moussa, M.G.; Ismael, M.A.; Wei, J.; Zhao, Y.; Wu, Y.; Hu, C. Earthworms, rice straw, and plant interactions change the organic connections in soil and promote the decontamination of cadmium in soil. Int. J. Environ. Res. Public Health 2018, 15, 2398. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.Q.; Zhang, W.; Li, J.; Lin, K.; Ji, R. Antioxidant and gene expression responses of Eisenia fetida following repeated exposure to BDE209 and Pb in a soil-earthworm system. Sci. Total. Environ. 2016, 556, 163–168. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Afzal, J.; Rana, M.S.; Imran, M.; Cai, M.; Hu, C. Phenanthrene Mitigates Cadmium Toxicity in Earthworms Eisenia fetida (Epigeic Specie) and Aporrectodea caliginosa (Endogeic Specie) in Soil. Int. J. Environ. Res. Public Health 2018, 15, 2384. [Google Scholar] [CrossRef] [Green Version]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.G.; You, X.W.; Chen, D.; Li, Y.Q.; Wang, F.L. Oxidative stress and gene expression of earthworm (Eisenia fetida) to clothianidin. Ecotoxicol. Environ. Saf. 2017, 142, 489–496. [Google Scholar] [CrossRef]
- OECD. Organization for Economic Co-Operation and Development, Test 207: Earthworm, Acute Toxicity Tests. In OECD Guidelines for Testing of Chemicals; Organization for Economic Co-operationand Development, Ed.; OECD: Paris, France, 1984. [Google Scholar]
- Xu, J.; Dong, F.S.; Liu, X.G.; Li, J.; Li, Y.B.; Shan, W.L.; Zheng, Y.Q. Determination of sulfoxaflor residues in vegetables, fruits and soil using ultraperformance liquid chromatography/tandem mass spectrometry. Anal. Methods 2012, 4, 4019–4024. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, S.M.; Wang, J.H.; Wang, J.; Shao, Y.T.; Zhu, L.S. Biochemical responses and DNA damage in earthworms (Eisenia fetida) induced by ionic liquid [Omim]PF6. Environ. Sci. Pollut. Res. 2016, 23, 6836–6844. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, L.S.; Wang, J.; Wang, J.H.; Liu, W.; Xie, H. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol. Biochem. 2009, 41, 905–909. [Google Scholar] [CrossRef]
- Štolfa, I.; Velki, M.; Vuković, R.; Ečimović, S.; Katanić, Z.; Lončarić, Z. Effect of different forms of selenium on the plant–soil–earthworm system. J. Plant Nutr. Soil Sci. 2017, 180, 231–240. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.L.; Wang, X.G.; Chen, D.; Li, Y.Q.; Wang, F.L. Comparative toxicity and bioaccumulation of two dinotefuran metabolites, UF and DN, in earthworms (Eisenia fetida). Environ. Pollut. 2018, 234, 988–996. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhang, L.; Feng, L.; Mao, L.G.; Jiang, H.Y. Oxidative stress of imidaclothiz on earthworm Eisenia fetida. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 191, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.H.; Zhao, X.P.; Wang, Q.; Qian, Y.Z. Comparative and combined acute toxicity of butachlor, imidacloprid and chlorpyrifos on earthworm, Eisenia fetida. Chemosphere 2014, 100, 111–115. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.G.; Xu, J.L.; You, X.W.; Chen, D.; Wang, F.L.; Li, Y.Q. Biochemical and genetic toxicity of dinotefuran on earthworms (Eisenia fetida). Chemosphere 2017, 176, 156–164. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.H.; Wang, G.C.; Zhu, L.S.; Wang, J. DNA damage and oxidative stress induced by imidacloprid exposure in the earthworm Eisenia fetida. Chemosphere 2016, 144, 510–517. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Zhang, Y.N.; Zhang, P.; Jiang, H.Y. Inhibition and recovery of biomarkers of earthworm Eisenia fetida after exposure to thiacloprid. Environ. Sci. Pollut. Res. 2015, 22, 9475–9482. [Google Scholar] [CrossRef]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.M.; Zhang, B.H.; Wang, C.X. Ecotoxicological effects on the earthworm Eisenia fetida following exposure to soil contaminated with imidacloprid. Environ. Sci. Pollut. Res. 2014, 21, 12345–12353. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Wang, Z.; Zhu, L. Toxicological effects of pyraclostrobin on the antioxidant defense system and DNA damage in earthworms (Eisenia fetida). Ecol. Indic. 2019, 101, 111–116. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Weaver, R.J.; Hill, G.E.; Kuan, P.-L.; Tseng, Y.-C. Copper exposure reduces production of red carotenoids in a marine copepod. Ecol. Indic. 2016, 70, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Matos, B.; Martins, M.; Samamed, A.C.; Sousa, D.; Ferreira, I.; Diniz, M.S. Toxicity Evaluation of Quantum Dots (ZnS and CdS) Singly and Combined in Zebrafish (Danio rerio). Int. J. Environ. Res. Public. Health 2020, 17, 232. [Google Scholar] [CrossRef] [Green Version]
- Parelho, C.; Rodrigues, A.; Bernardo, F.; do Carmo Barreto, M.; Cunha, L.; Poeta, P.; Garcia, P. Biological endpoints in earthworms (Amynthas gracilis) as tools for the ecotoxicity assessment of soils from livestock production systems. Ecol. Indic. 2018, 95, 984–990. [Google Scholar] [CrossRef]
- Koivula, M.J.; Kanerva, M.; Salminen, J.P.; Nikinmaa, M.; Eeva, T. Metal pollution indirectly increases oxidative stress in great tit (Parus major) nestlings. Environ. Res. 2011, 111, 362–370. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Zhu, Y. Mesotrione-induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Ecol. Indic. 2018, 95, 436–443. [Google Scholar] [CrossRef]
- Duryee, M.J.; Klassen, L.W.; Schaffert, C.S.; Tuma, D.J.; Hunter, C.D.; Garvin, R.P.; Anderson, D.R.; Thiele, G.M. Malondialdehyde-acetaldehyde adduct is the dominant epitope after MDA modification of proteins in atherosclerosis. Free Radic. Biol. Med. 2010, 49, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- Markad, V.L.; Gaupale, T.C.; Bhargava, S.; Kodam, K.M.; Ghole, V.S. Biomarker responses in the earthworm, Dichogaster curgensis exposed to fly ash polluted soils. Ecotoxicol. Environ. Saf. 2015, 118, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topal, A.; Alak, G.; Ozkaraca, M.; Yeltekin, A.C.; Comaklı, S.; Acıl, G.; Kokturk, M.; Atamanalp, M. Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 2017, 175, 186–191. [Google Scholar] [CrossRef]





| Biomarkers | Dose | Time | Dose × Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| OH− | 3 | 98.38 | 0.000 * | 5 | 25.22 | 0.000 * | 15 | 8.92 | 0.000 * |
| SOD | 3 | 10.61 | 0.000 * | 5 | 25.74 | 0.000 * | 15 | 7.62 | 0.000 * |
| CAT | 3 | 29.98 | 0.000 * | 5 | 22.76 | 0.000 * | 15 | 6.69 | 0.000 * |
| GST | 3 | 60.72 | 0.000 * | 5 | 30.59 | 0.000 * | 15 | 4.77 | 0.000 * |
| TBARS | 3 | 16.13 | 0.000 * | 5 | 10.09 | 0.000 * | 15 | 2.13 | 0.025 * |
| 8-OHdG | 3 | 307.90 | 0.000 * | 5 | 36.28 | 0.000 * | 15 | 22.65 | 0.000 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Liu, Y.; Fang, K.; Liu, T. The Toxic Effects of Sulfoxaflor Induced in Earthworms (Eisenia fetida) under Effective Concentrations. Int. J. Environ. Res. Public Health 2020, 17, 1740. https://doi.org/10.3390/ijerph17051740
Zhang X, Wang X, Liu Y, Fang K, Liu T. The Toxic Effects of Sulfoxaflor Induced in Earthworms (Eisenia fetida) under Effective Concentrations. International Journal of Environmental Research and Public Health. 2020; 17(5):1740. https://doi.org/10.3390/ijerph17051740
Chicago/Turabian StyleZhang, Xiaolian, Xiuguo Wang, Yalei Liu, Kuan Fang, and Tong Liu. 2020. "The Toxic Effects of Sulfoxaflor Induced in Earthworms (Eisenia fetida) under Effective Concentrations" International Journal of Environmental Research and Public Health 17, no. 5: 1740. https://doi.org/10.3390/ijerph17051740
APA StyleZhang, X., Wang, X., Liu, Y., Fang, K., & Liu, T. (2020). The Toxic Effects of Sulfoxaflor Induced in Earthworms (Eisenia fetida) under Effective Concentrations. International Journal of Environmental Research and Public Health, 17(5), 1740. https://doi.org/10.3390/ijerph17051740