Feasibility of Adjusting the S2O32−/NO3− Ratio to Adapt to Dynamic Influents in Coupled Anammox and Denitrification Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Operation of the Expanded Granular Sludge Bed Reactor (EGSB) Reactor
2.2. Batch Experiments
2.3. Effluent Sampling and Chemical Analysis Methods
2.4. Sludge Sampling and DNA Extraction
2.5. High-throughput Sequencing and Bioinformatic Analysis
3. Results and Discussion
3.1. Operating Performance of the A/SAD/HD System in Stage I
3.2. Operating Performance of the A/SAD/HD System in Stage II
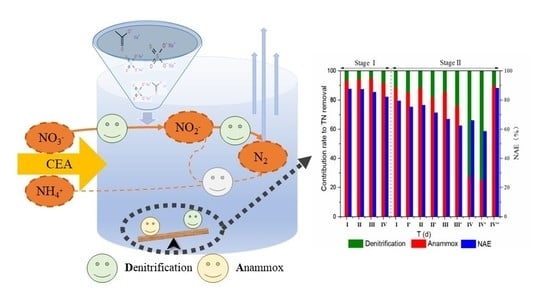
3.3. Cooperation and Competition between Anammox and Denitrification
3.4. Microbial Community Structure and Functional Bacteria Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jia, F.; Peng, Y.; Yang, Q. Competition and synergism between Anammox bacteria and other bacteria. Acta Sci. Circumstantiae 2014, 34, 1351–1361. [Google Scholar]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef]
- Kartal, B.; de Almeida, N.M.; Maalcke, W.J.; Op den Camp, H.J.M.; Jetten, M.S.M.; Keltjens, J.T. How to make a living from anaerobic ammonium oxidation. Fems. Microbiol. Rev. 2013, 37, 428–461. [Google Scholar] [CrossRef] [Green Version]
- Van der Star, W.R.L.; Abma, W.R.; Blommers, D.; Mulder, J.W.; Tokutomi, T.; Strous, M.; Picioreanu, C.; Van Loosdrecht, M.C.M. Startup of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007, 41, 4149–4163. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biot. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Lotti, T.; van der Star, W.R.L.; Kleerebezem, R.; Lubello, C.; van Loosdrecht, M.C.M. The effect of nitrite inhibition on the anammox process. Water Res. 2012, 46, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Park, J.; Kim, H. Determination of NH4+ in Environmental Water with Interfering Substances Using the Modified Nessler Method. J. Chem. Ny. 2013. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yuan, Y.; Huang, Y.; Bi, Z. Simultaneous removal of ammonia and nitrate by coupled S-0-driven autotrophic denitrification and Anammox process in fluorine-containing semiconductor wastewater. Sci. Total Environ. 2019, 661, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Amin, K.; Kim, S.; Yoon, S.; Kwon, K.; Bae, W. Autotrophic denitrification of nitrate and nitrite using thiosulfate as an electron donor. Water Res. 2014, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rios-Del Toro, E.E.; Cervantes, F.J. Coupling between anammox and autotrophic denitrification for simultaneous removal of ammonium and sulfide by enriched marine sediments. Biodegradation 2016, 27, 107–118. [Google Scholar] [CrossRef]
- Sun, X.B.; Du, L.F.; Hou, Y.Q.; Cheng, S.J.; Zhang, X.X.; Liu, B. Endogenous influences on anammox and sulfocompound-oxidizing autotrophic denitrification coupling system (A/SAD) and dynamic operating strategy. Bioresource Technol. 2018, 264, 253–260. [Google Scholar] [CrossRef]
- Dasgupta, S.; Wu, S.; Goel, R. Coupling autotrophic denitrification with partial nitritation-anammox (PNA) for efficient total inorganic nitrogen removal. Bioresource Technol. 2017, 243, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Li, W.F.; Li, X.C.; Zhao, D.F.; Ma, B.; Wang, Y.Q.; Liu, F.; Lee, D.J. Nitrite accumulation in continuous-flow partial autotrophic denitrification reactor using sulfide as electron donor. Bioresource Technol. 2017, 243, 1237–1240. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Ekama, G.A.; Cui, Y.-X.; Tang, C.-J.; van Loosdrecht, M.C.M.; Chen, G.-H.; Wu, D. Coupling of sulfur(thiosulfate)-driven denitratation and anammox process to treat nitrate and ammonium contained wastewater. Water Res. 2019, 163, 114854. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cao, S.B.; Wang, S.Y.; Niu, M.; Peng, Y.Z. Performance of partial denitrification (PD)-ANAMMOX process in simultaneously treating nitrate and low C/N domestic wastewater at low temperature. Bioresource Technol. 2016, 219, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Mccrady, M.H. Standard Methods for The Examination of Water and Waste-Water (12th ed). Am. J. Public Health Nations Health 1992, 56, 387–388. [Google Scholar] [CrossRef] [Green Version]
- Arbuckle, W.B.; Griggs, A.A. Determination of Biomass Mlvss in Pact Sludges. J. Water Pollut. Con. F. 1982, 54, 1553–1557. [Google Scholar]
- APHA. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Fernandes, S.O.; Javanaud, C.; Michotey, V.D.; Guasco, S.; Anschutz, P.; Bonin, P. Coupling of bacterial nitrification with denitrification and anammox supports N removal in intertidal sediments (Arcachon Bay, France). Estuar. Coast. Shelf. Sci. 2016, 179, 39–50. [Google Scholar] [CrossRef]
- Hallin, S.; Rothman, M.; Pell, M. Adaptation of denitrifying bacteria to acetate and methanol in activated sludge. Water Res. 1996, 30, 1445–1450. [Google Scholar] [CrossRef]
- Guven, D.; Dapena, A.; Kartal, B.; Schmid, M.C.; Maas, B.; van de Pas-Schoonen, K.; Sozen, S.; Mendez, R.; Op den Camp, H.J.M.; Jetten, M.S.M.; et al. Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl. Environ. Microb. 2005, 71, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Ruscalleda, M.; Lopez, H.; Ganigue, R.; Puig, S.; Balaguer, M.D.; Colprim, J. Heterotrophic denitrification on granular anammox SBR treating urban landfill leachate. Water Sci. Technol. 2008, 58, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Rattray, J.; van Niftrik, L.A.; van de Vossenberg, J.; Schmid, M.C.; Webb, R.I.; Schouten, S.; Fuerst, J.A.; Damste, J.S.S.; Jetten, M.S.M.; et al. Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst. Appl. Microbiol. 2007, 30, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; van Niftrik, L.; Rattray, J.; de Vossenberg, J.L.C.M.V.; Schmid, M.C.; Damste, J.S.S.; Jetten, M.S.M.; Strous, M. Candidatus ‘Brocadia fulgida’: An autofluorescent anaerobic ammonium oxidizing bacterium. Fems. Microbiol. Ecol. 2008, 63, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Takahashi, Y.; Fujii, N.; Yamada, Y.; Satoh, H.; Okabe, S. Nitrogen removal performance and microbial community analysis of an anaerobic up-flow granular bed anammox reactor. Chemosphere 2010, 78, 1129–1135. [Google Scholar] [CrossRef]
- Furukawa, K.; Inatomi, Y.; Qiao, S.; Quan, L.; Yamamoto, T.; Isaka, K.; Sumino, T. Innovative treatment system for digester liquor using anammox process. Bioresource Technol. 2009, 100, 5437–5443. [Google Scholar] [CrossRef]
- Jaroszynski, L.W.; Cicek, N.; Sparling, R.; Oleszkiewicz, J.A. Importance of the operating pH in maintaining the stability of anoxic ammonium oxidation (anammox) activity in moving bed biofilm reactors. Bioresource Technol. 2011, 102, 7051–7056. [Google Scholar] [CrossRef]
- Liu, C.S.; Zhao, D.F.; Yan, L.H.; Wang, A.J.; Gu, Y.Y.; Lee, D.J. Elemental sulfur formation and nitrogen removal from wastewaters by autotrophic denitrifiers and anammox bacteria. Bioresource Technol. 2015, 191, 332–336. [Google Scholar] [CrossRef]
- Chen, T.T.; Zheng, P.; Shen, L.D.; Ding, S.; Mahmood, Q. Kinetic characteristics and microbial community of Anammox-EGSB reactor. J. Hazard. Mater. 2011, 190, 28–35. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.Y.; Cao, T.H.; Peng, Y.Z. Optimization of three-stage Anammox system removing nitrogen from landfill leachate. Bioresource Technol. 2015, 185, 450–455. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.B.; Li, B.K.; Niu, M.; Wang, S.Y.; Peng, Y.Z. Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res. 2017, 108, 46–56. [Google Scholar] [CrossRef]
- Chamchoi, N.; Nitisoravut, S.; Schmidt, J.E. Inactivation of ANAMMOX communities under concurrent operation of anaerobic ammonium oxidation (ANAMMOX) and denitrification. Bioresource Technol. 2008, 99, 3331–3336. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Xing, B.S.; Yu, J.J.; Qin, T.Y.; Chen, S.X. The importance of the substrate ratio in the operation of the Anammox process in upflow biofilter. Ecol. Eng. 2013, 53, 130–137. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Z.M.; Li, J.; Zhao, B.H.; Bian, W.; Zhang, Y.Z.; Wang, X.J. Inhibition kinetics and granular sludge in an ANAMMOX reactor treating mature landfill leachate. Water Sci. Technol. 2016, 74, 2697–2707. [Google Scholar]
- Chen, F.M.; Li, X.; Yuan, Y.; Huang, Y. An efficient way to enhance the total nitrogen removal efficiency of the Anammox process by S-0-based short-cut autotrophic denitrification. J. Environ. Sci.—China 2019, 81, 214–224. [Google Scholar] [CrossRef]
- Guo, Q.; Hu, H.Y.; Shi, Z.J.; Yang, C.C.; Li, P.; Huang, M.; Ni, W.M.; Shi, M.L.; Jin, R.C. Towards simultaneously removing nitrogen and sulfur by a novel process: Anammox and autotrophic desulfurization-denitrification (AADD). Chem. Eng. J. 2016, 297, 207–216. [Google Scholar] [CrossRef]
- Liu, Y.W.; Ni, B.J. Appropriate Fe (II) Addition Significantly Enhances Anaerobic Ammonium Oxidation (Anammox) Activity through Improving the Bacterial Growth Rate. Sci. Rep.-UK 2015, 5, 8204. [Google Scholar] [CrossRef]
- Liang, B.; Wang, L.Y.; Mbadinga, S.M.; Liu, J.F.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation. Amb. Express. 2015, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Guo, J.B.; Li, H.B.; Han, Y.; Chen, Z.; Song, Y.Y.; Xing, Y.J.; Zhang, C.Q. A combined heterotrophic and sulfur-based autotrophic process to reduce high concentration perchlorate via anaerobic baffled reactors: Performance advantages of a step-feeding strategy. Bioresource Technol. 2019, 279, 297–306. [Google Scholar] [CrossRef]
- Kelly, D.P.; Wood, A.P. Confirmation of Thiobacillus denitrificans as a species of the genus Thiobacillus, in the beta-subclass of the Proteobacteria, with strain NCIMB 9548 as the type strain. Int. J. Syst. Evol. Micr. 2000, 50, 547–550. [Google Scholar] [CrossRef]
- Xing, W.; Li, J.L.; Cong, Y.; Gao, W.; Jia, Z.J.; Li, D.S. Identification of the autotrophic denitrifying community in nitrate removal reactors by DNA-stable isotope probing. Bioresource Technol. 2017, 229, 134–142. [Google Scholar] [CrossRef]
- Stinnesbeck, W.; Frey, E.; Zell, P.; Aviles, J.; Hering, F.; Frank, N.; Arps, J.; Geenen, A.; Gescher, J.; Isenbeck-Schroter, M.; et al. Hells Bells—unique speleothems from the Yucatan Peninsula, Mexico, generated under highly specific subaquatic conditions. Palaeogeogr. Palaeocl. 2018, 489, 209–229. [Google Scholar] [CrossRef]
- Montalvo, S.; Huilinir, C.; Galvez, D.; Roca, N.; Guerrero, L. Autotrophic denitrification with sulfide as electron donor: Effect of zeolite, organic matter and temperature in batch and continuous UASB reactors. Int. Biodeter. Biodegr. 2016, 108, 158–165. [Google Scholar] [CrossRef]
- Cao, S.B.; Du, R.; Li, B.K.; Ren, N.Q.; Peng, Y.Z. High-throughput profiling of microbial community structures in an ANAMMOX-UASB reactor treating high-strength wastewater. Appl. Microbiol. Biot. 2016, 100, 6457–6467. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Li, T.; Huang, K.L.; He, X.W.; Zhang, X.X. Roles and correlations of functional bacteria and genes in the start-up of simultaneous anammox and denitrification system for enhanced nitrogen removal. Sci. Total. Environ. 2019, 655, 1355–1363. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.H.; Qian, G.S.; Hu, X.; Ye, L.L. Performance and microbial community analysis of bio-electrocoagulation on simultaneous nitrification and denitrification in submerged membrane bioreactor at limited dissolved oxygen. Bioresource Technol. 2018, 258, 168–176. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.B.; Li, B.K.; Wang, S.Y.; Peng, Y.Z. Simultaneous domestic wastewater and nitrate sewage treatment by DEnitrifying AMmonium OXidation (DEAMOX) in sequencing batch reactor. Chemosphere 2017, 174, 399–407. [Google Scholar] [CrossRef]






| Phases | Stage I | Stage II | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | I′ | II | II′ | III | III′ | IV | IV′ | IV″ | |
| Days | 1–30 | 31–60 | 61–90 | 91–120 | 121–124 | 125–135 | 136–137 | 138–148 | 149–150 | 151–161 | 162–163 | 164–165 | 166–174 |
| NH4+-N (mg/L) | 192 | 192 | 192 | 192 | 175 | 175 | 166 | 166 | 158 | 158 | 149 | 149 | 64 |
| NO3−-N (mg/L) | 234 | 234 | 234 | 234 | 251 | 251 | 260 | 260 | 268 | 268 | 277 | 277 | 78 |
| CEA (%) | 55 | 55 | 55 | 55 | 59 | 59 | 61 | 61 | 63 | 63 | 65 | 65 | 55 |
| COD (mg/L) | 0.00 | 50.00 | 100.00 | 150.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50 |
| S/N | 1.4 | 1.24 | 1.08 | 0.92 | 1.24 | 1.3 | 1.24 | 1.34 | 1.24 | 1.38 | 1.24 | 1.42 | 1.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Cheng, S.; Wang, M.; Zhang, C.; Liu, B. Feasibility of Adjusting the S2O32−/NO3− Ratio to Adapt to Dynamic Influents in Coupled Anammox and Denitrification Systems. Int. J. Environ. Res. Public Health 2020, 17, 2200. https://doi.org/10.3390/ijerph17072200
Hou Y, Cheng S, Wang M, Zhang C, Liu B. Feasibility of Adjusting the S2O32−/NO3− Ratio to Adapt to Dynamic Influents in Coupled Anammox and Denitrification Systems. International Journal of Environmental Research and Public Health. 2020; 17(7):2200. https://doi.org/10.3390/ijerph17072200
Chicago/Turabian StyleHou, Yuqian, Shaoju Cheng, Mengliang Wang, Chenyong Zhang, and Bo Liu. 2020. "Feasibility of Adjusting the S2O32−/NO3− Ratio to Adapt to Dynamic Influents in Coupled Anammox and Denitrification Systems" International Journal of Environmental Research and Public Health 17, no. 7: 2200. https://doi.org/10.3390/ijerph17072200
APA StyleHou, Y., Cheng, S., Wang, M., Zhang, C., & Liu, B. (2020). Feasibility of Adjusting the S2O32−/NO3− Ratio to Adapt to Dynamic Influents in Coupled Anammox and Denitrification Systems. International Journal of Environmental Research and Public Health, 17(7), 2200. https://doi.org/10.3390/ijerph17072200