Air Pollution, Neonatal Immune Responses, and Potential Joint Effects of Maternal Depression
Abstract
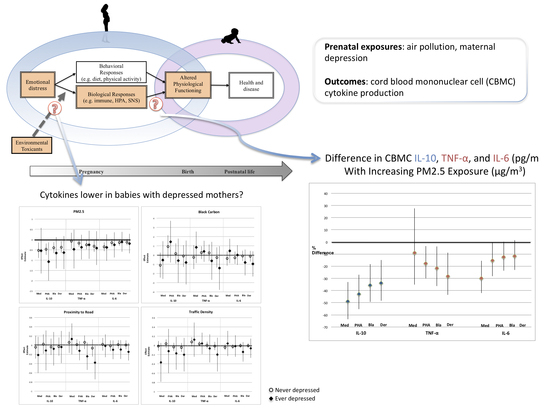
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Measures
2.2.1. Air Pollution
2.2.2. Immune Outcome Assessment
2.2.3. Depression Assessment
2.2.4. Covariate Assessment
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Air pollution and hospital admissions for heart disease in eight US counties. Epidemiology 1999, 10, 17–22. [Google Scholar] [CrossRef]
- Urch, B.; Speck, M.; Corey, P.; Wasserstein, D.; Manno, M.; Lukic, K.Z.; Brook, J.R.; Liu, L.; Coull, B.; Schwartz, J.; et al. Concentrated ambient fine particles and not ozone induce a systemic interleukin-6 response in humans. Inhal. Toxicol. 2010, 22, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, J.; Yang, X.; Zhang, Y.; Chen, Z. The role and potential pathogenic mechanism of particulate matter in childhood asthma: A review and perspective. J. Immunol. Res. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Yoshida, S.; Takano, H.; Nishikawa, M.; Miao, H.; Ichinose, T. Effects of fetal exposure to urban particulate matter on the immune system of male mouse offspring. Biol. Pharm. Bull. 2012, 35, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, K.A.; Secrest, J.R.; Lau, C.; Pulczinski, J.; Zamora, M.L.; Leal, J.; Langley, R.; Myatt, L.G.; Raju, M.; Chang, R.C.-A. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. USA 2019, 116, 3443–3448. [Google Scholar] [CrossRef] [Green Version]
- García-Serna, A.M.; Hernández-Caselles, T.; Jiménez-Guerrero, P.; Martín-Orozco, E.; Pérez-Fernández, V.; Cantero-Cano, E.; Muñoz-García, M.; Ballesteros-Meseguer, C.; de Los Cobos, I.P.; García-Marcos, L. Air pollution from traffic during pregnancy impairs newborn’s cord blood immune cells: The NELA cohort. Environ. Res. 2020. [Google Scholar] [CrossRef]
- Baïz, N.; Slama, R.; Béné, M.-C.; Charles, M.-A.; Kolopp-Sarda, M.-N.; Magnan, A.; Thiebaugeorges, O.; Faure, G.; Annesi-Maesano, I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy Childbirth 2011, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Hertz-Picciotto, I.; Herr, C.E.; Yap, P.-S.; Dostál, M.; Shumway, R.H.; Ashwood, P.; Lipsett, M.; Joad, J.P.; Pinkerton, K.E.; Šrám, R.J. Air pollution and lymphocyte phenotype proportions in cord blood. Environ. Health Perspect. 2005, 113, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Cowell, W.J.; Wright, R.J. Sex-Specific Effects of Combined Exposure to Chemical and Non-chemical Stressors on Neuroendocrine Development: A Review of Recent Findings and Putative Mechanisms. Curr. Environ. Health Rep. 2017, 4, 415–425. [Google Scholar] [CrossRef]
- Clougherty, J.E.; Rossi, C.A.; Lawrence, J.; Long, M.S.; Diaz, E.A.; Lim, R.H.; McEwen, B.; Koutrakis, P.; Godleski, J.J. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010, 118, 769. [Google Scholar] [CrossRef]
- Astell-Burt, T.; Maynard, M.J.; Lenguerrand, E.; Whitrow, M.J.; Molaodi, O.R.; Harding, S. Effect of air pollution and racism on ethnic differences in respiratory health among adolescents living in an urban environment. Health Place 2013, 23, 171–178. [Google Scholar] [CrossRef]
- Chen, E.; Schreier, H.M.; Strunk, R.C.; Brauer, M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ. Health Perspect. 2008, 116, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Clougherty, J.E.; Levy, J.I.; Kubzansky, L.D.; Ryan, P.B.; Suglia, S.F.; Canner, M.J.; Wright, R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 2007, 115, 1140–1146. [Google Scholar] [CrossRef] [Green Version]
- Morello-Frosch, R.; Shenassa, E.D. The environmental “riskscape” and social inequality: Implications for explaining maternal and child health disparities. Environ. Health Perspect. 2006, 114, 1150–1153. [Google Scholar] [CrossRef]
- Rosa, M.J.; Just, A.C.; Kloog, I.; Pantic, I.; Schnaas, L.; Lee, A.; Bose, S.; Chiu, Y.-H.M.; Hsu, H.-H.L.; Coull, B. Prenatal particulate matter exposure and wheeze in Mexican children: Effect modification by prenatal psychosocial stress. Ann. Allergyasthma Immunol. 2017, 119, 232–237.e231. [Google Scholar] [CrossRef]
- Hahn, J.; Gold, D.R.; Coull, B.A.; McCormick, M.C.; Finn, P.W.; Perkins, D.L.; Rich-Edwards, J.W.; Shiman, S.L.R.; Oken, E.; Kubzansky, L.D. Prenatal maternal depression and neonatal immune responses. Psychosom. Med. 2019, 81, 320–327. [Google Scholar] [CrossRef]
- Eaton, W.W.; Anthony, J.C.; Gallo, J.; Cai, G.; Tien, A.; Romanoski, A.; Lyketsos, C.; Chen, L.-S. Natural history of Diagnostic Interview Schedule/DSM-IV major depression: The Baltimore epidemiologic catchment area follow-up. Arch. Gen. Psychiatry 1997, 54, 993–999. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [Green Version]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oken, E.; Baccarelli, A.A.; Gold, D.R.; Kleinman, K.P.; Litonjua, A.A.; De Meo, D.; Rich-Edwards, J.W.; Rifas-Shiman, S.L.; Sagiv, S.; Taveras, E.M. Cohort Profile: Project Viva. Int. J. Epidemiol. 2014, 44, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloog, I.; Koutrakis, P.; Coull, B.A.; Lee, H.J.; Schwartz, J. Assessing temporally and spatially resolved PM 2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos. Environ. 2011, 45, 6267–6275. [Google Scholar] [CrossRef]
- Gryparis, A.; Paciorek, C.J.; Zeka, A.; Schwartz, J.; Coull, B.A. Measurement error caused by spatial misalignment in environmental epidemiology. Biostatistics 2009, 10, 258–274. [Google Scholar] [CrossRef]
- Karner, A.A.; Eisinger, D.S.; Niemeier, D.A. Near-roadway air quality: Synthesizing the findings from real-world data. Environ. Sci. Technol. 2010, 44, 5334–5344. [Google Scholar] [CrossRef]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Sioutas, C. Concentration and size distribution of ultrafine particles near a major highway. J. Air Waste Manag. Assoc. 2002, 52, 1032–1042. [Google Scholar] [CrossRef]
- Harris, M.H.; Gold, D.R.; Rifas-Shiman, S.L.; Melly, S.J.; Zanobetti, A.; Coull, B.A.; Schwartz, J.D.; Gryparis, A.; Kloog, I.; Koutrakis, P. Prenatal and Childhood Traffic-Related Pollution Exposure and Childhood Cognition in the Project Viva Cohort (Massachusetts, USA). Environ. Health Perspect. 2015, 123, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Zeka, A.; Melly, S.J.; Schwartz, J. The effects of socioeconomic status and indices of physical environment on reduced birth weight and preterm births in Eastern Massachusetts. Environ. Health Glob. Access Sci. Source 2008, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Gryparis, A.; Coull, B.A.; Schwartz, J.; Suh, H.H. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2007, 56, 183–209. [Google Scholar] [CrossRef]
- Zanobetti, A.; Austin, E.; Coull, B.A.; Schwartz, J.; Koutrakis, P. Health effects of multi-pollutant profiles. Environ. Int. 2014, 71, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Ebisu, K.; Bell, M.L. Airborne PM 2. 5 Chemical Components and Low Birth Weight in the Northeastern and Mid-Atlantic Regions of the United States. Environ. Health Perspect. 2012, 120, 1746–1752. [Google Scholar] [CrossRef] [Green Version]
- Baran, J.; Kowalczyk, D.; Oz, M.; Zembala, M. Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: Comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation. Clin. Diagn. Lab. Immunol. 2001, 8, 303–313. [Google Scholar] [CrossRef] [Green Version]
- De Groote, D.; Zangerlé, P.-F.; Gevaert, Y.; Fassotte, M.-F.; Beguin, Y.; Noizat-Pirenne, F.; Pirenne, J.; Gathy, R.; Lopez, M.; Dehart, I. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992, 4, 239–248. [Google Scholar] [CrossRef]
- Stites, D.P.; Carr, M.C.; Fudenberg, H.H. Development of cellular immunity in the human fetus: Dichotomy of proliferative and cytotoxic responses of lymphoid cells to phytohemagglutinin. Proc. Natl. Acad. Sci. USA 1972, 69, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Chan-Yeung, M.; Ferguson, A.; Chan, H.; Dimich-Ward, H.; Watson, W.; Manfreda, J.; Becker, A. Umbilical cord blood mononuclear cell proliferative response to house dust mite does not predict the development of allergic rhinitis and asthma. J. Allergy Clin. Immunol. 1999, 104, 317–321. [Google Scholar] [CrossRef]
- Clerici, M.; DePalma, L.; Roilides, E.; Baker, R.; Shearer, G. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J. Clin. Investig. 1993, 91, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Friberg, D.; Bryant, J.; Shannon, W.; Whiteside, T. In vitro cytokine production by normal human peripheral blood mononuclear cells as a measure of immunocompetence or the state of activation. Clin. Diagn. Lab. Immunol. 1994, 1, 261–268. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [Green Version]
- Milgrom, J.; Skouteris, H.; Worotniuk, T.; Henwood, A.; Bruce, L. The association between ante-and postnatal depressive symptoms and obesity in both mother and child: A systematic review of the literature. Women’s Health Issues 2012, 22, e319–e328. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kleinman, K.; Abrams, A.; Harlow, B.L.; McLaughlin, T.J.; Joffe, H.; Gillman, M.W. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J. Epidemiol. Community Health 2006, 60, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Willwerth, B.M.; Schaub, B.; Tantisira, K.G.; Gold, D.R.; Palmer, L.J.; Litonjua, A.A.; Perkins, D.L.; Schroeter, C.; Gibbons, F.K.; Gillman, M.W. Prenatal, perinatal, and heritable influences on cord blood immune responses. Ann. Allergyasthma Immunol. 2006, 96, 445–453. [Google Scholar] [CrossRef] [Green Version]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Graham, J.W.; Olchowski, A.E.; Gilreath, T.D. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev. Sci. 2007, 8, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Bolton, J.L.; Auten, R.L.; Bilbo, S.D. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brainbehav. Immun. 2014, 37, 30–44. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.-C.; Dao, K.; Roqué, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Guxens, M.; Garcia-Esteban, R.; Giorgis-Allemand, L.; Forns, J.; Badaloni, C.; Ballester, F.; Beelen, R.; Cesaroni, G.; Chatzi, L.; de Agostini, M. Air Pollution During Pregnancy and Childhood Cognitive and Psychomotor Development: Six European Birth Cohorts. Epidemiology 2014, 25, 636–647. [Google Scholar] [CrossRef] [Green Version]
- García-Serna, A.M.; Martín-Orozco, E.; Hernández-Caselles, T.; Morales, E. Prenatal and Perinatal Environmental Influences Shaping the Neonatal Immune System: A Focus on Asthma and Allergy Origins. Int. J. Environ. Res. Public Health 2021, 18, 3962. [Google Scholar] [CrossRef]
- van den Hooven, E.H.; de Kluizenaar, Y.; Pierik, F.H.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Lindemans, J.; Russcher, H.; Steegers, E.A.; Miedema, H.M.; et al. Chronic air pollution exposure during pregnancy and maternal and fetal C-reactive protein levels: The Generation R Study. Environ. Health Perspect. 2012, 120, 746–751. [Google Scholar] [CrossRef]
- Latzin, P.; Frey, U.; Armann, J.; Kieninger, E.; Fuchs, O.; Röösli, M.; Schaub, B. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS ONE 2011, 6, e23130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, J.L.; Huff, N.C.; Smith, S.H.; Mason, S.N.; Foster, W.M.; Auten, R.L.; Bilbo, S.D. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ. Health Perspect. 2013, 121, 1075. [Google Scholar] [CrossRef] [Green Version]
- Perera, F.P.; Wang, S.; Rauh, V.; Zhou, H.; Stigter, L.; Camann, D.; Jedrychowski, W.; Mroz, E.; Majewska, R. Prenatal Exposure to Air Pollution, Maternal Psychological Distress, and Child Behavior. Pediatrics 2013, 132, e1284–e1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, A.C.; Arbour, L. The Shared Pathoetiological Effects of Particulate Air Pollution and the Social Environment on Fetal-Placental Development. J. Environ. Public Health 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, R.J. Moving towards making social toxins mainstream in children’s environmental health. Curr. Opin. Pediatrics 2009, 21, 222. [Google Scholar] [CrossRef] [Green Version]
- Cao-Lei, L.; Laplante, D.P.; King, S. Prenatal Maternal Stress and Epigenetics: Review of the Human Research. Curr. Mol. Biol. Rep. 2016, 2, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.A.; Bloomberg, G.R.; Kattan, M.; Conroy, K.; Sandel, M.T.; Dresen, A.; Gergen, P.J.; Gold, D.R.; Schwarz, J.C.; Visness, C.M. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study). J. Allergy Clin. Immunol. 2011, 127, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, K.A.; Sillé, F.C. Environmental exposures during pregnancy: Mechanistic effects on immunity. Birth Defects Res. 2019, 111, 178–196. [Google Scholar] [CrossRef]
- Ly, N.P.; Rifas-Shiman, S.L.; Litonjua, A.A.; Tzianabos, A.O.; Schaub, B.; Ruiz-Pérez, B.; Tantisira, K.G.; Finn, P.W.; Gillman, M.W.; Weiss, S.T. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics 2007, 119, e171–e178. [Google Scholar] [CrossRef] [Green Version]
- Dahlhamer, J.M.; Zammitti, E.P.; Ward, B.W.; Wheaton, A.G.; Croft, J.B. Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1166–1169. [Google Scholar] [CrossRef] [Green Version]
- Shand, A.; Chen, J.; Selby, W.; Solomon, M.; Roberts, C. Inflammatory bowel disease in pregnancy: A population-based study of prevalence and pregnancy outcomes. BJOG: Int. J. Obstet. Gynaecol. 2016, 123, 1862–1870. [Google Scholar] [CrossRef]


| Characteristic | Overall n (%) | PM2.5 a,b | Distance to Road c | Traffic d | Black Carbon b |
|---|---|---|---|---|---|
| (n = 463) | (n = 322) | n = 461 | n = 459 | n = 455 | |
| Mother | |||||
| Household income ≤ $40,000/year | |||||
| No | 363 (78) | 12.1 ± 0.1 | 1107.7 ± 1.1 | 498.8 ± 1.1 | 0.69 ± 0.01 |
| Yes | 54 (12) | 12.3 ± 0.1 | 742.5 ± 1.2 | 1056.0 ± 1.2 | 0.80 ± 0.03 |
| Education ≤ high school | |||||
| No | 415 (90) | 12.0 ± 0.1 | 1074.9 ± 1.1 | 508.9 ± 1.1 | 0.69 ± 0.01 |
| Yes | 46 (10) | 12.1 ± 0.1 | 820.6 ± 1.3 | 1144.0 ± 1.2 | 0.81 ± 0.03 |
| Race/ethnicity | |||||
| Black | 62 (13) | 12.1 ± 0.1 | 544.6 ± 1.2 | 927.3 ± 1.1 | 0.82 ± 0.02 |
| Hispanic | 27 (6) | 12.2 ± 0.2 | 871.3 ± 1.4 | 686.9 ± 2.1 | 0.85 ± 0.03 |
| Other | 43 (9) | 12.0 ± 0.1 | 862.6 ± 1.2 | 766.8 ± 1.2 | 0.75 ± 0.03 |
| White | 329 (71) | 12.0 ± 0.1 | 1236.5 ± 1.1 | 469.8 ± 1.2 | 0.67 ± 0.01 |
| Pre-pregnancy BMI, kg/m2 | |||||
| Underweight, <18.5 | 19 (4) | 12.4 ± 0.3 | 614.0 ± 1.4 | 946.0 ± 1.2 | 0.76 ± 0.05 |
| Healthy weight, 18.5−<25 | 275 (59) | 12.1 ± 0.1 | 1053.6 ± 1.1 | 469.8 ± 1.2 | 0.69 ± 0.01 |
| Overweight, 25−<30 | 98 (21) | 11.9 ± 0.1 | 1339.4 ± 1.1 | 634.1 ± 1.2 | 0.71 ± 0.02 |
| Obese, >30 | 69 (15) | 12.2 ± 0.1 | 845.6 ± 1.2 | 766.8 ± 1.2 | 0.75 ± 0.03 |
| Age < 23 years | |||||
| No | 436 (94) | 12.0 ± 0.1 | 1074.9 ± 1.1 | 535.0 ± 1.1 | 0.70 ± 0.01 |
| Yes | 27 (6) | 12.3 ± 0.2 | 706.3 ± 1.3 | 974.8 ± 1.3 | 0.81 ± 0.04 |
| Pregnancy smoking status | |||||
| never | 322 (70) | 12.1 ± 0.1 | 1022.5 ± 1.1 | 503.8 ± 1.2 | 0.70 ± 0.01 |
| former | 82 (18) | 11.9 ± 0.1 | 1141.4 ± 1.2 | 579.5 ± 1.2 | 0.67 ± 0.02 |
| pregnancy | 54 (12) | 12.2 ± 0.1 | 1043.1 ± 1.2 | 899.9 ± 1.2 | 0.77 ± 0.04 |
| Cesarean delivery | |||||
| No | 370 (80) | 12.1 ± 0.1 | 1053.6 ± 1.1 | 573.8 ± 1.1 | 0.71 ± 0.01 |
| Yes | 87 (19) | 12.0 ± 0.1 | 1096.6 ± 1.2 | 438.0 ± 1.3 | 0.67 ± 0.02 |
| Probable depression in pregnancy | |||||
| No | 308 (67) | 12.1 ± 0.8 | 1152.9 ± 3.5 | 455.9 ± 14.6 | 0.68 ± 0.21 |
| Yes | 53 (11) | 12.0 ± 0.7 | 1074.9 ± 3.6 | 766.8 ± 4.0 | 0.76 ± 0.26 |
| Ever depressed | |||||
| No | 281 (61) | 12.1 ± 0.1 | 1152.9 ± 1.1 | 433.7 ± 1.2 | 0.68 ± 0.01 |
| Yes | 80 (17) | 12.1 ± 0.1 | 1096.6 ± 1.2 | 782.3 ± 1.2 | 0.75 ± 0.03 |
| Child | |||||
| Season of birth | |||||
| Winter | 116 (25) | 11.7 ± 0.1 | 1043.1 ± 1.1 | 420.8 ± 1.3 | 0.73 ± 0.02 |
| Spring | 118 (25) | 12.1 ± 0.1 | 1224.1 ± 1.1 | 474.5 ± 1.3 | 0.73 ± 0.02 |
| Summer | 123 (27) | 12.3 ± 0.1 | 1074.9 ± 1.1 | 615.4 ± 1.2 | 0.70 ± 0.02 |
| Fall | 106 (23) | 12.2 ± 0.1 | 862.6 ± 1.2 | 798.1 ± 1.2 | 0.65 ± 0.02 |
| Child sex | |||||
| Male | 253 (55) | 12.0 ± 0.1 | 1043.1 ± 1.1 | 603.2 ± 1.1 | 0.72 ± 0.01 |
| Female | 210 (45) | 12.1 ± 0.1 | 1053.6 ± 1.1 | 503.8 ± 1.2 | 0.69 ± 0.01 |
| Cytokine | Exposure | Main Effect, PM2.5 e | Main Effects, Additive Model f |
|---|---|---|---|
| % Change (95% CI) | % Change (95% CI) | ||
| IL-10 | Med g | ||
| PM2.5 | −40.12 (−57.10, −16.61) | −40.17 (−57.07, −16.61) | |
| Ever depressed | 0.26 (−52.70, 112.53) | ||
| PHA | |||
| PM2.5 | −42.83 (−55.36, −26.79) | −41.75 (−54.46, −25.49) | |
| Ever depressed | −44.3 (−66.62, −7.07) | ||
| Bla | |||
| PM2.5 | −35.59 (−49.3, −18.18) | −34.50 (−48.39, −16.88) | |
| Ever depressed | −42.34 (−65.05, −4.86) | ||
| Der b | |||
| PM2.5 | −33.85 (−48.52, −14.99) | −32.99 (−47.85, −13.91) | |
| Ever depressed | −34.56 (−60.57, 8.61) | ||
| TNF | Medium | ||
| PM2.5 | −9.16 (−35.23, 27.41) | −10.16 (−36.00, 26.12) | |
| Ever depressed | 39.9 (−31.01, 183.66) | ||
| PHA | |||
| PM2.5 | −17.77 (−29.92, −3.52) | −17.1 (−29.36, −2.72) | |
| Ever depressed | −22 (−44.33, 9.29) | ||
| Bla | |||
| PM2.5 | −21.68 (−36.34, −3.65) | −21.02 (−35.82, −2.81) | |
| Ever depressed | −23.44 (−51.05, 19.76) | ||
| Der | |||
| PM2.5 | −28.45 (−43.74, −9.00) | −27.77 (−43.21, −8.12) | |
| Ever depressed | −27.38 (−59.10, 28.94) | ||
| IL-6 | Medium | ||
| PM2.5 | −29.95 (−42.06, −15.31) | −30.16 (−42.26, −15.51) | |
| Ever depressed | 9.53 (−27.43, 65.31) | ||
| PHA | |||
| PM2.5 | −15.33 (−28.29, −0.02) | −14.97 (−28.03, 0.46) | |
| Ever depressed | −12.49 (−38.38, 24.3) | ||
| Bla | |||
| PM2.5 | −12.52 (−23.60, 0.17) | −12.30 (−23.45, 0.46) | |
| Ever depressed | −8.25 (−30.88, 21.78) | ||
| Der | |||
| PM2.5 | −11.76 (−23.13, 1.29) | −11.52 (−22.95, 1.61) | |
| Ever depressed | −8.99 (−32.06, 21.91) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, J.; Gold, D.R.; Coull, B.A.; McCormick, M.C.; Finn, P.W.; Perkins, D.L.; Rifas Shiman, S.L.; Oken, E.; Kubzansky, L.D. Air Pollution, Neonatal Immune Responses, and Potential Joint Effects of Maternal Depression. Int. J. Environ. Res. Public Health 2021, 18, 5062. https://doi.org/10.3390/ijerph18105062
Hahn J, Gold DR, Coull BA, McCormick MC, Finn PW, Perkins DL, Rifas Shiman SL, Oken E, Kubzansky LD. Air Pollution, Neonatal Immune Responses, and Potential Joint Effects of Maternal Depression. International Journal of Environmental Research and Public Health. 2021; 18(10):5062. https://doi.org/10.3390/ijerph18105062
Chicago/Turabian StyleHahn, Jill, Diane R. Gold, Brent A. Coull, Marie C. McCormick, Patricia W. Finn, David L. Perkins, Sheryl L. Rifas Shiman, Emily Oken, and Laura D. Kubzansky. 2021. "Air Pollution, Neonatal Immune Responses, and Potential Joint Effects of Maternal Depression" International Journal of Environmental Research and Public Health 18, no. 10: 5062. https://doi.org/10.3390/ijerph18105062
APA StyleHahn, J., Gold, D. R., Coull, B. A., McCormick, M. C., Finn, P. W., Perkins, D. L., Rifas Shiman, S. L., Oken, E., & Kubzansky, L. D. (2021). Air Pollution, Neonatal Immune Responses, and Potential Joint Effects of Maternal Depression. International Journal of Environmental Research and Public Health, 18(10), 5062. https://doi.org/10.3390/ijerph18105062